Abstract
Background Gastric cancer may be considered the final step of a progressive imbalance between mucosal cell proliferation and apoptosis. CDC25 phosphatases comprise a multigene family, including CDC25A and CDC25B, that plays a crucial role in the control of cell cycle progression and has been linked to the development of human cancers. The role of CDC25 phosphatases in the pathogenesis of gastric cancers is, however, still largely unknown. Material and methods Immunohistochemical expression of CDC25A and CDC25B was investigated in matched normal and cancerous tissues from 70 patients with gastric cancer (52 intestinal and 18 diffuse type). Results In non-cancerous gastric tissues the expression of CDC25A and CDC25B was absent or weak. In gastric cancer tissues, the enhanced immunoreactivity of CDC25 phosphatases was independent of intestinal or diffuse type of gastric cancer. However, the intensity of immunostaining was related to the grade of differentiation of the tumors. Interestingly, c-myc expression was directly correlated with CDC25A and B expression. Conclusions The overexpression of CDC25A and B seems to be a common and very early event in the development of both intestinal and diffuse types of gastric cancer and may play an important role in gastric carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite a dramatic reduction in incidence and mortality rates, gastric cancer is the second most frequent cause of cancer-related death worldwide [1]. Over the last decades it has become clear that carcinogenesis evolves from progressive genotypic changes that modify original cell morphology, generating biologically transformed cells that are characterized by uncontrolled growth and by progressive de-differentiation of the histological and cytological structure [2–4]. Gastric carcinoma can be divided into two distinct types: the intestinal type and the diffuse type that can be separated by characteristic histological features [5]. Although some differences exist in the histological intermediary stages and in the frequency and timing of certain molecular alterations, both diffuse and intestinal type gastric cancers are accompanied by important common cellular changes. These include an increase in cell proliferation and alteration in apoptosis [6]. Cell cycle checkpoints are crucial in controlling cell proliferation [7]. They are frequently disrupted in tumors by activation of oncogenes and inactivation of tumor suppressor genes [8]. Thus, identification and characterization of abnormalities occurring in components of these checkpoints may extend our understanding of the multistep process of gastric carcinogenesis.
CDC25 phosphatases play key roles in cell cycle progression by controlling the activation of cyclin-dependent kinases [9–11]. CDC25 is a multigene family, comprising CDC25A, CDC25B and CDC25C [12, 13]. However, only CDC25A and CDC25B possess oncogenic properties [14]. Several studies have demonstrated overexpression of CDC25A and CDC25B in different primary human tumors supporting the key role of these genes in tumorigenesis [15–20]. Interestingly, several groups have demonstrated that the expression of CDC25 phosphatases may be induced by c-myc [21–33].
Materials and methods
Tissue samples
Tissue samples for immunohistochemical analysis were collected from patients who underwent gastric cancer surgery. The study population consisted of 70 patients, 50 men, 20 women. The mean age was 59.9 years (SD: 10.6 years, range: 26–86 years). The clinical and histological characteristics of the study population are shown in Table 1.
Histology
According to the Lauren’s classification [5], we classified the carcinoma tissues into diffuse and intestinal types of tumor. There were 52 carcinomas of the intestinal type and 18 carcinomas of the diffuse type. The grade of differentiation was poor in 41 gastric cancers, moderate in 23 and well differentiated in six tumors. The tumors were confined to the mucosa or submucosa in two cases (all of the intestinal type) and advanced in 68 cases.
Immunohistochemistry
Sections (4 μm) were dewaxed and rehydrated, then the endogenous peroxydase activity was inhibited by incubation with 0.3% H202-methanol solution (30 min at room temperature). To enhance immunostaining, sections were treated with an antigen retrieval solution (0.1 M trinatriumcitrat-dihydrate pH 6.0 and 0.1 M citric-acid solution) and heated two times in a microwave oven at high power for 7 min.
To reduce non-specific background staining, slides were incubated with normal goat serum for 20 min at room temperature. Finally, the slides were incubated with the appropriate primary antisera in humidity chambers over night at 4°C. The following primary antibodies were used: anti-human CDC25A (sc-97) polyclonal IgG, CDC25B (sc-326) polyclonal IgG, c-myc (sc-40) monoclonal IgG (Santa Cruz Biotechnology, CA).
Detection of the bound primary antibody was performed using the standard HRP-Streptavidin System (Kirkegaard & Perry Laboratories, USA). Peroxidase activity was detected with diaminobenzidine as substrate. Finally the slides were weakly counterstained with hematoxylin and mounted with a mounting medium.
Furthermore, to ensure the specificity of immunostaining we performed immunohistochemistry using consecutive sections in the absence of the primary antibody and with preimmune serum. In all of these cases, no immunostaining was detected. The degree of immunopositivity was evaluated semiquantitatively. The number of positive cells was counted and expressed as percentages. The immunoreactivity was graded as negative (0–5%), low positivity (5–25%), moderate positivity (25–50%), and high positivity (>50%).
