Abstract
Celiac disease is associated with decreased bone density, but there are conflicting data regarding fracture risk. We determined the fracture incidence relative to matched controls in a population-based cohort with celiac disease before and after diagnosis. Olmsted County residents with celiac disease (n = 83) diagnosed between 1950 and 2002 were compared with 166 gender and age matched controls. Fracture histories were ascertained from each subject’s medical records. Celiac disease is linked to an increased fracture risk before and after diagnosis. Before the index date, cases had a fracture rate twice that of controls (CI: 1.0–3.9, P = 0.045) and 2.5-fold greater after the index date (CI: 1.1–5.6, P = 0.026). Appendicular and axial fractures were 2.5 (CI: 0.9–6.5) and 3.2 times more likely (CI: 1.0–10.5) after the index date. These observations support a rationale for earlier detection of celiac disease, and active management of bone disease before bone effects have occurred, to reduce the persistent risk of fractures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Celiac disease is a chronic inflammatory disorder of the small intestine resulting from the ingestion of gluten and related substances found in cereal grains such as wheat, barley and rye [1]. The prevalence of diagnosed celiac disease varies widely from 1 per 5,000 to 2 per 300 [2, 3]. Recent studies have indicated that the prevalence of diagnosed celiac disease has increased from 1 per 4,800 to 1 per 2,000 in the United States [4]. This probably still greatly underestimates the true prevalence as judged by population-based screening studies [5]. Still considered a rare clinical entity in this country, potential celiac disease is often ignored by clinicians, despite the fact that the condition predominantly affects Caucasians, who comprise over 190 million residents of the United States.
Osteomalacia and osteoporosis have both been associated with celiac disease [6–10]. This has been postulated to result from secondary hyperparathyroidism due to vitamin D deficiency as well as systemic inflammation seen with celiac disease [4, 11, 12]. Indeed, decreased bone mineral density (BMD) has been reported even in patients with clinically silent celiac disease [13]. Despite this known correlation, few studies have investigated long-term fracture risk in this group of patients. One study has reported an increased risk of peripheral fractures in those with celiac disease. However, most fractures were recorded prior to diagnosis or in those non-compliant with their gluten-free diet [14]. Another study challenged this finding by reporting no increased risk of fractures before or after the diagnosis, yet verification of the diagnosis of celiac disease was low (78%) [15]. Another study using a matched cohort design reported no increase in fracture risk in celiac disease compared with controls. However, this was based on patient recall and there was a lower response rate among controls than cases [16]. A recent study from the UK used the general practitioner database to show a moderately increased risk of fracture in those who were prescribed gluten-free products [17]. Similarly, it has previously been demonstrated that Crohn’s disease does not put patients at increased risk for fracture compared with controls, despite the frequent presence of osteoporosis in patients with Crohn’s disease [18]. Without population-based data on fractures in known celiac patients, the true burden of osteoporosis remains unclear [19].
The aim of this study was to determine the risk of fractures in a well-defined, population-based cohort of individuals with celiac disease relative to matched controls derived from the same community. By performing this study on a well-established cohort of Olmsted County, Minnesota, residents with a diagnosis of celiac disease confirmed by standard clinical and histologic criteria, it was the researchers’ hope that this issue might be more accurately assessed. If an increase in fracture risk were clearly identified, then intervention via measurement and replacement of vitamin D along with calcium supplementation or other pharmacotherapy may prove beneficial. We also sought to specifically examine the risk of fractures before and after diagnosis since the phenomenon of reversing osteopenia with effective treatment raised the possibility that any increased fracture risk in celiac patients could be more pronounced in the time period before diagnosis [20].
Methods
Data source
Olmsted County is well suited for studies of disease associations such as this because comprehensive medical records for the residents are available for review and because these records are accessible through a centralized index of diagnoses made by essentially all medical care providers used by the local population [21]. Most gastrointestinal and trauma care, for example, is provided by the Mayo Clinic, which has maintained a common medical record with its two affiliated hospitals in the community (St. Marys and Rochester Methodist) for the past 100 years. This dossier-type record contains both inpatient and outpatient data [22]. Medical records of other providers who serve the local population, most notably the Olmsted Medical Center (with its affiliated hospital), are also indexed into this system and are available for use in approved studies [20]. This medical records linkage system (Rochester Epidemiology Project) permitted the identification of all health care records containing a diagnosis of celiac disease whether resulting from inpatient or outpatient care, death certification, or autopsy.
