Abstract
Ginseng, the root of Panax ginseng C.A. Meyer, has been reported to exert preventive effects on gastropathy via anti-oxidative and anti-inflammatory actions. In this study, we investigated the protective effects of ginseng against ethanol-induced gastric damages in rat. To examine the preventive effect of ginseng, rats received two different ginseng extracts, A and B, 1 h prior to the administration of ethanol. Pretreatment of rats with ginseng extract A and B attenuated the ethanol-induced gastric lesions by 111 ± 48 and 142 ± 47 mm2 compared to control group (164 ± 54 mm2). Significant induction of cytoprotective heat-shock proteins HSP27 and HSP70 was found in the ginseng-administrated rats, suggesting that the restoration of the proteins might contribute to prevention of ethanol-induced gastric injuries. It is, therefore, suggested that ginseng has a protective effect against ethanol-induced gastric damages by induction of heat-shock proteins 70 and 27.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric ulcerative injuries are provoked by etiologic factors such as ethanol consumption, physiological stress, nonsteroidal anti-inflammatory drugs (NSAIDs), and Helicobacter pylori infection [1]. Excessive ethanol ingestion leads to gastric lesions characterized by mucosa edema, subepithelial hemorrhages, cellular exfoliation and inflammatory cell infiltration. Consequently, ethanol abuse impairs the integrity of the gastric mucosa barrier and histology [2–3]. Pharmacotherapy against ethanol-induced gastric damages has primarily been focused on the enhancement of mucosal defense mechanisms, thereby accelerating healing and preventing relapses [4–7]. Enhancement of mucosa secretion, blood flow, cell regeneration, and the release of endogenous protective molecules such as prostaglandins and epidermal growth factors have been considered as treatment for ethanol-induced gastric mucosa damages.
Ginseng has been used for traditional herbal medicine for over 2,000 years in Asian countries including Korea, Japan, and China, and is believed to exert beneficial effects on health and to promote longevity. Recently, intensive biochemical and molecular studies have shown pharmacological effects of ginseng in the central nervous system and cardiovascular, endocrine, and immune systems as well as in cases of cancer [8–12]. These studies suggest that the preventive effects of ginseng are due to its anti-inflammation, anti-oxidation, and cytoprotective activities [13–14]. Especially for alcohol-associated human diseases, ginseng has been shown to accelerate alcohol clearance in blood by increasing the activities of alcohol dehydrogenase and aldehyde dehydrogenase [15], attenuating plasma ethanol level [16], and reducing impairment of acquisition by ethanol intake [17]. However, the molecular mechanism of ginseng has not yet been fully elucidated.
In this study, we investigated the preventive effect of ginseng against ethanol-induced gastric damages in rat and attempted to elucidate its underlying molecular mechanisms. Our results demonstrated that pretreatment of rats with ginseng prevented ethanol-induced gastric lesions. Furthermore, COX-2 expression was significantly attenuated, and cytoprotective heat-shock proteins HSP27 and HSP70 were induced in rats who received ginseng extracts. These results suggested that ginseng has a cytoprotective effect against ethanol-induced gastric damages by induction of heat-shock proteins.
Methods
Reagents
Ginseng extracts were supplied by Ginseng Research Group, Korea Food Research Institute (Songnam, Korea). In brief, ginseng extract A was prepared from a 4-year-old Panax ginseng C.A. Meyer (Keumsan, Choongchungnam-do, Korea). The aqueous extract of ginseng was prepared by decoction in a 20-fold amount of water at 120°C for 3 h and then filtered. The residue was further extracted with a 20-fold amount of 80% ethanol for 3 h, and the organic solvent was removed. The aqueous extract was combined with the ethanol extract. The mixture was concentrated under vacuum and lyophilized with a yield of about 58% of the original ginseng powder. For preparation of ginseng extract B, 2 g of ginseng powder was extracted twice with 50 ml of water-saturated butanol (n-BuOH) at 80°C for 1 h. The extract was filtered and evaporated under vacuum. The concentrate was extracted with 50 ml of ether, and the solvent was decanted. The residue was dried at 105°C for 30 min and cooled in a desiccator. The final products were used as crude saponin fraction (ginseng extract B) of white ginseng.