Statistical analysis
Spearman test was used to evaluate the correlation of CDC25A and CDC25B with the grade of differentiation of the cancers as well as the correlation between the expression of CDC25A and CDC25B. The expression of CDC25A and CDC25B in relation to histotype and degree of differentiation was determined by Fisher’s exact test and non-parametric one-way ANOVA (Kruskal–Wallis test), respectively. A P-value below 0.05 was taken as the level of significance.
Results
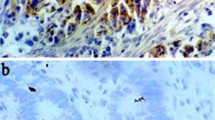
In non-neoplastic gastric mucosa the surface epithelial cells exhibited no CDC25 phosphatase immunoreactivity. However, areas with intestinal metaplasia exhibited strong CDC25 expression (Fig. 1). In the gastric cancer cells CDC25 immunoreactivity was detected in the cytoplasm of the cancer cells. Interestingly, the expression of c-myc was also observed in the cytoplasm of gastric cancer cells (Fig. 1).
Immunohistochemical analysis of CDC25A and c-myc expression in gastric cancer and intestinal metaplasia. Strong immunoreactivity specific for CDC25A was present in gastric cancer cells (A). Furthermore, areas with intestinal metaplasia also exhibited strong CDC25A immunoreactivity (B). C-myc expression was detected in gastric cancer cells (C) and areas with intestinal metaplasia (D) as well. Magnifications: A, C, 120×; B, D, 400×
In gastric cancer CDC25A and CDC25B were expressed in 87.1% and 92.9% of the cases, respectively, but the overexpression of each one of these proteins was not significantly different in relation to the histotype of gastric cancer (Table 2).
The intensity of immunostaining of CDC25A and CDC25B was directly related to the grade of differentiation of the tumors (Tables 3–5), and the immunoreactivity of each one of these markers was significantly lower in poorly differentiated than in well or moderately differentiated adenocarcinomas (P < 0.001). Furthermore, the co-expression of the two CDC phosphatases was highly statistically significant (Table 6).
Discussion
Gastric cancer is the end result of a long-term process accompanied by a sequence of molecular changes that include increased cell proliferation and alteration in apoptosis [2–4]. The imbalance between apoptosis and cell proliferation has also been observed in premalignant conditions of the stomach such as Helicobacter pylori-associated chronic gastritis, gastric atrophy, intestinal metaplasia, and dysplasia [34].
However, alterations and mutations of cell cycle regulating genes occur typically in the cancer cells resulting in a loss of the homeostasis of gastric epithelial cells [6].
CDC25 phosphatases comprise a multigene family, consisting of CDC25A, CDC25B, and CDC25C, that plays a crucial role in the control of cell cycle progression by activating cyclin-dependent kinases. CDC25A is required for entry into S phase, while CDC25B seems to be the mitotic starter phosphatase [9–11]. It has been suggested that both CDC25A and CDC25B, but not CDC25C possesses oncogenic properties [14].
The proto-oncogene c-myc has been shown to play a key role in growth control, differentiation and apoptosis and has been proposed to induce expression of CDC25A and CDC25B [33].
We examined the immunohistochemical expression of CDC25A, CDC25B and c-myc in tumoral and non-tumoral mucosa of seventy patients with primary gastric cancer. In normal mucosa we found absent or weak immunopositivity, but in tumoral areas CDC25A, CDC25B, and c-myc were frequently overexpressed. These results confirm the key role of CDC25A, CDC25B, and c-myc in gastric carcinogenesis. Frequency of overexpression of these proteins was not significantly different between the two histological types, intestinal and diffuse, of gastric cancer. However, there was strong association between CDC25A, CDC25B and c-myc overexpression and higher histological grade of differentiation.
Taken together, these data suggest that the overexpression of CDC25A, CDC25B and c-myc may contribute to the development of gastric cancer. Furthermore, the overexpression of these proteins seems to be a very early and common event in carcinogenesis of intestinal and diffuse types of gastric cancer.
The association between loss or weak expression of these molecules and poor differentiation may be explained by a possible clonal selection of these aggressive cancer cells.
Although several studies support the concept that the overexpression of CDC25A and CDC25B may play an important role in carcinogenesis, the mechanisms leading to such overexpression are still unclear [15–20]. A recent study showed that CDC25 phosphatases are direct transcriptional targets of c-myc, and overexpression of CDC25A and CDC25B is associated with overexpression of c-myc in several types of human cancers [33].
In our study we found a significant and direct relation between c-myc and CDC25 phosphatase overexpression. These results indicate that in gastric cancer the overexpression of CDC25A and B may also be a downstream event of c-myc overexpression.
In conclusion, the overexpression of CDC25A, CDC25B, and c-myc seems to be a common and very early event in the carcinogenesis of both intestinal and diffuse types of gastric cancer. Because of the direct correlation, it is likely that CDC25A and B overexpression in gastric cancer is caused by c-myc.
