Abstract
The application of metallic stents for benign stenosis is limited due to long-term complications. We report here the results of the implantation of a novel biodegradable poly-l-lactic acid (PLLA) esophageal stent in two patients with benign esophageal stenosis after endoscopic submucosal dissection (ESD). Case 1 was a 64-year-old man who received ESD for an early squamous esophageal cancer in the middle esophagus. The mucosal defect was seven-eighths of the circumference, and the distal margin of the resection scar formed the stenosis. After balloon dilatation, the PLLA esophageal stent was endoscopically placed; for 6 months, he has not experienced any symptoms of re-stenosis. Case 2 consisted of a 62-year-old man who developed an early squamous esophageal cancer in the middle esophagus. The lesion was resected by ESD, and the mucosal defect was seven-eighths of the circumference. The resection scar formed the stenosis, and the PLLA esophageal stent was endoscopically placed. He also has not experienced any symptoms of re-stenosis for 6 months. In conclusion, the PLLA esophageal stent provides a new possibility for the management of benign esophageal strictures after ESD. Due to the biodegradable features of this stent, longer term studies are necessary to investigate the relationship between the expected disappearance of the stent and the patency of the stricture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The placement of self-expanding metallic stents is routinely used to maintain esophageal patency in patients with malignancy who either have unresectable disease or are poor candidates for surgery [1–3]. Many reports have documented the clinical effectiveness of these tools, particularly covered stents [2, 3]. However, the application of metallic stents for benign stenosis is limited due to the relatively little information that is currently available on the long-term complications, including migration, the formation of new strictures, fistula formation and hyperplastic tissue reactions [4–6].
In patients with benign esophageal strictures, it is desirable that if a stent be kept in the proper position during the repair process [7] and then be easily removed as this would reduce the chance of re-stenosis. In other words, if a stent could be constructed from a biodegradable material, then a subsequent stent removal operation would not be necessary. In addition, the loss of biodegradable stents after the proper time period may not be injurious to the body.
In Japan, the majority of esophageal cancers are squamous cell carcinomas. Since lymph node metastasis is considered to be rare in esophageal mucosal cancers, endoscopic treatment for esophageal mucosal cancers, such as endoscopic submucosal dissection (ESD), is the recommended approach in Japan [8]. ESD is a recently developed technique that enables the surgeon to perform an en bloc resection of a mucosal lesion. The quality of life after an endoscopic resection is also much better than that after an esophagectomy. However, a mucosal resection over three-fourths of the circumference may cause stenotic changes and requires balloon dilatation.
In this study, we report the results of the implantation of a novel biodegradable poly-l-lactic acid (PLLA) esophageal stent in two patients with benign esophageal stenosis after ESD. PLLA has been used for orthopedic applications in humans and has generally been found to be bioabsorbed over a few months [9].
Case reports
Description of the stent and endoscopic implantation
The PLLA esophageal stent (Tanaka-Marui stent; Marui Textile Machinery Co., Osaka, Japan) is composed of a PLLA monofilament (molecular mass: 183 kDa; diameter: 0.23 mm) with a machine-knitted design that resembles the ultra-flex metallic stent (Fig. 1a). The length and diameter of the PLLA stent was designed according to the esophageal lesion of each individual patient. One end of the PLLA stent was reduced to a diameter of 5 mm by tying with silk sutures, and the PLLA stent was fitted over an endoscope (Fig. 1b).
The Ethics Committee of Shiga University of Medical Science approved this project, and written informed consent was obtained from each patient.