The ginseng extract A or B was suspended in 0.5% carboxymethylcellulose (CMC, Sigma, St. Louis, MO) and administered to rats at a concentration of 10 mg/kg body weight (BW).
Experimental design and treatment of animals
Sprague Dawley male rats (5 months old and 200–250 g BW) were purchased from Central Lab Animal (Seoul, Korea). Animals were housed in climate-controlled conditions (24 ± 1°C at 50% relative humidity) with 12 h dark-light cycle. The experimental protocol was approved by the Animal Care and Use Committee of Ajou University and complied with the international criteria for humane use of animals in research. All animals were fed standard chow and tap water ad libitum and acclimatized for 1 week before experiment. Rats were fasted for 24 h, and then 10 mg of ginseng extract A or B suspended in 0.5% CMC/kg was orally administered. One hour later, 1 ml of absolute ethanol was orally given to the rats. The animals were sacrificed by anesthesia with excessive ether 1 h after ethanol administration.
Assessment of ethanol-induced gastric mucosal damages
The stomach was isolated quickly, opened along the greater curvature, rinsed in ice-cold saline and flattened on a glass slide. Macroscopic gastric injuries were photographed with a digital camera, and gastric lesion area (mm2) was measured. The isolated stomach tissues were further used for Western blot analysis and immunohistochemical evaluation. For microscopic histopathological evaluation, the stomach was fixed in 10% formalin solution and embedded in paraffin, and the standard hematoxylin-eosin stain was performed. The slides were assessed according to the following criteria of O’Brien: degree of mucosa edema, subepithelial hemorrhages, cellular exfoliation and inflammatory cell infiltration.
Immunohistochemical stain
For immunohistochemical analysis, 10% buffered formalin-fixed, paraffin-embedded sections were deparaffinized, rehydrated, and then boiled in 100 mM Tris-buffered saline (pH 7.6) with 5% urea in an 850-W microwave oven for 5 min, followed by two more treatments of 5 min each. Then, the sections were stained with Histostain-Plus kit (Zymed Laboratories, San Francisco, CA) according to the manufacturer’s instructions. Primary antibodies recognizing COX-2 (C-20) and iNOS (C-11) were purchased from Santa Cruz Biotechnology (California), and antibody against nitrotyrosine (HM11) was obtained from Zymed. The sections were counterstained with hematoxylin.
TUNEL assay
Genomic DNA damages by ethanol exposure were analyzed by TUNEL assay using a frozen section of rat stomach, and the assay was performed with Fluorescent FragEL DNA fragment detection kit (Oncogene, Boston, MA) according to the manufacturer’s instructions. Fluorescenin-labeled cells by TdT enzyme were visualized under a confocal microscope (Olympus, BX50F, Japan).
Western blot analysis
Proteins isolated from gastric mucosa of rats or AGS cells were electrophoresed on 12% SDS-PAGE gels, and transferred to PVDF membranes by using a semidry transfer system (Hoeffer Phamacia Biotech, San Francisco, CA). Membranes were blocked by 5% nonfat dry milk for 1 h and immunoprobed with specific antibodies. Antibodies used in this study were purchased from Santa Cruz Biotechnology [HSP27 (C-20), HSP70 (W27), HSP90 (F-8)] or Calbiochem (α-tubulin, DM1A).
Cell culture and cell viability assay
Human gastric epithelial AGS cells were cultured in RPMI 1640 medium (Gibco BRL, Grand Island, NY), containing 10% fetal bovine serum and 100 units/ml penicillin, in a humidified 5% CO2 atmosphere. The cells were exposed to various concentrations of ethanol for the indicated time and used for cell viability assay and Western blot analysis. To evaluate the effect of ethanol on viability of gastric epithelial AGS cells, the cells were exposed to various concentrations of ethanol for 16 h, harvested, and stained with trypan blue. Survival (%) was calculated by the following formula: no. of viable cells (dye-excluding cells)/no. of total cells × 100.