References
Pisani P, Parkin DM, Bray F, Ferlay J (1999) Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 83:18–29
Correa P, Shiao YH (1994) Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res 54(7 Suppl):1941s–1943s
Farber E (1988) Cancer development and its natural history. A cancer prevention perspective. Cancer 62(8 Suppl):1676–1679
Farber E (1980) Reversible and irreversible lesions in processes of cancer development. IARC Sci Publ (27):143–151
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Path Microbiol Scand 64:31–49
Moss SF (1998) Cellular markers in the gastric precancerous process. Aliment Pharmacol Ther 12(Suppl 1):91–109
Nurse P (1994) Ordering S phase and M phase in the cell cycle. Cell 79:547–550
Ebert MP, Yu J, Sung JJ, Malfertheiner P (2000) Molecular alterations in gastric cancer: the role of Helicobacter pylori. Eur J Gastroenterol Hepatol 12:795–798
Nigg EA (1995) Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays 17:471–480
Morgan D (1995) Principles of cdk regulation. Nature 374:131–134
Pines J (1993) Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci 18:195–197
Galaktionov K, Beach D (1991) Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell 67:1181–1194
Nagata A, Igarashi M, Jinno S, Suto K, Okayama H (1991) An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol 3:959–968
Galaktionov K, Jessus C, Beach D (1995) Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev 9:1046–1058
Hernandez S, Hernandez L, Bea S, Pinyol M, Nayach I, Bellosillo B et al (2000) Cdc25a and the splicing variant cdc25b2, but not cdc25b1, -b3 or -c are over-expressed in aggressive human non-Hodgkin’s lymphomas. Int J Cancer 89:148–152
Wu W, Fan YH, Kemp BL, Walsh G, Mao L (1998) Overexpression of cdc25A and cdc25B is frequent in primary non-small cell lung cancer but is not associated with overexpression of c-myc. Cancer Res 58:4082–4085
Hernandez S, Hernandez L, Bea S, Cazorla M, Fernandez PL, Nadal A et al (1998) Cdc25 cell cycle-activating phosphatases and c-myc expression in human non-Hodgkin`s lymphomas. Cancer Res 58:1762–1767
Gasparotto D, Maestro R, Piccinin S, Vukosavljevic T, Barzan L, Sulfaro S et al (1997) Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res 57:2366–2368
Yao Y, Slosberg ED, Wang L, Hibshoosh H, Zhang YJ, Xing WQ et al (1999) Increased susceptibility to carcinogen-induced mammary tumors in MMTV-Cdc25B transgenic mice. Oncogene 18:5159–5166
Broggini M, Buraggi G, Brenna A, Riva L, Codegoni AM, Torri V et al (2000) Cell cycle-related phosphatases CDC25A and B expression correlates with survival in ovarian cancer patients. Anticancer Res 20:4835–4840
Askew DS, Ashmun RA, Simmons BC, Cleveland JL (1991) Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene 6:1915–1922
Shi Y, Glynn JM, Guilbert LJ, Cotter TG, Bissonnette RP, Green DR (1992) Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science 257:212–214
Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M et al (1992) Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119–128
Yokota J, Tsunetsugu-Yokota Y, Battifora H, Le Fevre C, Cline MJ (1986) Alterations of myc, myb, and rasHa proto-oncogenes in cancers are frequent and show clinical correlation. Science 231:261–265
Korc M, Meltzer P, Trent J (1986) Enhanced expression of epidermal growth factor receptor correlates with alterations of chromosome 7 in human pancreatic cancer. Proc Natl Acad Sci USA 83:5141–5144
Kozbor D, Croce CM (1984) Amplification of the c-myc oncogene in one of five human breast carcinoma cell lines. Cancer Res 44:438–441
Alitalo K, Schwab M, Lin CC, Varmus HE, Bishop JM (1983) Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci USA 80:1707–1711
Collins S, Groudine M (1982) Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature 298:679–681
Kim YJ, Ghu HD, Kim DY, Kim HJ, Kim SK, Park CS (1993) Expression of cellular oncogenes in human gastric carcinoma: c-myc, c-erb B2, and c-Ha-ras. J Surg Oncol 54:167–170
Ninomiya I, Yonemura Y, Matsumoto H, Sugiyama K, Kamata T, Miwa K et al (1991) Expression of c-myc gene product in gastric carcinoma. Oncology 48:149–153
Han S, Kim HY, Park K, Cho HJ, Lee MS, Kim HJ et al (1999) C-myc expression is related with cell proliferation and associated with poor clinical outcome in human gastric cancer. J Korean Med Sci 14:526–530
Sanz-Ortega J, Steinberg SM, Moro E, Saez M, Lopez JA, Sierra E et al (2000) Comparative study of tumor angiogenesis and immunohistochemistry for p53, c-ErbB2, c-myc and EGFr as prognostic factors in gastric cancer. Histol Histopathol 15:455–462
Galaktionov K, Chen X, Beach D (1996) Cdc25 cell-cycle phosphatase as a target of c-myc. Nature 382:511–517
Anti M, Armuzzi A, Gasbarrini G (1998) Epithelial cell turnover and apoptosis. Ital J Gastroenterol Hepatol 30(Suppl 3):S276–S278
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xing, X., Chen, J. & Chen, M. Expression of CDC25 Phosphatases in Human Gastric Cancer. Dig Dis Sci 53, 949–953 (2008). https://doi.org/10.1007/s10620-007-9964-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9964-4