Abstract
The purpose of this study was to examine the expression of Wnt pathway-related genes in patients with ulcerative colitis (UC). RNA from colonoscopic biopsies from noninflammatory bowel disease (non-IBD) subjects and UC patients were obtained and examined with a Wnt-specific microarray for the expression of Wnt pathway-related genes. Paired samples from uninflamed and inflamed areas of the colon were obtained for the UC patients. WNT2B, WNT3A, WNT5B, WNT6, WNT7A, WNT9A, and WNT11 exhibited significantly increased expression in UC compared to non-IBD patients. Frizzled 3 (FZD3) and FZD4 exhibited significantly increased expression, and FZD1 and FZD5 exhibited significantly decreased expression in UC patients. Genes with increased expression in inflamed mucosa included DKK4, DVL2, SOX17, and COL1A1. There was no difference in the expression of a panel of Wnt target genes. The expression of inducible nitric oxide synthase (INOS) was variably influenced by inflammation. Significant differences in extracellular and cell-surface components of the Wnt pathway exist in the colonic mucosa of patients with UC compared with non-IBD patients, which may influence the strength or specificity of Wnt signaling. In inflammation, inhibitory components of the Wnt pathway exhibit increased expression, but no changes in Wnt pathway target gene expression are seen. The role and complex regulation of Sox17 and iNOS in IBD warrant further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Wnt signaling pathway is involved in colon carcinogenesis and in control of proliferation and differentiation in the colonic stem cell compartment [1, 2]. Activating mutations in this pathway are found in more than 80% of sporadic forms of colon cancer [3, 4], as is dysregulation of other components of the Wnt pathway, including Wnt2, Wnt5a, frizzled (Fz) receptors, and the downstream transcriptional regulator LEF1 [5, 6]. Whether this pathway plays an important role in inflammatory-bowel-disease (IBD)-associated colon cancers, or in IBD in general, remains to be specifically defined.
Signaling through the Wnt pathway begins with a Wnt ligand, a secreted growth factor that interacts with a serpentine cell-surface Fz receptor and LRP 5/6 coreceptor to initiate the signal cascade [7]. Members of the disheveled (Dsh or dvl in human nomenclature) family interact with Fz [8], APC, and axin, which leads to inhibition of glycogen synthase kinase-3β, preventing phosphorylation and degradation of β-catenin. β-catenin then accumulates and is translocated to the nucleus where it binds to members of the lymphoid-enhancer-factor/T-cell factor (LEF/TCF) family of HMG-box transcription factors [9], inducing the transcription of many target genes including MYC [10], cyclooxygenase-2 (COX-2) [11], and cyclin D1 (CCND1) [12]. β-catenin-mediated Wnt signaling is referred to as the canonical Wnt pathway. Signaling can be blocked by soluble Fz-related proteins (sFRPs, FzBs), which bind to Wnt ligands extracellularly [13] and by proteins of the dickkopf (dkk) family, which bind to the LRP surface molecule [14].
Whereas mutations that result in the stabilization of β-catenin, either in APC or β-catenin itself, are extremely common in sporadic colon cancer, such mutations appear to be rare in tumors from patients with ulcerative colitis (UC) [15–18]. Ulcerative-colitis-associated rat-colon carcinogenesis induced by 1-hydrozyanthraquinone and methylazoxymethanol acetate does not involve APC mutations [19, 20], though β-catenin mutations may be seen [21]. Nucleotide array analysis comparing inflamed intestinal mucosa of patients with ulcerative colitis to inflamed mucosa of patients with Crohn’s disease revealed increased expression of three Wnt pathway genes, SARP1 (SFRP2), frizzled (FZD), and disheveled (DVL), in samples from patients at highest risk for colon cancer—those with longstanding ulcerative colitis [22]. Recent work from our laboratory indicates that the pattern of DVL family-member expression is distinct in IBD-associated colon cancers in comparison with sporadic colon cancer [23]. Differential expression of FZD, DVL, and SFRP2 suggests that Wnt signaling may contribute to colon carcinogenesis in patients with IBD.
In this study, microarray techniques were utilized to evaluate the expression of Wnt pathway-related genes in patients with UC in order to define differences in expression between normal and IBD patients and between uninflamed and inflamed colonic mucosa in patients with UC.