Study sample
Following approval by the Institutional Review Boards of Mayo Clinic and the Olmsted Medical Center, the complete inpatient and outpatient medical records of each candidate case were retrieved and reviewed for the diagnosis of celiac sprue between 1950 and 2002 by study criteria previously used in an epidemiologic review of celiac patients in Olmsted County [4].
The Olmsted County residents with celiac disease (cases) were matched by age (±2 years), sex, and closest clinic number to two county residents who did not have celiac disease (controls). The pool of controls included all Olmsted County residents who were medically attended during the calendar year of diagnosis of each case; because almost all residents have at least one contact with the medical system in any 3-year period, this pool is essentially an enumeration of the population [20]. Among potential controls, the one with the closest medical registration number was chosen. These clinic numbers are unique to each individual and are assigned at the initial visit for each patient, so this procedure matches for the duration of documented clinical history before the index date (the date of diagnosis for cases and the date of closest clinic visit for controls).
Data collection
The 83 case and 166 matched control sets were then followed forward (retrospective cohort study) and backward (retrospective case-control study) in time through their linked medical records in the community. All cases and controls had provided authorization for review of their medical records for research [23]. Each subject’s complete inpatient and outpatient record at each local provider of medical care was searched for the occurrence of any fracture. Follow-up continued until death or the most recent clinical contact documented in community medical records. The records contained the clinical history and the radiologist’s report of each fracture, but the original images were not consistently available for review. Consequently, the diagnosis of vertebral fracture was accepted on the basis of the radiologist’s report of compression or collapse of one or more thoracic or lumbar vertebrae [24]. Fractures were also classified according to the circumstance of the injury; by convention, falls from standing height or less are considered moderate trauma, whereas motor vehicle accidents and falls from a height are deemed severe trauma. Fractures of the hip, spine, or distal forearm that result from minimal or moderate trauma in patients 35 years or older are considered to represent osteoporotic fractures [25].
Statistical analysis
Comorbidity was estimated using the clinically based and convenient measure developed by Charlson et al. [26]. This comorbidity measurement tool is a weighted index which takes into account the number and seriousness of 17 chronic comorbid diseases and was readily available for this population. With each increased level of the Charlson comorbidity score, a significant step-wise increase in cumulative mortality has been demonstrated [27], therefore representing a valid approach to estimating illness burden (defined as risk of death) attributable to comorbid disease. In order to address whether comorbid conditions could have affected the risk of fractures in our analyses, we estimated the accumulated level of comorbidity in our study population, from birth to the index date, by analyzing the frequency of the comorbid conditions in cases and controls using the computerized database available through the Rochester Epidemiology Project database as previously described [18].
Stratified Cox proportional hazards models were used to compare cases and controls on the time to the first fracture after the index date [28]. Each individual case and the two matched controls form a stratum. In this analysis, the follow-up of all members of a stratum was censored at the earliest event or follow-up date of any member of the stratum. These models were estimated for any type of fracture and for peripheral, axial, and osteoporotic fractures, both unadjusted (no covariates) and adjusted for the Charlson comorbidity score. Hazard ratios (with 95% confidence intervals) were used to estimate the relative risk of fractures in cases relative to controls, and the effect of age at diagnosis of celiac disease. Kaplan–Meier cumulative incidence plots were also provided. In these plots, the follow-up times of all members of a matched set were censored as in the modeling setting.
We also assessed the risk for a fracture prior to the index date in cases relative to controls for each fracture type (any, peripheral, axial, and osteoporotic) using conditional logistic regression [29]. We estimated separate models for each fracture type, with and without adjusting for the Charlson comorbidity score. Odds ratios (with 95% confidence intervals) were used to estimate the risk of a prior fracture in cases relative to controls.
