Abstract
Objective The purpose was to investigate the expression of musashi-1 (msi-1) and its significances in small intestinal mucosa that was severely damaged by high-dose 5-FU. Methods A total of 40 adult C57BL/6J mice were divided into two groups: the control group (n = 8, group A) and experimental group (n = 32). The mice in the control group were treated with PBS by intraperitoneal injection, and the other mice were treated with high-dose 5-FU (150 mg/kg body weight for 5 consecutive days) by intraperitoneal injection. At the 1st (group B), 3rd (group C) and 5th (group D) day after treatment with high-dose 5-FU, the dying mice were killed, HE staining and immunohistochemical techniques were used to detect the expression of the putative marker of intestinal epithelial stem cells, msi-1, in samples of the middle intestine from these mice, and the percentage of the msi-1-positive cells from the intestinal mucosal cells of the mice in group B was detected by FACS. Results After treatment with high-dose 5-FU, the intestinal mucosa suffered severe damage: the villi and crypts disappeared, the number of msi-1-positive cells increased greatly, the intestinal epithelial cells could be divided into two fractions by FACS, and the percentage of msi-1-positive cells was up to 67.75% in the fraction in which the value of FSC was higher. Conclusions After treatment with high-dose 5-FU, the percentage of intestinal stem cells had increased significantly, which was useful for the further isolation and enrichment of intestinal epithelial stem cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intestinal stem cells are found just above the crypt base and play a critical role in self-renewing when intestinal mucosa are in a physiological status and repairing when intestinal mucosa suffer injury [1–3]. However, because there are no specific cell surface markers for intestinal stem cells, it seemed impossible to isolate and enrich purity intestinal stem cells, and the biologic feature of intestinal stem cells cannot be understood thoroughly at present [4]. Msi-1 is a RNA-binding protein and has been reported first as a marker for neural stem cells. Recently, some research papers have revealed that msi-1 is expressed specifically in the crypt base, just above Paneth’s cells, where the predicted intestinal epithelial stem cell region exists, and the number of cells expressing msi-1 greatly conforms with intestinal stem cells; since then, msi-1 has been considered as a strong candidate marker for intestinal stem cells [5, 6]. Msi-1 was first found on the neural stem cells and plays a critical role in the asymmetric cell division of stem cells [5]. For intestinal stem cells, asymmetric cell division also occurs; one stem cell is divided into two different daughter cells: one is a stem cell, which keeps the number of stem cells homeostatic; the other is known as a transit-amplifying cell (TA), which proliferates rapidly and has a limited capacity of differentiation. The cells derive from TA, then go up to the mouth of the crypt, become terminal differentiated cells, and continue their path up to the villus tip. The terminal cells are absorptive enterocytes, enteroendocine and goblet cells [7, 8]. For asymmetric cell division, the region and the number of msi-1-expressed cells, msi-1 is considered as the marker of intestinal stem cells.
5-Fluoro-2,4(1H,3H)pyrimidinedione(5-FU), as one of the anti-tumor drugs, has a great effect on the cells of S phase and can especially kill the rapidly proliferating cells, but has relative insensitivity to cells of G0/G1 phase. During clinical chemotherapy with 5-FU, the intestinal mucosa is usually damaged, because the intestinal mucosa renew quickly all the time, and the proportion of rapidly proliferating cells is very high; as the result of this, 5-FU causes severe side effects in the intestinal mucosa, which has been confirmed by many reports [9, 10]. However, based on previous studies, it could be revealed that most of the stem cells were in G0/G1 phase and expressed rich ABC (ATP banding cassette) transporter, which could pump the cell toxicant out of the cells [11]; therefore, we could hypothesize that 5-FU had little effect on intestinal stem cells. In this paper, to investigate the characterization of intestinal stem cells, we made a mouse model of intestinal mucosa severely damaged by intraperitoneally injected high-dose 5-FU and tried to clear most of the intestinal mucosal cells, while keeping the intestinal stem cells left with this animal model; in addition, the expression of msi-1 was detected.
Materials and Methods
Animals
A total of 40 adult C57BL/6J mice (22–26 g) were obtained from the experimental animal center of Southern Medical University and allowed to acclimatize to our laboratory conditions for 7 days before any experimental manipulation. All animals were housed under constant temperature and humidity in a room with an artificial 12-h light/dark cycle, fed a standard laboratory diet, and had free access to tap water. All protocols were approved and followed by the established guidelines of the Institutional Animal Care and Use Committee of Southern Medical University.