Case 1
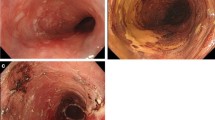
A 64-year-old man was admitted to Shiga University Hospital (Otsu, Japan) due to an early squamous esophageal cancer in the middle esophagus. The lesion consisted of two parts: one was 40 mm in diameter on the posterior wall, and the other one was 7 mm in diameter on the anterior wall (Fig. 2a). The lesion occupied three-fourths of the circumference, and endoscopic ultrasound sonography indicated that the depth of the invasion partially reached the muscularis mucosa. In accordance with the patient’s request, ESD was performed [8]. The lesion was resected en bloc, and the mucosal defect was seven-eighths of the circumference (Fig. 2b). Seven days after the resection, an episode of dysphagia appeared. As shown in Fig. 2c, the distal margin of the resection scar formed a stenosis, and a fiberscope could not pass through it. Balloon dilatation was repeated, but the stenotic changes recurred. To improve the stenosis, the PLLA esophageal stent was placed. The stenotic lesion was initially dilated to a diameter of 12–14 mm by balloon dilatation, and the PLLA esophageal stent was then fitted over a fiberscope and inserted through the stenotic portion. When the tip of the endoscope reached a point 20 mm from the anal side of the stenosis, the PLLA stent was released by pulling it out from the fiberscope (Fig. 2d). The oral end of the PLLA stent was fixed to the esophageal wall by five endoscopic clips (HX-110LR; Olympus medical System Corp. Tokyo, Japan) (Fig. 2e), and the silk suture at the distal margin was then cut by scissor forceps (FS-3L-1; Olympus) (Fig. 2f). An endoscopic follow-up study after 2 months revealed that the PLLA stent had remained at the proper position. At the time of writing – 6-month follow-up – the patient has not reported any symptoms of re-stenosis.
Endoscopic findings of the PLLA stent (case 1). Endoscopic findings of early esophageal cancer (a), the mucosal defect after ESD (b), the stenotic portion of the distal margin (c), the released PLLA stent (d), fixation of the oral side by endoscopic clips (e), the opened distal side (f), and the final view of the implanted PLLA stent (g)
Case 2
A 62-year-old man was admitted to Shiga University Hospital (Otsu, Japan) due to an early squamous esophageal cancer in the middle esophagus. The lesion occupied three-fourths of the circumference, and the depth of invasion was limited within the mucosa. The lesion was resected en bloc, and the mucosal defect was seven-eighths of the circumference. Four weeks after the resection, an episode of dysphagia appeared. The resection scar had formed a stenosis, and this was dilated by a balloon apparatus. The PLLA esophageal stent was ultimately endoscopically placed. Endoscopic follow-up study after 2 months revealed that PLLA stent had remained at the proper position. At the time of writing – 6-month follow-up – the patient has not reported any symptoms of re-stenosis.
Discussion
A biodegradable esophageal stent would appear to be the ideal approach to treating benign esophageal stenosis because it would provide patency without any long-term complications. We report here the usefulness of biodegradable PLLA stents for benign esophageal strictures after an en bloc resection of early esophageal cancer by ESD. The PLLA is polyglycoside which can be hydrolyzed and absorbed in tissues [9–11]. The same material is used in absorbable sutures. To our knowledge, the current study is the first report on the feasibility and safety of esophageal biodegradable stents in humans. No major clinical events associated with PLLA stent implantation were observed in the two patients within a 6-month follow-up and, in addition, the patency of the stenosis was well maintained and there were no complaints of re-stenotic symptoms. Although the number of patients was small and the follow-up period was short, the initial and 6-month results are quite encouraging.
PLLA has been used for orthopedic applications in humans and has generally been found to be biocompatible for at least the first few months after implantation [12]. It has been applied experimentally for various stents, including the coronary artery [13–16], bronchus [17], urinary, and biliary tracts in animals [18, 19], and investigations into their clinical use are ongoing. In humans, PLLA has already been used as coronary and urethral stents. The PLLA stent is bioabsorbed in 8 months over tissue plants [9, 11]. PLLA is flexible, which allows it to adapt to the wall of the esophageal stenosis. The radial force exerted by the PLLA esophageal stent is over 20 g/mm per centimeter, and this is enough to maintain patency and to prevent re-stenosis in esophageal benign strictures.
There is an increasing number of patients with early esophageal cancers who receive complete resection by endoscopic procedures such as ESD [8]. This treatment is less invasive than traditional surgical approaches, but when the mucosal defect covers over three-fourths of the circumference, then stenosis frequently occurs [8]. A mucosal defect that covers the entire circumference always causes stenosis. Thus, the number of patients with esophageal stenosis after ESD is increasing, and these patients are sometimes resistant to repeated balloon dilatation. In these patients, the application of biodegradable esophageal stents seems to be a reasonable option. The PLLA esophageal stent effectively relieved their stenotic symptoms. A detailed clinical follow-up of the biodegradable stent should be performed over a longer period.