Results
Pretreatment of rats with ginseng extracts prevents ethanol-induced gastric lesions
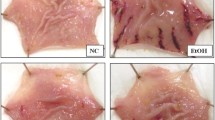
To test the protective effect of ginseng extracts on ethanol-induced gastric injuries, ginseng extract A or B at 10 mg/kg body weight was orally administered to rats 1 h before the intake of absolute ethanol. The administration of absolute ethanol induced severe hemorrhagic necrotic lesions of 164.0 ± 54.6 mm2 in the gastric mucosa (Fig. 1A and B, left panel) of control rats. Furthermore, Fig. 1B indicated that rats given absolute ethanol showed apparent changes such as formation of multifocal and diffuse epithelial erosion by exfoliation of the superficial gastric epithelium, extensive hemorrhage in mucosa, and significant edema with inflammatory cell infiltration into the mucosa and submucosa. However, rats pretreated with ginseng extracts A or B showed reduction in gross gastric lesions of 111.0 ± 45.0 and 142.0 ± 47.0 mm2, respectively (Fig. 1A and B, left panel), demonstrating that the severity of microscopic gastric injuries by ethanol intake was attenuated in the ginseng-treated rats. These findings indicate that pretreatment of rats with ginseng extracts prevents ethanol-induced gastric damages, macroscopically and microscopically.
Preventive effect of ginseng extracts against ethanol-induced gastric injury in rats. (A) Gross lesion area. Rats were pretreated orally with ginseng extract A or B at 10 mg/kg body weight prior to exposure to absolute ethanol. After 1 h of ethanol exposure, stomachs were isolated from the rats, and the area of gastric gross lesions was measured by using transparent 1 mm2 grids. Lesion area (mm2) is presented as mean ± SD. (B) Histopathology. The left panel shows macroscopic gross findings and the right panels represent microscopic histological appearances with hematoxylin and eosin stain. The gastric mucosa of rats treated with absolute ethanol shows severe erosive hemorrhagic lesions, accompanied by disruption and exfoliation of the superficial gastric mucosa (1), extensive hemorrhage (2), and inflammatory-cell infiltration (3) under high magnification (× 100)
COX-2, iNOS, and nitrotyrosine are down-regulated in rats that received ginseng extracts
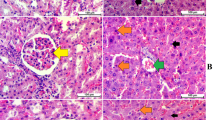
COX-2, iNOS, and nitrotyrosine have been considered as key mediators involved in the process of inflammation and oxidative stress [18–19]. Therefore, they are immunohistochemically evaluated by using antibodies recognizing them. Figure 2A showed that strong immunoreactivity of COX-2 was observed in the damaged gastric mucosa of ethanol-treated rats compared to normal rats. COX-2 was predominantly located in the cytoplasm of mucosa neck cells and parietal cells. However, the expression of COX-2 was significantly reduced in rats pretreated with ginseng extract A or B. As seen in Fig. 2B, the ginseng-treated group showed low immunoreactivity against iNOS, while moderate reactivity was observed in the ethanol-treated control group. Nitrotyrosine, an indicator of the production of NO in cell damage and inflammation processes, was found to be widely expressed, ranging from the superficial gastric epithelium to the base of the gastric gland of the control group (Fig. 2C). However, the intensity was slightly reduced in the mucosa of ginseng-treated rats. This result indicated that ethanol in the stomach imposed a burden of oxidative stress on the gastric mucosa, resulting in acute gastric lesion that could be prevented by ginseng pretreatment.