Model of Severe Damage to the Intestinal Mucosa Induced by High-dose 5-FU and the Preparation of Samples
The mice in the experimental group (32/40) were treated with high-dose 5-FU (150 mg/kg body weight for 5 consecutive days) by intraperitoneal injection, and eight of them were selected as the empty control group (group A) and treated with PBS by intraperitoneal injection. At the 1st (group B), 3rd (group C) and 5th (group D) day after treatment with high-dose 5-FU, the dying mice in the experimental group were killed, and the middle small intestines (about 4 cm in length) were obtained by cutting open the belly. The mice in the control group were killed at the 1st day after treatment, and the middle small intestines (about 4 cm in length) were obtained. All specimens were fixed in phosphate buffer saline (PBS) containing 4% polyoxymethylene, and the fixed tissues were subsequently embedded in paraffin for the HE straining and immunochemical detection.
Mucosal Cell Preparations
Mucosal cells were prepared from the intestine of mice in group B (about 6 cm in length) for detecting the percentage of msi-1-positive cells by FACS. The individual mucosal cells were prepared according to the method of Dekaney et al. [12]. Briefly, the small intestines were flushed with Hank’s buffered saline solution (HBSS) and split open lengthwise, then cut into about 1-mm pieces and washed six to eight times with HBSS. Next, the pieces were shaken (150 rpm) in HBSS containing 0.5 U/ml dispase (Invitrogen) and 15 U/ml type III collagenase (Sigma) at room temperature for 1 h. After finishing digestion, the small pieces of tissue were pipetted up and down for 10 min gently, and then the fetal bovine serum was added to 5% to inhibit dispase/collagenase activity. The tissue was kept sedimented under gravity for 1 min, then the supernatant was removed and centrifuged at 500 rpm for 5 min, then the pellets was resuspended with HBSS and through a 70-μm filter. Finally, the HBBS-containing individual intestinal mucosal cells were prepared, and then these mucosal cells were centrifuged again and were resuspended with fixation buffer at 4°C.
Immunohistochemistry
The intestines were embedded in paraffin and sectioned to 4-μm thick. The immunostraining of msi-1 was performed according to the protocol of the Vectastain Elit ABC kit (Vector Laboratories, Burlingame, CA). After paraffin was removed, the sections were immersed in methanol containing 0.3% (v/v) H2O2 for 20 min to inactive intrinsic peroxidase and then heated in 0.01 M citric acid buffer, PH 6.0, using a microwave for 30 min. The sections were blocked with 3% BSA for 10 min, and then immersed in PBS containing 0.2% Triton X-100 for 1 h at room temperature. Then, the sections were incubated with biotinylated anti-msi-1 14H1 antibody (1:250, rat monoclonal, kindly supplied by Prof. Okano, Keio University, Tokyo) that recognizes amino acids 235–244 on msi-1 overnight at 4°C. After being washed with PBS three times, the sections were incubated with ovidin-conjugate peroxidase (Vestastain ABC Elit kit) for 30 min at room temperature, and then washed with PBS three times again. Finally, the sections were strained blown by DAB in a dark room for 3 min, incubated with hematoxylin for nuclear staining, dehydrated with ethanol, and then sealed with balsam after washing with xylene.
Flow Cytometric Analysis
After being washed three times with FACS buffer (PBS/0.2% BSA/0.02%NaN2), the fixed intestinal mucosal cells were resuspended with permeabilization wash buffer at 0.5–1.0 × 106 per 100 μl for 20 min, then incubated with anti-msi-1 rat Ig G monoclonal antibody (1:500, kindly supplied by Prof. Okano, Keio University, Tokyo) for 30 min at room temperature; after being washed three times with FACS buffer, the cells were incubated with second antibody, FITC labeled anti-rat goat Ig G monoclonal antibody (1:750, Vector Laboratories, Burlingame, CA) for 30 min in the dark; after being washed three times with FACS buffer, cells were analyzed using a FACS Calibur flow cytometer (BD Biosciences Franklin Lakes, NJ) equipped with a 15-mW, air-cooled 488-nm argon ion laser for excitation of FITC. FITC fluorescence (FL1) was collected after passing a 530/30-nm band pass filter (BP). A minimum of 10,000 events was collected on each sample. Finally, data were analyzed using CELLQuest 3.2.1.f1 software (BD Biosciences).
Statistical Analysis
Numerical results for different groups were expressed as mean ± SD. Statistical analysis was performed using one-way ANOVA and LSD-t test to compare individual groups. A P value < 0.05 was considered significant.