Materials and methods
Patients and sample preparation
Following informed consent, biopsies of normal colonic mucosa were obtained from non-IBD patients undergoing surveillance colonoscopy and from patients with UC undergoing annual colonoscopic screening. From the latter group of patients, biopsies were obtained from both uninflamed and inflamed colonic mucosa. All biopsies were performed in duplicate from an individual area and were uniquely identified. From each matched pair of biopsy specimens, one sample was fixed and submitted for histologic analysis and one sample immediately placed in RNA-later (Qiagen, Inc., Valencia, CA, USA). Samples submitted for histologic analysis were reviewed by an experienced pathologist (FL) who was blinded from the information regarding biopsy inflammation status. Samples from non-IBD patients were utilized for microarray analysis only if deemed histologically normal. Samples from UC patients were utilized for microarray analysis only if paired samples from an uninflamed area and from an inflamed area, based on the histologic review, were both available. In this study, no biopsy specimens were read as having significant dysplasia, and no malignancies or adenomas were identified.
Microarray analysis
Six samples of colonic mucosa from non-IBD patients (normal) and six paired samples from UC patients (six uninflamed IBD and six inflamed IBD) were available for Wnt-specific microarray analysis. Biopsy samples were incubated in RNA-later at 4°C for 24 h, and RNA was then isolated utilizing Trizol reagent. cDNA probes were synthesized using a GeArray AmpoLabeling-LPR kit and hybridized to the GEArray Q Series Human Wnt Signaling Pathway Membrane-based Gene Array (SuperArray Bioscience, Frederick, MD, USA) according to the manufacturers’ protocols. These arrays are pathway focused and designed to systematically profile the expression of genes involved in and downstream of Wnt signaling. The array includes the Wnt ligands and their receptors, intracellular signaling molecules, and representative target genes. It can be used to determine the pathway activation profile by chemiluminescence image analysis with a CCD camera system. The GEArray expression analysis suite, an online image data acquisition and analysis software, was utilized to facilitate background normalization, correction for different degrees of exposure, and normalization with multiple housekeeping gene controls on each membrane. Gene expression from individual arrays and collectively from multiple Wnt microarrays were analyzed and compared.
Statistical analysis
Direct comparison between gene expression in tissue between normal and IBD uninflamed and between IBD uninflamed and IBD inflamed was made for the entire population (n = 6 in each case). The proportion of patients expressing a specific marker was compared with the proportion of patients with nonexpression using a two-sided Fisher’s exact test. Let:
Significance was set at α = 0.05 to test the null hypothesis π1 = π2 vs. π1 ≠ π2. An unpaired t test was utilized for comparison of expression in normal mucosa vs. IBD uninflamed mucosa. A paired t-test was utilized for matched samples in individual UC patients comparing IBD uninflamed to IBD inflamed mucosa. The level of gene expression was designated in arbitrary units following background correction and normalization, as indicated above, and the mean and standard deviation (SD) calculated for each individual gene marker.
Results
Wnt ligand and Fz receptor expression in IBD
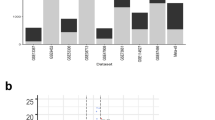
There were multiple significant differences in the expression of Wnt ligands and Fz receptors in uninflamed IBD colonic mucosa compared with normal, non-IBD mucosa (Table 1). In normal colonic mucosa, WNT1, WNT5A, and WNT10A had the highest levels of expression. In IBD uninflamed colon, WNT1, WNT2B, WNT3A, WNT5B, and WNT9A had the highest levels of expression. There was significantly higher expression in the IBD samples compared with normal for WNT2B, WNT3A, WNT5B, WNT6, WNT7A, WNT9A, and WNT11 (Fig. 1A; all P values < 0.05; WNT3A had the most significant difference, P < 0.0001). None of the Wnt ligands showed a decreased level of expression in IBD when compared with normal. The predominantly expressed Fz receptors in the normal colon were FZD1, FZD5, and FZD9, and in the IBD uninflamed mucosa FZD3, FZD4, and FZD9. Fz receptors with significantly decreased expression in IBD compared with normal included FZD1 and FZD5 (Fig. 1B; P = 0.006, P = 0.012 respectively). Fz receptors with significantly increased expression in IBD compared with normal included FZD3 and FZD4 (Fig. 1B; P = 0.002, P = 0.029, respectively).