Paraffin section of stomachs was immunohistochemically stained using specific antibodies recognizing COX-2 (A), iNOS (B), and nitrityrosin (C). The figures represent immunoreactivity of each group: normal; control, rats treated with absolute ethanol; ginseng A, rats administered orally with ginseng extract A at 10 mg/kg body weight 1 h prior to ethanol treatment; and ginseng B, rats administered orally with ginseng extract B at 10 mg/kg body weight 1 h prior to ethanol treatment. The inserted photographs were taken under high magnification (× 100)
Administration of ginseng extracts rescues gastric mucosa cells from ethanol-induced cell death and strongly induces the expression of cytoprotective heat-shock proteins HSP27 and HSP70
TUNEL assay was performed to examine cell death accompanied by genomic DNA fragmentation. As shown in Fig. 3, many FITC-positive cells were found in the mucosa of rats treated with ethanol; their location corresponded to that of gastric erosive lesion in the stomach, demonstrating that gastric lesions induced by ethanol exposure were accompanied by cell death with genomic DNA damages. Ginseng pretreatment significantly rescued the gastric mucosa cells from ethanol-induced cell death. Interestingly, as shown in Fig. 4, cytoprotective heat-shock proteins HSP70 and HSP27 were overexpressed in the ginseng–pretreated rats in contrast to low expression in the control rats. The intensity of HSP27 and HSP70 in the ginseng extract A-treated group showed 2.4- and 2.8-fold increase compared to that of control group (Fig. 4B). In the group that received ginseng extract B, the increase in HSP27 was 2.1-fold and 4.25-fold for HSP70. HSP90 and α-tubulin did not display apparent differences between the groups. These data suggest that overexpression of HSP27 and HSP70 may play a cytoprotective role in ethanol-induced injuries of gastric mucosa.
Attenuation of ethanol-induced cell death with ginseng pretreatment. To evaluate cell death with genomic DNA breakage by ethanol treatment, TUNEL assay was performed. Frozen sections of rat stomach were permeabilized with 1% Triton X-100. Cells having the 3′-OH ends of DNA fragment were labeled with fluorescenin by TdT enzyme and visualized by photography (× 40 magnification). The inserted photograph was taken under high magnification (× 100). Figures are representative of each group: control, rats treated with absolute ethanol; ginseng A, rats administered orally with ginseng extract A at 10 mg/kg body weight 1 h prior to ethanol treatment; and ginseng B, rats administered orally with ginseng extract B at 10 mg/kg body weight 1 h prior to ethanol treatment
Overexpression of cytoprotective heat-shock proteins, HSP70 and HSP27, by ginseng pretreatment. Western blot analysis was performed with gastric mucosa of rats. Proteins extracted from gastric mucosa were electrophoresed, transferred to PVDF membranes and probed with antibodies recognizing HSP27, 70, 90 and α-tubulin (A). Alpha-tubulin was employed as a loading control to check the quality and quantity of proteins. Amount of protein expression was measured by densitometry and presented as average of relative intensity (x-fold) compared to that of the control group administered with ethanol (B)
HSP 27 expression confers a cytoprotective action against ethanol-provoked cell death in vitro
As shown in Fig. 3, treatment of rats with ethanol induced significant cell death in the gastric mucosa. We first examined the effect of ethanol on viability of gastric epithelial cells in cell culture system and found that ethanol exposure remarkably reduced cell viability in a dose-dependent manner (Fig. 5). Ethanol in concentrations greater than 16% showed significant cytotoxic effects on gastric epithelial cells, evidenced by marked attenuation of AGS cell viability (0% cell viability by 16% ethanol and 0.77% cell viability by 32% ethanol). On the other hand, the lower-than-2% ethanol showed no effect on cell survival.