Results
Effects of High-dose 5-FU on the General Status of Mice
High-dose 5-FU had significant effects on the mice of the experimental group. After injecting high-dose 5-FU for a second time, the food intake of mice was decreased, and after the third time, all the mice in the experimental group refused to eat and had severe diarrhea; meanwhile, the body weight decreased significantly. At the last injection, one mouse died, and at the 1st day after treatment, three mice died, and six of the surviving mice were dying. At the 3rd day after treatment, three mice died, and eight mice were dying. At the 5th day after treatment, four mice died, and seven mice were dying. However, there was no difference in the control group. Thus, there were eight mice in group A, six mice in group B, eight mice in group C and seven mice in group D.
Effects of 5-FU on Histo-morphology
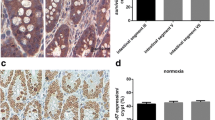
Tissue sections from the middle intestines of mice in all groups were subjected to H&E staining. In group A, the intestinal tissues were normal, and the villi, crypts and submucous structure were as they should be. However, the intestines of mice in the experimental group were damaged severely by high-dose 5-FU. On macroscopic observation, the enteric wall became thinner, and a great deal of mucous accumulation was found in the enteric cavity. The microscopic observation showed that the villi and crypts of intestines from the mice in the experimental groups had disappeared. In the enteric cavity of the mice in group D, some short villi had appeared. In addition, many cells with bigger size and the higher nuclear–cytoplasmic ratio ratios had exited in the cavity of the intestines of mice in group B, and in group D, those cells were located between the short villi (Fig. 1).
Histo-morphological changes of the intestines from the mice in different groups (HE staining). (a) Representative sections of intestines from the mice in the control group. (b) The enteric lumen of intestines from the mice in group B was covered by flat monolayer epithelial cells whose cell size was bigger than others. (c) The enteric lumen of mice in group D had grown some short villi between which were cells of bigger size
Tissue Expression of msi-1
The expression of msi-1 was very weak in the intestines of mice in group A, and only several msi-1-positive cells were located on the base of the crypt, just above the Paneth’s cell (Fig. 2a). After treatment with high-dose 5-FU, the percentage of msi-1-positive cells had increased significantly (P < 0.01, Table 1). In the enteric cavity of the mice in group B, it was shown that all cells whose size was bigger and nuclear–cytoplasmic ratio higher expressed msi-1 protein, and in group D, these cells appeared at the base of two consecutive villi (Fig. 2b, c and d).
The expression of msi-1 in the enteric lumen of mice in different groups. (a) In the enteric lumen of mice in the control group, the msi-1-positive cells were on the base of crypts, just above the Paneth’s cells. (b, c) On the surface of the enteric lumen of mice in group B, there were many msi-1-positive cells whose cell size also was bigger than others. (d) On the surface of the enteric lumen from the mice in group D, the cells that expressed msi-1 and whose cell size was bigger were located between the villi
Mucosal Cells Expression of msi-1 by FACS
After treatment of high-dose 5-FU, based on the results of FACS, the mucosal cells were found to be divided into two fractions based on their size. The fraction with the higher value of forward scatter (FSC) contained more msi-1-positive cells, and the proportion of msi-1-positive cells in this fraction was up to 67.75%; the mean value of fluorescence was 852.19. However, in the other fraction, the proportion of msi-1-positive cells was only 1.23%, and the mean value of fluorescence was 72.44 (Fig. 3).
The percentage of msi-1-positive cells in the mucosal cells from the intestines of mice in group B. The cell fraction whose value of FCS height was high expressed rich msi-1, and the percentage of msi-1-positive cells was up to 67.75%. However, the other cell fraction expressed few msi-1, and the percentage of msi-1-positive cells was only 1.23%
Discussion
The intestinal epithelium is the single layer of cells that lines the intestinal tract, and it is characterized by the highest rate of 2–3 days' renewal. Based on the high rate of self-renewal, it can be presumed that the proportion of the proliferating cells was very high, and these cells were sensitive to 5-FU. Generally, TA, the daughter cell of intestinal epithelial stem cells, produced all of the mature cells; thus, most TA cells had the status of proliferation; meanwhile, it was also revealed that in the region of TA, there were many proliferation cell nuclear antigen (PCNA) positive cells (Fig. 4), which means TA cells were on the S phase of the cell cycle [13, 14]. After treatment with high-dose 5-FU, most TA cells can be killed, and the mature cells derived from TA cells, such as enterocytes, enteroendocine, and goblet cells, will not be renewed in time, which might be the reasons for the damage induced by high-dose 5-FU. From the results of our studies, in the intestines of mice from group B, high-dose 5-FU had significant effects on the intestinal epithelium, the villi had disappeared, and the structure of the crypts also could not be found, instead of which there were flat single columnar epithelial cells embedded in connective tissue. The morphology of cells on the surface of the enteric lumen was similar to the stem cells according to the cell size, such as the big cell nucleus and the high of nuclear–cytoplasmic ratio value. The intestinal epithelial stem cells play a critical role in the procedure of regenerating the damaged intestinal mucous. It was reported that most of the stem cells were in the G0/G1 phase and expressed rich ABC transporter such as p-glycoprotein 1 and ABCG2/BCRP, which had the ability to pump the cell toxicant out of the cells [15–17]; thus, the intestinal stem cells might be insensitive to 5-FU. Our results revealed that the stem cells had survived and exited on the surface of the enteric lumen after intestinal epithelial cells were cleared by high-dose 5-FU. When the 5-FU injection was stopped, these cells began to proliferate and differentiate to regenerate the damaged intestinal epithelium induced by 5-FU. During the procedure of regeneration, the stem cells were located between the villi, and the crypt structure was formed.