Panel A: expression of Wnt ligands with statistically significant differential expression between normal [noninflammatory bowel disease (IBD)] colonic mucosa and uninflamed IBD colonic mucosa (mean ± SEM). Panel B: expression of frizzled (Fz) receptors with statistically significant differential expression between normal (non-IBD) colonic mucosa and uninflamed IBD colonic mucosa (mean ± SEM)
Wnt pathway-related gene expression in inflamed mucosa in IBD
In individual patients, multiple genes showed differential expression between inflamed and uninflamed mucosa (Table 2). All samples were paired so as to account for differences in clinical characteristics and treatments that may have been present for different patients. When combining microarray data from all six patients, a smaller subset of genes (Fig. 2A) was consistently noted to be differentially expressed, including DKK4; P = 0.008), DVL2; P = 0.03), SOX17 [sex-determining region Y (SRY)-box 17; P = 0.029], and collagen type I α1 (COL1A1; P = 0.015). Expression of each of these genes was increased in inflamed mucosa compared with uninflamed mucosa. Four genes were expressed at very high levels in uninflamed mucosa and had slightly decreased levels of expression in inflamed mucosa, which were statistically significant, but expression in the latter remained very high [SFRP2, WISP3, WNT3A, and CCND3; P values all <0.05]. Interestingly, the presence of inflammation had no effect on the expression of Wnt ligands or Fz receptors in the IBD patients.
Panel A: Wnt pathway-related genes with statistically significant differential expression between uninflamed inflammatory bowel disease (IBD) colonic mucosa and inflamed IBD colonic mucosa (mean ± SEM). Panel B: Wnt pathway target genes in uninflamed IBD colonic mucosa and inflamed IBD colonic mucosa (mean ± SEM). None of the differences are statistically significant
The expression of multiple Wnt target genes was examined, including CNND1, CD44, fibroblast growth factor-4 (FGF4), c-JUN, LEF1, matrix metalloproteinase-26 (MMP26), MMP7 and c-MYC (Fig. 2B). None of these genes had differential expression in inflamed IBD mucosa compared with uninflamed IBD mucosa. The expression of MMP26 was decreased but did not reach statistical significance for this cohort of six paired samples. Specifically, there was no consistent increase in any of the target genes that might indicate augmentation of Wnt pathway signaling.
Inducible nitric oxide synthase (iNOS) has been associated with the initiation and maintenance of inflammation in human IBD, is part of the intestinal antibacterial response [24], and has recently been shown to be a target gene of Wnt/β-catenin signaling [25]. Therefore, the expression of iNOS was carefully examined in each of the uninflamed and inflamed samples from IBD patients. For one patient, iNOS expression was more than three-fold higher in inflamed mucosa. However, for two patients, levels were roughly equivalent and, in three patients, expression levels in inflamed mucosa were markedly reduced (Fig. 3).
Discussion
The Wnt pathway is involved in regulation of homeostasis within the colonic stem cell compartment controlling, along with Notch signaling, the balance between proliferation and differentiation [1, 2]. Colonic mucosal proliferation is increased in UC, especially in the setting of high-grade dysplasia [26]. However, whereas some studies have suggested that Wnt signaling is involved in UC-related colon cancer [23], others suggest that UC-associated colon cancers arise along a different pathway than sporadic, Wnt/APC driven, colon cancers [27]. Characteristics of Wnt signaling in UC mucosa compared with colonic mucosa of non-UC patients has not been previously defined.
We demonstrate here significant differences in the expression of Wnt ligands and Fz receptors in patients with UC compared with non-UC normal controls. Because the function of different Wnt ligands and Fz receptors is poorly understood, it is unclear what functional significance these differences portend or whether the differential expression is a result of, or contributes to, the pathophysiology of IBD. Interestingly, Fz4 has significantly higher expression in the mucosa from the UC patients, and this receptor, in addition to binding Wnt ligands, can bind to the non-Wnt protein norrin to initiate a signaling cascade [28]. The expression of norrin in patients with UC has not been reported and was not tested in this study.
The lack of increase in the expression of numerous Wnt target genes in inflamed UC mucosa suggests that Wnt signaling is not of critical importance in the inflammatory process. Despite a prior report that CCND1 is increased in active UC [29], no increase was seen in this study. Two Wnt-related genes that did exhibit increased expression in inflammation were DKK4 and DVL2. DKK4 inhibits Wnt signaling and DVL2, because it functions to transduce signals to the β-catenin destruction complex, is also a negative regulator of Wnt throughput. SOX17, a HMG box transcription factor, was found to be increased in inflamed mucosa. Sox17 is known to interact directly with β-catenin and, therefore, also inhibits the Wnt pathway throughput by sequestering β-catenin so that it is unavailable to bind with LEF/TCF transcription factors [30, 31]. Sox17 affects differentiation [32] and it is expression is required for the development of gut endoderm in the mouse [33]. Overexpression of Sox17 may be a response to inflammation-induced mucosa damage in active UC.