Results
Baseline characteristics (at index date)
The median age at diagnosis of celiac disease was 46 years (range 1–84 years), and 58 (70%) were female. The sex and age distribution of the controls was similar because of the matching. Table 1 demonstrates clinical characteristics for patients with and without celiac disease. Patients with celiac disease were more likely to have used bisphosphonates (5% vs 2%), corticosteroids (13% vs 7%) and supplemental calcium per se (28% vs 18%), though these rates were not significantly different (P = 0.264, 0.063, and 0.074, respectively). The diagnosis of osteopenia or osteoporosis was significantly more prevalent in cases than controls (17% vs 7%, P = 0.016) but rates of thyroid disease did not differ appreciably (5% vs 9%, P = 0.238). The mean period of observation between the date of first registration and the index date was 9.7 years in both groups due to matching, and median follow-up was 5.1 years for cases and 4.5 years for controls.
Overall fracture occurrence
A total of 39 cases had one or more fractures, with 40% occurring prior to their diagnosis date. A total of 45 controls had one or more fractures, with 32% occurring prior to the index date. Of the patients with celiac disease, 53% had no fracture, 23% had one fracture, 15% had two fractures, and 10% had three or more fractures. Among controls, 73% had no fracture, 18% had one fracture, 5% had two fractures, and 4% had three or more fractures.
Fractures before the index date
The odds that a patient with celiac disease had a fracture before the index date was greater than in controls (adjusted odds ratio = 2.0; CI: 1.0–3.9; P = 0.044; Table 2). There were similarly increased odds for appendicular (peripheral) and axial (central) fractures, although the latter was not statistically significant. A seven-fold elevation in the odds for a prior osteoporotic fracture had a large confidence interval and was not statistically significant (Table 2).
Fractures after the index date
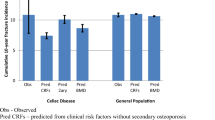
The cumulative incidence of an initial fracture (of any type) following the index date is displayed in Fig. 1. Based on the stratified proportional hazards model with comparable duration of follow-up in the two groups, the relative risk (RR) of having a fracture after the index date was greater in patients diagnosed with celiac disease than in their matched controls (adjusted RR = 2.5; CI 1.1–5.6; P = 0.030; Table 3). Figure 2 demonstrates the cumulative incidence of axial fractures in celiacs and controls. Celiac cases had an increased risk for axial fractures after the index date relative to controls (RR = 4.2; CI: 1.0–17.6; P = 0.050). A borderline statistically significant increase in the incidence of initial peripheral fractures (Fig. 3) was also observed in celiac cases (RR = 2.7; CI: 1.0–7.8; P = 0.066; Table 3). Although the cumulative incidence of osteoporotic fractures appeared to increase late in the follow-up for celiac patients this was not significantly different (Table 3).
Fractures were similarly distributed among peripheral (64 and 67%) and axial (36 and 33%) regions in cases and in controls respectively. In cases, a greater predominance of fractures affected small peripheral bones of the upper limb (30% vs 15%), whereas fractures of the lower limb long bones predominated in the controls (25% vs 12% in cases). Non-vertebral fractures could be clearly lateralized in almost all patients except five controls for whom definite lateralization information was not obtainable. Interestingly, almost two-thirds (61%) of non-vertebral fractures were left-sided in cases versus less than half (42%) in the control population.
A greater percentage of the fractures observed in celiac cases occurred “spontaneously” in the absence of a significant traumatic event (17% vs 8%) or were fractures of unknown causation (32% vs 26%).
Effect of age at diagnosis on fracture risk
There was an increased risk of fracture with age at diagnosis of celiac disease [HR = 1.050 95% CI (1.011, 1.090) P = 0.0110]. Among celiac disease cases, the age of diagnosis as a predictor of having any future fractures is significantly predictive even after controlling for Charlson count. That is, the older one’s age at diagnosis of CD, the higher one’s risk of developing at least one fracture [OR = 1.04 (per 1 year change), 95% CI: (1.01, 1.07), P = 0.0004], or alternatively, the younger the age, the lower the risk.