Msi-1 was first demonstrated to be a specific marker for neural stem cells by Okano et al. [5, 18]. Msi-1 regulates the procedure of asymmetric cell division of stem cells by being involved in the Notch signal pathway. The Notch signal pathway maintains the stem cells in the status of undifferentiation. The Numb protein, which is a downstream protein in the Notch pathway, can bind the intercellular domain of Notch protein and leads to the degradation of Notch protein, finally downregulating the strength of the Notch pathway. Msi-1 binds the mRNA of the Numb protein and decreases the translation of Numb protein [19, 20]. Known as a cell marker of intestinal stem cells because of not being a cell surface protein, msi-1 cannot be used for isolating living intestinal stem cells, but it has been extensively used for identifying the intestinal stem cells, and it can be thought that the cells expressing msi-1 were just intestinal stem cells. It also can be found that, in the intestines of mice in group A, the cells expressed msi-1 just located on the base of the crypts, just above the Paneth’s cells, and the number of msi-1-positive cells was 6.17 ± 0.87/crypt; the percentage of them was 2.06 ± 0.13%. After intestinal epithelium was severely damaged by high-dose 5-FU, the normal intestinal epithelial structure disappeared, and a flat single layer of epithelial cells was left. The epithelium still had little potential for regeneration; at the 5th day after treatment, lumen had formed some short and big villi on the surface of the enteric, and some small intercessions had exited in the villi. The results of immunohistochemistry showed that the cells on the surface of the enteric lumen of the intestines of mice in group B had expressed msi-1 strongly, and the percentage of msi-1-positive cells reached 25.15 ± 0.49%. During the procedure of regeneration, the percentage of msi-1-positive cells increased; at the 5th day after treatment, the percentage went down to 16.20 ± 0.39%. The above-mentioned cells whose size was bigger than others all expressed msi-1, and these cells can be considered to be intestinal stem cells. Asai and his colleagues found that during the development of mouse intestines during the embryo period, the msi-1 expressed on the surface of enteric lumen, when the intestinal epithelium of 13.5 (E13.5) day fetuses still was a single layer of flat epithelial cells, just as in the intestines of mice in group A in our studies. On E15.5, msi-1 was found located between newly grown short villi, just as in the intestines of mice in group D in our studies [21]. During the development of the mouse intestine, the intestinal stem cells that express msi-1 produce their daughter cells and differentiate into the mature cells, such as absorptive enterocytes, enteroendocine and goblet cells, and finally the villi and crypt structures are formed [22, 23]. In this study, the damaged intestinal epithelium was repaired by the cells that expressed msi-1, and these cells might be just intestinal epithelial stem cells.
Unfortunately, the dearth of known cell surface markers for intestinal epithelial stem cells has precluded the used of the approach based on the use of markers and respective antibodies. For these reasons, isolating the pure intestinal epithelial stem cells seems impossible at present. Msi-1 has been considered as a specific marker for intestinal epithelial stem cells, but because of exiting in the cytoplasm and cell nucleus, msi-1 cannot be used for isolating living intestinal epithelial stem cells by FACS or magnetic-activated cell sorting (MACS). It was referred to that stem cells express rich ABC transporter, and the ABC transporter maintains a high efflux capability for not only cell toxicant, but also fluorescent dyes such as Hoechst 33342 and Rhodamine 123. These dyes have been proven remarkably powerful tools in the purification and characterization of stem cells, and the Hoechst 33342 low-staining cell fraction was called a side population (SP). The SP cells from different tissues contained rich stem cells [24–26]. Dekanery and his colleague successfully sorted SP cells from intestinal mucous by FACS, and this cell fraction expressed rich msi-1, which meant it might contain rich intestinal epithelial stem cells [11]. However, isolating SP fraction by FACS was very difficult, and the procedure of sorting had a great effect on the activity of the cells. In this paper, it can be found that the percentage of mis-1 expressed in the cell fraction whose value of FCS was high and came close to 67.75%, and we can presume that the cells whose cell size was bigger in the intestines of mice when injected with high-dose 5-FU also contained rich intestinal epithelial stem cells; based on their size, we could sort the cell fraction more easily by density gradient centrifugation. If we can successfully sort the cells whose size is bigger, we might reach the goal of isolating many more rich intestinal epithelial stem cells, and this is useful for the further study of the biological characterization of intestinal epithelial stem cells.