Inducible nitric oxide synthase (iNOS) is positively regulated by canonical Wnt signaling [25] and has been implicated in the pathogenesis of IBD. Increased expression of iNOS has been reported in inflamed colonic IBD tissue [34–36] which may be beneficial during acute inflammation though potentially detrimental if upregulation is sustained [24]. Lack of iNOS, however, has been implicated in the development of polyps and dysplasia in the IL10(−/−) murine model of IBD, suggesting that iNOS may be protective for the development of colonic malignancy [37]. We demonstrate significant but not consistent changes in iNOS expression in inflamed mucosa compared with uninflamed mucosa in the six patients studied. Three patients demonstrated a marked decrease in iNOS expression, one a five-fold increase and two others minimally changed. As there was no evidence of activation of Wnt signaling in inflamed mucosa in our study, regulation of iNOS in this setting appears to be Wnt independent and may vary dependent upon specific patient characteristics.
In summary, significant differences in extracellular and cell-surface components of the Wnt pathway exist in the colonic mucosa of patients with UC compared with non-IBD patients. These may influence the strength or specificity of Wnt signaling in the colon. In inflammation, inhibitory components of the Wnt pathway exhibit increased expression and no changes in Wnt pathway throughput, as measured by the expression of a panel of target genes, is seen. Finally, the role and complex regulation of Sox17 and iNOS in IBD warrant further investigation.
References
de Lau W, Barker N, Clevers H (2007) WNT signaling in the normal intestine and colorectal cancer. Front Biosci 12:471–491
Pinto D, Clevers H (2005) Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 306:357–363
Miyoshi Y, Nagase H, Ando H, Ichii S, Nakatsura S, Aoki T, Miki Y, Mori T, Nakamura Y (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1:229–233
Potter JD (1999) Colorectal cancer: molecules and populations. J Nat’l Cancer Inst 91:916–932
Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML (2001) Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet 28:53–57
Holcombe RF, Marsh JL, Waterman ML, Lin F, Milovanovic T, Truong T (2002) Expression of Wnt ligands and frizzled receptors in colonic mucosa and in colon carcinoma. Mol Pathol 55:220–226
Cadigan KM, Nusse R (1997) Wnt signaling: a common theme in animal development. Genes Dev 11:3286–3305
Klingensmith J, Nusse R, Perrimon N (1994) The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev 8:118–130
Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of β-catenin with the transcription factor LEF1. Nature 382:638–642
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Howe LR, Subbaramaiah K, Chung WJ, Dannenberg AJ, Brown AM (1999) Transcriptional activation of cyclooxygenase-2 in Wnt-1-transformed mouse mammary epithelial cells. Cancer Res 59:1572–1577
Stutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestall R, Ben-Ze’ev A (1999) The cyclin D1 gene is a target of the beta-catenin/LEF1 pathway. PNAS 96:5522–5527
Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA (1999) Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem 274:16180–16187
Zorn AM (2001) Wnt signaling: antagonistic dickkopfs. Curr Biol 11:R592–R595
Aust DE, Terdiman JP, Willenbucher RF, Chang CG, Molinaro-Clark A, Baretton GB, Loehrs U, Waldman FM (2002) The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas: a mutational analysis. Cancer 94:1421–1427
Umetani N, Sasaki S, Watanabe T, Shinozaki M, Matsuda K, Ishigami H, Ueda E, Muto T (1999) Genetic alterations in ulcerative colitis-associated neoplasia focusing on APC, K-ras gene and microsatellite instability. Jpn J Cancer Res 90:1081–1087
Tarmin L, Yin J, Harpaz N, Kozam M, Noordzij J, Antonio LB, Jiang HY, Chan O, Cymes K, Meltzer SJ (1995) Adenomatous polyposis coli gene mutations in ulcerative colitis-associated dysplasias and cancers versus sporadic colon neoplasms. Cancer Res 55:2035–2038
Tamura K (2003) Alterations of CDKNZA and mismatch repair deficiency may be important as early events of colorectal tumors associated with ulcerative colitis. Proc AACR 44:1477
Tanaka T, Kohno H, Murakami M, Shimada R, Kagami S (2000) Colitis-related rat colon carcinogenesis induced by 1-hydrozy-antraquinone and methylazoxymethanol acetate. Oncol Rep 7:501–508