An “interaction model” was also ran using age, case status, and case by age using ALL subjects (i.e. the strata are the matched sets of case and two controls, with follow-up adjusted for early “censoring” in the matched set). This model again indicated an age effect, but not a case status effect, and most importantly, no case by age interaction. Thus, it would appear that the “age” effect in cases is essentially a general one, the older at index date one is the higher the fracture risk.
Discussion
Osteoporosis and osteomalacia are commonly associated with celiac disease [30, 31]. Whilst osteomalacia may be the first presentation of celiac disease, many patients with otherwise asymptomatic celiac disease are found to have significantly diminished bone density [32, 33]. Numerous factors have been postulated to effect BMD in celiac disease. For example, celiac disease has been linked to calcium malabsorption with secondary hypersecretion of PTH [6, 34–36]. Vitamin D activity is also impaired, with reduced vitamin D dependent transporter protein seen in active celiac disease [37–39]. Celiac disease has also been linked to impaired zinc absorption, leading to a reduction of IGF-1 that has been correlated with decreased BMD [12, 40–43]. Celiac disease has also been associated with amenorrhea or male hypogonadism which could also contribute to decrease bone mass [44, 45].
While it is well accepted that reduced bone density may increase fracture risk, it is less clear that the diagnosis of celiac disease per se is associated with a substantially increased risk of fractures [7, 46]. This risk is greater than that reported in a study from England [47], while it is similar to the risk of first hip fractures in Swedish children with celiac disease [48]. In this population-based study, we demonstrate that fracture risk is, in fact, elevated among patients with celiac disease. Most significantly, the increase in fracture risk persists after the diagnosis of celiac disease. This suggests the bone disease at diagnosis is often sufficiently advanced as to preclude a return to health even with subsequent diet restriction and medical management [47–49]. It is also possible that the persistent risk of fractures is due to other factors such as muscle weakness or neurologic disturbance, though that would not necessarily explain the trend for increased frequencies of spontaneous and axial fractures in the celiac cases. Based on studies involving other skeletal disorders, axial sites would be expected to show greater response to gluten-free diet, with previous studies showing a greater increase in axial BMD than appendicular BMD [50–54].
With the increase in osteoporotic fractures, as well as the increased diagnosis of osteopenia and osteoporosis in celiac patients, generalized osteoporosis would appear to be a pressing problem in these patients. Even in clinically silent disease, celiac patients without treatment have lower BMD than treated patients, with bone density increasing over time after initiation of a gluten-free diet [53–56]. Physicians need to give attention to the early detection and treatment of celiac disease, as well as active detection and management of bone disease, secondary hyperparathyroidism and vitamin D deficiency in celiac disease [17, 18, 55]. Celiac disease patients diagnosed and treated at a young age are more likely to return to normal bone density than those diagnosed and treated later in adulthood [56–59]. Given the general tendency for advancing age to increase fracture risk in both cases and controls, physicians should consider early measurement of bone mineral density in celiac patients, as well as measuring serum vitamin D levels with therapy including supplementation with calcium or vitamin D, as appropriate.
The present study has a number of strengths. The study subjects represented a population-based inception cohort, and we examined comprehensive medical records from both before and after the time that their celiac disease was first recognized. Because of the unique records linkage system in Olmsted County, which provides access to the medical records of the entire community [20], we obtained a complete ascertainment of celiac disease to the extent that the condition came to clinical attention. This allowed for inclusion of all known patients in a defined population base [4]. The resulting fractures were documented in the detailed inpatient and outpatient records that spanned each subject’s entire period of residency in the community. Fracture ascertainment was as complete as possible given the detailed clinical records reviewed (including radiology reports) for each case. As osteoporosis is not the only determinant of risk for fractures, we also addressed comorbidities using the Charlson comorbidity index convenient surrogate for secondary osteoporosis which is an important predictor of fractures in the elderly [27]. This showed that accounting for common disorders which impact morbidity did not affect the substantive increase in fracture risk seen among the celiac cases. However, the Charlson Index may be limited as a measure of all fracture risks in this population.