Reference
Booth C, Potten CS (2000) Gut instincts: thought on intestinal epithelial stem cells. J Clin Invest 105(11):1493–1499
Marshman E, Booth C, Potten CS (2002) The intestinal epithelial stem cell. Bioessays 24(1):91–98
Brittan M, Wright NA (2004) Stem cell in gastrointestinal structure and neoplastic development. Gut 53(6):899–910
Bjerknes M, Cheng H (2006) Intestinal epithelial stem cells and progenitors. Methods Enzymol 419:337–383
Nakamura M, Okano H, Blendy JA et al (1994) Musashi, a neural RNA-binding protein required for Drosophila adult eternal sensory organ development. Neuron 13:67–81
Potten CS, Booth C, Tudor GL et al (2003) Identification of putative intestinal stem cells and early lineage marker; musashi-1. Differentiation 71(28):28–41
Slorach EM, Campbell FC, Dorin JR (1999) A mouse model of intestinal stem cell function and regeneration. J Cell Sci 112(Pt 18):3029–3038
Potten CS, Owen G, Booth D (2002) Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115(Pt 11):2381–2388
Kim KA, Kakitani M, Zhao J et al (2005) Mitogenic influence of human R-spondin1 on the intestinal epitheliu. Science 309(5738):1256–1259
Ottewell PD, Duckwoth CA, Varro A et al (2006) Gastrin increases murine intestinal crypt regeneration following injury. Gastroenterology 130(4):1169–1180
Buning KD (2002) ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 20(1):11–20
Dekaney CM, Rodriguez JM, Graul MC et al (2005) Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 129(5):1567–1580
Fukui T, Takeda H, Shu HJ et al (2006) Investigation of musashi-1 expressing cells in the murine model of dextran sodium sulfate-induced colitis. Dig Dis Sci 51(7):1260–1268
Van Zant G (1984) Studies of hematopoietic stem cells spared by 5-fluorouracil. J Exp Med 159(3):679–690
Zhou S, Schuetz JD, Bunting KD et al (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7(9):1028–1034
Uchida N, Dykstra B, Lyons K et al (2004) ABC transporter activities of murine hematopoietic stem cells vary according to their developmental and activation status. Blood 103(12):4487–4495
Challen GA, Little MH (2006) A side order of stem cells: the SP phenotype. Stem Cells 24(1):3–12
Lovell MA, Geiger H, Van Zant GE et al (2006) Isolation of neural precursor cells from Alzheimer’s disease and aged control postmortem brain. Neurobiol Aging 27(7):909–917
Okano H, Kawahara H, Toriya M et al (2005) Function of RNA-binding protein musashi-1 in stem cells. Exp Cell Res 306(2):349–356
Potten CS, Boot C, Tudor GL et al (2003) Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71(1):28–41
Asai R, Okano H, Yasugi S (2005) Correlation between musashi-1 and c-hairy-1 expression and cell proliferation activity in the developing intestine and stomach of both chicken and mouse. Dev Growth Differ 47(9):501–510
Crosnier C, Stamataki D, Lewis J (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7(5):349–359
Kayahara T, Sawada M, Takaishi S et al (2003) Candidate markers for stem and early progenitor cells, musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett 535(1–3):131–135
Goodell MA, Brose K, Paradis G et al (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183:1797–1806
Liadaki K, Kho AT, Sanoudou D et al (2005) Side population cells isolated from different tissues share transcriptome signatures and express tissue-specific markers. Exp Cell Res 303(2):360–374
Asakura A, Rudnicki MA (2002) Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol 30(11):1339–1345
Acknowledgement
This study was supported by the Natural Science Foundation of Guangdong Province (032901).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuqi, L., Chengtang, W., Ying, W. et al. The Expression of Msi-1 and Its Significance in Small Intestinal Mucosa Severely Damaged by High-Dose 5-FU. Dig Dis Sci 53, 2436–2442 (2008). https://doi.org/10.1007/s10620-007-0155-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-0155-0