Suzui M, Ushijima T, Yoshimi N, Nakagama H, Hara A, Sugimura T, Nagao M, Mori H (1997) No involvement of APC gene mutations in ulcerative colitis-associated rat colon carcinogenesis induced by 1-hydrozyanthraquinone and methylazixymethanol acetate. Mol Carcinog 20:389–393
Suzui M, Ushijima T, Dashwood RH, Yoshimi N, Sugimura T, Mori H, Nagao M (1999) Frequent mutations of the rat beta-catenin gene in colon cancers induced by methylazoxymethanol acetate plus 1-hydrozyanthraquinone. Mol Carcinog 24:232–237
Uthoff SM, Eichenberger MR, Lewis RK, Fox MP, Hamilton CJ, McAuliffe TL, Grimes HL, Falandiuk S (2001) Identification of candidate genes in ulcerative colitis and Crohn’s disease using cDNA array technology. Int J Oncolo 19:803–810
You J, Bryant PJ, Jurnak F, Holcombe RF (2007) Expression of Wnt pathway components frizzled and disheveled in colon cancer arising in patients with inflammatory bowel disease. Oncol Rep 18(3):691–694
Kollios G, Valatas V, Ward SG (2004) Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology 113:427–437
Du Q, Park KS, Guo Z, He P, Nagashima M, Shao L, Sahai R, Geller DA, Hussain SP (2006) Regulation of human nitric oxide synthase 2 expression by Wnt β-catenin signaling. Cancer Res 66:7024–7031
Kullmann F, Fadaie M, Gross V, Knuchel R, Bocker T, Steinbach P, Scholmerich J, Ruschoff J (1996) Expression of proliferating cell nuclear antigen (PCNA) and Ki-67 in dysplasia in inflammatory bowel disease. Eur J Gastroenterol Hepatol 8:371–379
Mikami T, Mitomi H, Hara A, Yanagisawa N, Yoshida T, Tsuruta O, Okayasu I (2000) Decreased expression of CD44, alpha-catenin, and deleted colon carcinoma and altered expression of beta-catenin in unlcerative colitis-associated dysplasia and carcinoma, as compared with sporadic colon neoplasms. Cancer 89:733–740
Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J (2004) Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116:883–895
Wong NA, Mayer NJ, Anderson CE, McKenzie HC, Morris RG, Diebold J, Mayr D, Brock IW, Royds JA, Gilmour HM, Harrison DJ (2003) CyclinD1 and p21 in ulcerative colitis-related inflammation and epithelial neoplasia: a study of aberrant expression and underlying mechanisms. Hum Pathol 34:580–588
Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowdky MW, Varmus HE (1999) Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell 4:487–498
Sinner D, Rankin S, Lee M, Zorn AM (2004) Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development 131:3069–3080
Park KS, Wells JM, Zorn AM, Wert SE, Whitsett JA (2006) Sox17 influences the differentiation of respiratory epithelial cells. Dev Biol 294:192–202
Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Uonekawa H, Yazaki K, Tam PP, Hayashi Y (2002) Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129:2367–2379
Lundberg S, Holst M, Hellstrom PM (2006) Expression of iNOS mRNA associated with suppression of colonic contraction in rat colitis. Acta Physiol 187:489–494
Ljung T, Lundberg S, Varsanyi M, Johansson C, Schmidt PT, Herulf M, Lundberg JO, Hellstrom PM (2006) Rectal nitric oxide as a biomarker in the treatment of inflammatory bowel disease: responders versus nonresponders. World J Gastroenterol 12:3386–3392
Porras M, Martin MT, Torres R, Vergara P (2006) Cyclical upregulated iNOS and long-term downregulated nNOS are the bases for relapse and quiescent phases in a rat model of IBD. Am J Physiol Gastrointest Liver Physiol 290:G423–G430
Zhang R, Ma A, Urbanski SJ, McCafferty DM (2006) Induction of inducible nitric oxide synthase: a protective mechanism in colitis-induced adenocarcinoma. Carcinogenesis 28(5):1122–1130
Acknowledgements
The authors thank Dr. Kestus Planutis and Dr. Chris Hope for their advice on experimental methodologies. This work supported by NIH grant DK-65642 (RFH) and a grant from the Broad Medical Research Foundation (IBD-0065R, RFH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions: Dr. You and Dr. Nguyen performed microarray experiments and analysis; Dr. Albers participated in tissue acquisition; Dr. Lin provided pathological review of all samples; Dr. Holcombe provided oversight and direction for all aspects of the research.
Rights and permissions
About this article
Cite this article
You, J., Nguyen, A.V., Albers, C.G. et al. Wnt Pathway-Related Gene Expression in Inflammatory Bowel Disease. Dig Dis Sci 53, 1013–1019 (2008). https://doi.org/10.1007/s10620-007-9973-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9973-3