Conversely, there are also clear limitations of this retrospective study based on medical records. The relatively small numbers of cases diagnosed in the community setting limited the power to analyze some subcategories. However, the precision of case identification and the rigorous evaluation of fracture history before and after diagnosis would be difficult to obtain in other settings. Although it is not possible in a medical record study such as this to blind the reviewers to the subject’s status (case or control), it was only necessary to record the occurrence of specific fractures so this end-point is not prone to bias. Measurements of BMD were not routinely performed, and the role of bone loss or elevated bone turnover in fracture risk could not be directly assessed. However, BMD is largely used as a surrogate measure of fracture risk whereas this study directly assessed actual fracture occurrence. Other potentially important risks for fractures such as body mass index or smoking status were not addressed and may be important factors. Although the celiac disease population in general is largely Caucasian, it may also be difficult to generalize these data from the largely white, educated community in Olmsted County to other ethnic groups in society [20]. Despite these limitations, these data quantify the risk of fracture associated with an initial diagnosis of celiac disease and point to a need for much earlier detection and treatment of celiac disease if excess fractures are to be avoided both before and after diagnosis. The current relatively advanced age at diagnosis of celiac disease in this cohort and its evident association with excess fractures would suggest strategies to detect presymptomatic disease may be needed.
References
Farrell RJ, Kelly CP (2002) Celiac sprue. N Engl J Med 346:180–188
Ascher H, Kristiansson B (1997) The highest incidence of celiac disease in Europe: the Swedish experience. J Pediatr Gastroenterol Nutr 24:S3–6
Talley NJ, Valsovinols M, Petterson TM, Carpenter HA, Melton LJ (1994) Epidemiology of celiac sprue: a community-based study. Am J Gastroenterol 89:843–846
Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ III (2003) Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol 1:19–22
Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K (2003) Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Int Med 163:286–292
Selby PL, Davies M, Adams JE, Mawer EB (1999) Bone loss in celiac disease is related to secondary hyperparathyroidism. J Bone Miner Res 14:652–657
Sategna-Guidetti C, Grosso SB, Mengozzi G, Aimo G, Zaccaria T, Di Stefano M, Isaia GC (2000) The effects of 1-year withdrawal on bone mass, bone metabolism and nutritional status in newly-diagnosed adult coeliac disease patients. Aliment Pharmacol Ther 14:35–43
Mather KJ, Meddings JB, Beck PL, Scott RB, Hanley DA (2001) Prevalence of IgA-antiendomysial antibody in asymptomatic low bone mineral density. Am J Gastroenterol 96:120–125
Meyer D, Stavropolous S, Diamond B, Shane E, Green PH (2001) Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol 96:112–119
Stenson W, Newberry R, Lorenz R, Baldus C, Civitelli R (2005) Increased prevalence of celiac disease and need for routine screening among patients with osteoporosis. Arch Int Med 165:393–399
Sugai E, Chernavsky A, Pedreira S, Vazquez H, Niveloni S, Mazure R, Mauriro E, Ravinovich GA, Bai JC (2002) Bone-specific antibodies in sera from patients with celiac disease: characterization and implications in osteoporosis. J Clin Immunol 22:353–362
Fornari MC, Pedreira S, Niveloni S, Gonzalez D, Diez RA, Vazquez H (1998) Pre- and post-treatment serum levels of cytokines IL-1 beta, IL-6, and IL-1 receptor antagonist in celiac disease. Are they related to the associated osteopenia? Am J Gastroenterol 93:412–418
Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR (1999) The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol 94:691–696