Effect of ethanol treatment on viability of gastric epithelial AGS cells. Human gastric epithelial AGS cells were exposed to various concentrations of ethanol (0, 2, 4, 8, 16, 32%, v/v) for 16 h, and cell viability was measured by trypan blue exclusion assay. Survival (%) was calculated by the following formula: no. of viable cells (dye-excluded cells)/no. of total cells × 100. The graph shows a mean of three independent experiments
Next, we examined the possibility of whether restoration of the heat-shock proteins by ginseng, as shown in Fig. 4, could confer a cytoprotective role in ethanol-induced cell death. Thus, to evaluate the relationship between HSP27 expression and cell death, we checked the cell viability after ethanol treatment in the presence or absence of siRNA specific to HSP27. Human gastric epithelial AGS cells abundantly expressed endogenous HSP27. Incubation of AGS cells with siRNA HSP27 (10 μM) for 24 h attenuated HSP27 expression by 38% compared with control AGS. Treatment of AGS cells with 8% ethanol reduced cell viability to 79.49% (Fig. 6). On the other hand, down-regulation of HSP27 by siRNA HSP27 significantly increased ethanol-induced cell death, evidenced by 45.84% cell viability. However, siRNA HSP27 itself did not alter the cell viability (98.7%). These results indicate that HSP27 confers cytoprotection in ethanol-induced cell death.
Enhancement of ethanol-induced cell death by down-regulation of heat-shock protein 27. (A) Cell viability assay. AGS cells were transfected with siRNAHSP27 for 24 h and then exposed to 8% ethanol for 16 h. After ethanol treatment, cell viability was determined. The graph is a representative of three independent experiments. (B) After measurement of cell viability, the cells were used for Western blot of HSP27
Discussion
Excess alcohol consumption has been associated with multiple pathologies. In the digestive apparatus, alcohol has commonly been considered for its toxic effects on liver and pancreas [20–21]. However, relatively little attention has been paid to its actions within the stomach. Nevertheless, studies demonstrated that alcohol abuse is associated with development of gastric diseases such as gastritis, gastric peptic ulcer disease, and even gastric cancer [22]. Alcohol stimulates acute gastritis as a result of direct mucosal damages that are further aggravated by other important risk factors such as nonsteroidal anti-inflammatory drugs (NSAIDs), acid, Helicobacter pylori infection, and physiological stress [1]. Despite widespread interest in alcohol-induced gastrointestinal diseases, the effects of acute and chronic exposure of the stomach to alcohol remain to be fully elucidated. Animal models serve as an essential tool for analyzing molecular mechanisms involved in alcohol-induced gastric diseases. Intragastric application of ethanol in animals causes acute gastric mucosal injuries characterized by linear hemorrhages, mucosal erythema and edema, scattered petechiae and erosive changes [2–3]. Furthermore, ethanol-induced gastric damages have been shown to be associated with signaling molecules that are involved in the process of inflammation, apoptosis as well as oxidative stress. Activation of inflammation-associated transcriptional factors such as NF-kB, AP-1, and C/EBPβ and expression of its target genes such as COX-2, iNOS, and several pro-inflammatory cytokines have also been proposed to be responsible for alcohol-induced cell injuries [23–24]. Notable increase in lipid peroxidation (LPO), MDA, and MPO and depletion of glutathione (GSH) have also been reported in gastric mucosa exposed to ethanol [25–26]. In the present study, we also confirmed the induction of COX-2 and iNOS after ethanol treatment of gastric mucosa in rats.
The focus on pharmacotherapy would be the enhancement of mucosal defense mechanisms to accelerate healing and prevent relapses. Mucoprotective agents such as prostaglandin, glutathione, ornoprostil, sucralfate, and misoprostol have been found to exert protective effects on ethanol-induced gastric damages [27]. In addition to these compounds, natural extracts such as green tea extracts, wogonin, and curcumin showed a potential protective effect against alcoholic diseases [28–30]. Our present findings suggest that ginseng, the root of Panax ginseng C.A. Meyer, could be a potential protective source against ethanol-induced gastric damages. Pretreatment of animals with ginseng extracts significantly ameliorated pathological changes in gastric mucosa induced by ethanol and remarkably induced cytoprotective heat-shock proteins HSP 27 and HSP70.