In conclusion, the PLLA esophageal stent represents a new option for the management of benign esophageal strictures. Due to the biodegradable features of this stent, longer term studies are necessary to investigate the relationship between the expected disappearance of the stent and the patency of the stenosis. We are now investigating a larger number of patients with benign esophageal stenosis to determine the long-term safety and efficacy of PLLA esophageal stents.
References
Holt AP, Patel M, Ahmed MM (2004) Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 60:1010–1017
Song HY, Do YS, Han YM, Sung KB, Choi EK, Sohn KH, Kim HR, Kim SH, Min YI (1994) Covered, expandable esophageal metallic stent tubes: experiences in 119 patients. Radiology 193:689–695
Song HY, Park SI, Do YS, Yoon HK, Sung KB, Sohn KH, Min YI (1997) Expandable metallic stent placement in patients with benign esophageal strictures: results of long-term follow-up. Radiology 203:131–136
Ackroyd R, Watson DI, Devitt PG, Jamieson GG (2001) Expandable metallic stents should not be used in the treatment of benign esophageal strictures. J Gastroenterol Hepatol 16:484–487
Evrard S, Le Moine O, Lazaraki G, Dormann A, El Nakadi I, Deviere J (2004) Self-expanding plastic stents for benign esophageal lesions. Gastrointest Endosc 60:894–900
Mercer CD, Hill LD (1986) Surgical management of peptic esophageal stricture. Twenty-year experience. J Thorac Cardiovasc Surg 91:371–378
Karas SP, Gravanis MB, Santoian EC, Robinson KA, Anderberg KA, King SB 3rd (1992) Coronary intimal proliferation after balloon injury and stenting in swine: an animal model of restenosis. J Am Coll Cardiol 20:467–474
Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y (2005) Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 3:S67–S70
Kulkarni RK, Pani KC, Neuman C, Leonard F (1966) Polylactic acid for surgical implants. Arch Surg 93:839–843
Schakenraad JM, Dijkstra PJ (1991) Biocompatibility of poly (dl-lactic acid/glycine) copolymers. Clin Mater 7:253–269
Schakenraad JM, Hardonk MJ, Feijen J, Molenaar I, Nieuwenhuis P (1990) Enzymatic activity toward poly(l-lactic acid) implants. J Biomed Mater Res 24:529–545
Bostman OM (1991) Absorbable implants for the fixation of fractures. J Bone Joint Surg Am 73:148–153
Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S, Uehata H (2000) Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 102:399–404
Tsuji T, Tamai H, Igaki K, Kyo E, Kosuga K, Hata T, Okada M, Nakamura T, Komori H, Motohara S, Uehata H (2001) Biodegradable polymeric stents. Curr Interv Cardiol Rep 3:10–17
Vogt F, Stein A, Rettemeier G, Krott N, Hoffmann R, von Dahl J, Bosserhoff AK, Michaeli W, Hanrath P, Weber C, Blindt R (2004) Long-term assessment of a novel biodegradable paclitaxel-eluting coronary polylactide stent. Eur Heart J 25:1330–1340
Yamawaki T, Shimokawa H, Kozai T, Miyata K, Higo T, Tanaka E, Egashira K, Shiraishi T, Tamai H, Igaki K, Takeshita A (1998) Intramural delivery of a specific tyrosine kinase inhibitor with biodegradable stent suppresses the restenotic changes of the coronary artery in pigs in vivo. J Am Coll Cardiol 32:780–786
Robey TC, Eiselt PM, Murphy HS, Mooney DJ, Weatherly RA (2000) Biodegradable external tracheal stents and their use in a rabbit tracheal reconstruction model. Laryngoscope 110:1936–1942
Chepurov AK, Krivoborodov GG, Zubarev AV, Zaitsev NV, Markina N (2003) Biodegradable ureteral stents in treating patients with infravesical obstruction. Urologiia 44–50
Knutson T, Pettersson S, Dahlstrand C (2002) The use of biodegradable PGA stents to judge the risk of post-TURP incontinence in patients with combined bladder outlet obstruction and overactive bladder. Eur Urol 42:262–267
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, Y., Tanaka, T., Andoh, A. et al. Novel Biodegradable Stents for Benign Esophageal Strictures Following Endoscopic Submucosal Dissection. Dig Dis Sci 53, 330–333 (2008). https://doi.org/10.1007/s10620-007-9873-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9873-6