Vasquez H, Mazure R, Gonzalez D, Flores D, Pedreira S, Niveloni S, Smecuol E, Maurino E, Bai JC (2000) Risk of fractures in celiac disease patients: a cross-sectional, case-control study. Am J Gastroenterol 95:183–189
Vestergaard P, Mosekilde L (2002) Fracture risk in patients with celiac disease, Crohn’s disease, and ulcerative colitis: a nation-wide follow-up of 16,416 patients in Denmark. Am J Epidemiol 156:1–10
Thomason K, West J, Logan RF, Coupland C, Holmes GK (2003) Fracture experience patients with coeliac disease: a population based survey. Gut 52:518–522
West J, Logan RF, Card TR, Smith C, Hubbard R (2003) Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology 125:429–436
Loftus EV, Crowson CS, Sandborn WJ, Tremaine WJ, O’Fallon WM, Melton LJ III (2002) Long-term fracture risk in patients with Crohn’s disease: a population-based study in Olmsted County, Minnesota. Gastroenterology 123:468–475
Bernstein CN, Leslie WD, Leboff MS (2003) AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 124:795–841
Valdimarsson T, Toss G, Ross I, Lofman O, Strom M (1994) Bone mineral density in coeliac disease. Scand J Gastroenterol 29:457–461
Melton LJ III (1996) History of the Rochester epidermiology program. Mayo Clin Proc 713:266–274
Kurland LT, Mogaard CA (1981) The patient record in epidermiology. Sci Am 245:54–63
Melton LJ III (1997) The threat to medical-records research. N Engl J Med 337:1466–1470
Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ III (1992) Incidence of clinically diagnosed vertebral fractures: a population based study in Rochester, Minnesota (1985)–1989. J Bone Miner Res 7:221–227
Melton LJ III (1995) Epidemiology of fractures. In: Riggs BL, Meltion LJ (eds) Osteoporosis: etiology, diagnosis, and management, 2nd edn. Lippincott-Raven, Philadelphia, pp 225–247
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 40:373–383
Gabriel SE, Crowson CS, O’Fallon WM (1999) Comorbidity in arthritis. J Rheumatol 26:2475–2479
Hosmer DW, Lemeshow S (1999) Applied survival analysis. Wiley, New York
Hosmer DW, Lemeshow S (1989) Applied logistic regression. Wiley, New York
Meyer D, Stavropolous S, Diamond B, Shane E, Green P (2001) Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol 96:112–119
Delco F, El Serag HB, Sonnenberg A (1999) Celiac sprue among US military veterans: association disorders and clinical manifestations. Dig Dis Sci 44:966–972
Fickling WE, McFarlane XA, Bhalla AK, Robertson DAF (2001) The clinical impact of metabolic bone disease in coeliac disease. Postgrad Med J 77:33–36
Walters J, Banks L, Butcher G, Fowler C (1995) Detection of low bone mineral density by dual energy X ray absorptionmetry in unsuspected suboptimally treated coeliac disease. Gut 37:220–224
Corazza GR, Di Sario A, Cecchetti L, Jorizzo RA, Di Stefano M, Minguzzi L, Brusco G, Bernardi M, Gasbarrini G (1996) Influence of pattern of clinical presentation and of gluten-free diet on bone mass and metabolism in adult coeliac disease. Bone 18:525–530
Keaveny AP, Freaney R, McKenna MJ, Masterson J, O-Donoghue DP (1996) Bone remodeling indices and secondary hyperparathyroidism in celiac disease. Am J Gastroenterol 91:1226–1231
Corazza GR, Di Sario A, Cecchetti L, Tarozzi C, Corrao G, Bernardi M, Gasbarrini G (1995) Bone mass and metabolism in patients with celiac disease. Gastroenterology 109:122–128
Vestergaard P (2003) Bone loss associated with gastrointestinal disease: prevalence and pathogenesis. Eur J Gastroenterol Hepatol 15:851–856
Staun M, Jarnum S (1988) Measurement of the 10,000-molecular weight calcium-binding protein in small-intestinal biopsy specimens from patients with malabsorption syndromes. Scand J Gastroenterol 23:827–832
Caraceni MP, Molteni N, Bardella MT, Ortolani S, Nogara A, Bianchi PA (1988) Bone and mineral metabolism in adult celiac disease. Am J Gastroenterol 83:274–277
Jameson S (2000) Coeliac disease, insulin-like growth factor, bone mineral density, and zinc. Scand J Gastroenterol 35:894–896