Heat-shock proteins (HSPs) are crucial for the maintenance of cell integrity during both normal cell growth and pathophysiological conditions [31]. By controlling binding and release, HSPs function mainly as molecular chaperones that participate in the folding and assembly of nascent and unfolding proteins and facilitate protein transport to subcellular compartments. These HSPs are classified into four major families according to their biological activities and apparent molecular weights: HSP90, HSP70, HSP60, and small HSPs including HSP27. While HSP60, HCS70 and HSP90 are constitutively expressed, HSP70 and HSP27 are induced by various conditions, including heat, oxidative stress, or drug exposure [32]. The type of HSP induced and its level of expression can determine the fate of a cell in response to stress or stimulus. HSPs may also play a cytoprotective role in gastrointestinal tissue. For example, guinea-pig gastric mucosal cells in which HSPs were induced by heat-shock treatment showed considerable resistance against injury induced by 7.5% ethanol [33]. In addition, oral administration of geranylgeranylacetone (GGA), an anti-ulcer drug, rapidly induced HSP60, heat-shock cognate protein 70, HSC70, and HSP90 in rat gastric mucosal cells, and these induced HSPs contributed to the suppression of ulcer formation induced by water-immersion restraint stress [34]. Taken together, our findings suggest that induction of HSPs by ginseng treatment may positively contribute to gastric mucosal defense and participate in cytoprotection.
In conclusion, our results demonstrated that pretreatment of rats with ginseng prevented ethanol-induced gastric lesions, and rats that received ginseng extract demonstrated significant induction of cytoprotective heat-shock proteins HSP27 and HSP70. Down-regulation of HSP27 by siRNA HSP27 also resulted in a significant increase in ethanol-induced cell death. These findings suggest that ginseng has a cytoprotective effect on ethanol-induced gastric damages through induction of heat-shock proteins.
References
Behrman SW (2005) Management of complicated peptic ulcer disease. Arch Surg 140:201–208
Guslandi M (1987) Effects of ethanol on the gastric mucosa. Dig Dis 5:21–32
Laine L, Weinstein WM (1988) Histology of alcoholic hemorrhagic “gastritis”: a prospective evaluation. Gastroenterology 94:1254–1262
Guth PH, Paulsen G, Nagata H (1984) Histologic and microcirculatory changes in alcohol-induced gastric lesions in the rat: effect of prostaglandin cytoprotection. Gastroenterology 87:1083–1090
Chamberlain CE (1993) Acute hemorrhagic gastritis. Gastroenterol Clin North Am 22:843–873
Tepperman BL, Soper BD (1993) Interaction of nitric oxide and salivary gland epidermal growth factor in the modulation of rat gastric mucosal integrity. Br J Pharmacol 110:229–234
Cho CH, Chen BW, Hui WM, Luk CT, Lam SK (1990) Endogenous prostaglandins: its role in gastric mucosal blood flow and ethanol ulceration in rats. Prostaglandins 40:397–403
Attele AS, Wu JA, Yuan CS (1999) Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58:1685–1693
Tsang D, Yeung HW, Tso WW, Peck H (1985) Ginseng saponins: influence on neurotransmitter uptake in rat brain synaptosomes. Planta Med 51:221–224
Ota T, Fujikawa-yamamoto K, Zong ZP, Yamazaki M, Odashima S, Kitagawa I, Abe H, Arichi S (1987) Plant-glycoside modulation of cell surface related to control of differentiation in cultured B16 melanoma cells. Cancer Res 47:3863–3867
Kenarova B, Neychev H, Hadjiivanova C, Petkov VD (1990) Immunomodulating activity of ginsenoside Rg1 from Panax ginseng. Jpn J Pharmacol 54:447–454
Mehendale SR, Wang CZ, Shao ZH, Li CQ, Xie JT, Aung HH, Yuan CS (2006) Chronic pretreatment with American ginseng berry and its polyphenolic constituents attenuate oxidant stress in cardiomyocytes. Eur J Pharmacol 553:209–214