Devine A, Rosen C, Mohan S, Baylink D, Prince RL (1998) Effects of zinc and other nutritional factors on insulin-like growth factor I and insulin-like growth factor-binding proteins in postmenopausal women. Am J Clin Nutr 68:200–206
Wood RJ, Zheng JJ (1997) High dietary calcium intakes reduce zinc absorption and balance in humans. Am J Clin Nutr 65:1803–1809
Valdimarsson T, Arnqvist HJ, Toss G, Jarnerot G, Nystrom F, Strom M (1999) Low circulating insulin-like growth factor I in coeliac disease and its relation to bone mineral density. Scand J Gastroenterol 34:904–908
Farthing MJ, Rees LH, Dawson AM (1983) Male gonadal function in coeliac disease III pituitary regulation. Clin Endocrinol 19:661–671
Sher KS, Jayanthi V, Probert CS, Steward CR, Mayberry JF (1994) Infertility, obstetric and gynecological problems in coeliac sprue. Dig Dis 12:186–190
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259
West J, Logan RF, Card TR, Smith C, Hubbard R (2003) Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology 125:429–436
Ludvigsson JF, Michaelsson K, Ekkom A, Montgomery SM (2007) Coeliac disease and the risk of fractures—a general population-based cohort study. Aliment Pharmacol Ther 25:273–285
McFarlane XA, Bhalla AK, Reeves DE, Morgan LM, Robertson DA (1995) Osteoporosis in treated adult coeliac disease. Gut 36:710–714
Bode S, Hassager C, Gudmand-Hoyer E, Christiansen C (1991) Body composition and calcium metabolism in adult treated coeliac disease. Gut 32:1342–1345
Pistorium LR, Sweldan WH, Purdle DW, Steel SA, Howey S, Bennett JR, Sutton D (1995) Coeliac disease and bone mineral density in adult female patients. Gut 37:639–642
Mautalen C, Gonzalez D, Mazure R, Vazquez H, Lorenzetti MP, Maurino E, Niveloni S, Pedreira S, Smecuol E, Boerr LA, Bai JC (1997) Effect of treatment on bone mass, mineral metabolism and body composition in untreated celiac disease patients. Am J Gastroenterol 92:313–318
Ciacci C, Maurelli L, Klain M, Savino G, Salvatore M, Mazzacca G, Cirillo M (1997) Effects of dietary treatment on bone mineral density in adults with celiac disease: factors predicting response. Am J Gastroenterol 92:992–996
Bai JC, Gonzalez D, Mautalen C, Mazure R, Pedreira S, Vasquez H, Smecuol E, Siccardi A, Cataldi M, Niveloni S, Boerr LA, Maurino E (1997) Long-term effect of gluten restriction on bone mineral density of patients with coeliac disease. Aliment Pharmacol Ther 11:157–164
Mustalahti K, Collin P, Sievanen H, Salmi J, Maki M (1999) Osteopenia in patients with clinically silent coeliac disease warrants screening. Lancet 354:744–745
Gonzalez D, Mazure R, Mautalen C, Vasquez H, Bai J (1995) Body composition and bone mineral density in untreated and treated patients with celiac disease. Bone 16:231–234
Scott EM, Gaywood I, Scott BB (2000) Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. Gut 46(Suppl 1):I1–I8
Mora S, Weber G, Barera G, Bellini A, Pasolini D, Prinster C, Bianchi C, Chiumello G (1993) Effect of gluten-free bone mineral content in growing patients with celiac disease. Am J Clin Nutr 57:224–228
Rea F, Polito C, Marotta A, Di Toro A, Iovene A, Collini R, Rea L, Sessa G (1996) Restoration of body composition in celiac children after one year of gluten-free diet. J Pediatr Gastroenterol Nutr 23:408–412
Acknowledgments
The authors would like to thank Kathryn Jensen and Sughra Naqvi for assistance with manuscript preparation. This study was supported in part by research grants DK 57982 and AR 30582 and MO1 RR00585 to the University of Rochester General Clinical Research Center from the National Institutes of Health, US Public Health Service. The funding source had no role in the design or execution of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jafri, M.R., Nordstrom, C.W., Murray, J.A. et al. Long-term Fracture Risk in Patients with Celiac Disease: A Population-Based Study in Olmsted County, Minnesota. Dig Dis Sci 53, 964–971 (2008). https://doi.org/10.1007/s10620-007-9976-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9976-0