Rausch WD, Liu S, Gille G, Radad K (2006) Neuroprotective effects of ginsenosides. Acta Neurobiol Exp (Wars) 66:369–375
Yun TK (2001) Panax ginseng—a non-organ-specific cancer preventive? Lancet Oncol 2:49–55
Lee YJ, Pantuck CB, Pantuck EJ (1993) Effect of ginseng on plasma levels of ethanol in the rat. Planta Med 59:17–19
Lee FC, Ko JH, Park JK, Lee JS (1987) Effects of Panax ginseng on blood alcohol clearance in man. Clin Exp Pharmacol Physiol 14:543–546
Lee SC, Moon YS, You KH (2000) Effects of red ginseng saponins and nootropic drugs on impaired acquisition of ethanol-treated rats in passive avoidance performance. J Ethnopharmacol 69:1–8
Altura BM, Gebrewold A, Zhang A, Altura BT (2002) Ethanol induces rapid lipid peroxidation and activation of nuclear factor-kappa B in cerebral vascular smooth muscle: relation to alcohol-induced brain injury in rats. Neurosci Lett 325:95–98
Nanji AA, Jokelainen K, Fotouhinia M, Rahemtulla A, Thomas P, Tipoe GL, Su GL, Dannenberg AJ (2001) Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am J Physiol Gastrointest Liver Physiol 281:G1348–G1356
McCullough AJ, O’Connor JF (1998) Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol 93:2022–2036
Schenker S, Montalvo R (1998) Alcohol and the pancreas. Recent Dev Alcohol 14:41–65
Bujanda L (2000) The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol 95:3374–3382
Gukovskaya AS, Mouria M, Gukovsky I, Reyes CN, Kasho VN, Faller LD, Pandol SJ (2002) Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology 122:106–118
Wu D, Cederbaum AI (2003) Role of p38 MAPK in CYP2E1-dependent arachidonic acid toxicity. J Biol Chem 278:1115–1124
Shaw S, Herbert V, Colman N, Jayatilleke E (1990) Effect of ethanol-generated free radicals on gastric intrinsic factor and glutathione. Alcohol 7:153–157
Hernández-Muñoz R, Montiel-Ruíz C, Vázquez-Martínez O (2000) Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab Invest 80:1161–1169
Laudanno OM, Bedini OA, San Miguel P, Cesolari JA (1987) Gastric cytoprotection induced by prostaglandin-dopaminergic mechanism. Acta Gastroenterol Latinoam 17:299–303
Lee JS, Oh TY, Kim YK, Baik JH, So S, Hahm KB, Surh YJ (2005) Protective effects of green tea polyphenol extracts against ethanol-induced gastric mucosal damages in rats: stress-responsive transcription factors and MAP kinases as potential targets. Mutat Res 579:214–224
Park S, Hahm KB, Oh TY, Jin JH, Choue R (2004) Preventive effect of the flavonoid, wogonin, against ethanol-induced gastric mucosal damage in rats. Dig Dis Sci 49:384–394
Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ (2003) Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol 284:G321–G327
Ranford JC, Henderson B (2002) Chaperonins in disease: mechanisms, models, and treatments. Mol Pathol 55:209–213
Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858
Nakamura K, Rokutan K, Marui N, Aoike A, Kawai K (1991) Induction of heat shock proteins and their implication in protection against ethanol-induced damage in cultured guinea pig gastric mucosal cells. Gastroenterology 101:161–166
Hirakawa T, Rokutan K, Nikawa T, Kishi K (1996) Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology 111:345–357
Acknowledgements
This work was partly supported by a grant of the Korea Heath 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A010383).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeo, M., Kim, DK., Cho, S.W. et al. Ginseng, the Root of Panax ginseng C.A. Meyer, Protects Ethanol-Induced Gastric Damages in Rat through the Induction of Cytoprotective Heat-Shock Protein 27. Dig Dis Sci 53, 606–613 (2008). https://doi.org/10.1007/s10620-007-9946-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9946-6