Abstract
Stellate cells are activated by free radicals, and synthesize collagen. N-acetylcysteine (NAC) is a precursor of reduced glutathione and a potent scavenger of hydroxyl radicals and has potential antifibrotic effects. We aimed to test the effects of NAC on bile duct ligation (BDL) induced liver damage in rats. Forty-seven Wistar rats were divided into 5 groups: group 1, BDL + NAC (n = 10); group 2, BDL (n = 10); group 3, sham + NAC (n = 10); group 4, sham (n = 10); and group 5, control group (n = 10). NAC (50 μmol/kg per day) or saline of single doses were administered intraperitoneally for 28 days. Serum biochemical and liver oxidative stress parameters were studied. Liver collagen level was determined by the method of Lopez de Leon and Rojkind. Liver slides were stained by hematoxylin and eosin and Masson trichrome\Gomory reticulum staining. Aspartate aminotransferase (AST) and alkaline phosphatase levels in the BDL + NAC group were lower than the BDL group and were higher than the control groups (all P < .001). Malondialdehyde, luminal, and glutathione levels in group 1 were lower than the BDL group (P = .01, P = .002, and P < .001) and higher than the control groups (all P < .001). NAC had no effect on alanine aminotransferase (ALT), gammaglutamyl transferase, bilirubin, albumin, or lucigenin levels. Liver collagen levels were higher in the BDL groups (P < .001); however, NAC had no effect on the collagen levels. The BDL groups showed stage 3 fibrosis; all the control groups were normal. NAC improved some biochemical parameters (AST, alkaline phosphatase) and oxidative stress parameters (malondialdehyde, luminol, glutathione) in the BDL model. NAC was found to be effective on cholestasis-induced hepatotoxicity. However, NAC was inefficient as an antifibrotic agent within a 1-month period of administration in the BDL model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Current clinical data indicate that oxidative stress is associated with the activation of hepatic stellate cells (HSC), which are the central mediators in the pathogenesis of fibrosis and synthesize collagen [1]. Lipid peroxidation has also been shown to stimulate collagen production in fibroblasts and HSC [2].
At present, no effective treatment of liver fibrosis is available for clinical use. Some experimental studies reported partial success with some substances such as melatonin [3], pegylated interferon [4], malotilate [5], halofuginone [6], and Sho-saiko-to [7], a Far East herbal therapeutic, in this setting. An effective therapeutic strategy against the development of hepatic fibrosis is still needed.
Antioxidant agents or glutathione (GSH) precursors have been shown to exert protective effects against HSC activation [8, 9]. However, the role of oxidative stress and the beneficial effects of antioxidant agents during the initial phases of liver fibrosis have not been fully investigated.
The antifibrotic effect of N-acetylcysteine (NAC) was demonstrated in various experimental models of fibrosis and there are some studies especially emphasizing its antifibrotic effects and underlying its properties in controlling collagen levels in lung tissue [10–12]. In a previous study, Vendemiale et al. [13] showed that NAC was effective on dimethylnitrosamine (DMN)-induced liver damage in rats.
In this study, our aims were to evaluate the contribution of oxidative processes in the early stages of rat hepatic fibrogenesis induced by bile duct ligation (BDL) and to assess the protective role of the antioxidant agent NAC against the development of hepatic fibrosis on BDL-induced liver damage in rats, as a new therapeutic challenge.
Materials and methods
Procedures related to experimental animals
This experimental protocol had the full approval of the Ethical Committee on Animal Research, Marmara University School of Medicine, Turkey, and complied with International Guidelines for animal research. All animals received humane care.
Forty-seven male Wistar rats, between 12 and 14 weeks of age and 220–340 g, were obtained from Marmara University Animal Research Laboratory. Animals were kept at a constant temperature (22 ± 1°C) with 12-hour light and dark cycles, in the same unit and allowed to acclimatize to their new conditions for 1 week before beginning the study. All animals received humane care in compliance with the National Institutes of Health criteria for laboratory animals. Rats had free access to standard rat chow and water.
Under general pentobarbital anesthesia, 40 male Wistar rats underwent BDL or sham operation. Briefly, the common bile duct was exposed after laparotomy. Subsequently, 2 double knots were placed proximally and distally and the part of the bile duct between the 2 double knots was excised. In the sham-operated rats, the abdomen was closed without BDL. Twenty of the rats underwent BDL and 20 were sham operated. BDL rats were divided into 2 groups. Sterile saline (1 mL/kg per day) was administered intraperitoneally for 28 days to the first group (BDL group; n = 10). The second group (BDL + NAC; n = 10) received 50 μmol/kg per day NAC intraperitoneally (Asist ampoules, Hüsnü Arsan İlaçları A.Ş. İstanbul, Turkey). The sham-operated rats were also divided into 2 groups. After the sham operation, sterile saline was administered to the sham group (n = 10) and NAC was given to the sham + NAC group (n = 10). During this administration, the same doses of NAC were given to the BDL + NAC group and saline was administered to the BDL group immediately after BDL. An additional group of 7 healthy control rats were studied as the control group. At the end of the study period, rats were weighed and decapitated; their trunk blood was collected, centrifuged (3000 rpm, 10 min, 4°C) and serum samples were obtained for biochemical analyses of AST, ALT, alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), total bilirubin, and direct bilirubin. The serum samples were stored at −80°C and measured with automated standardized procedures (Roche Hitachi 917/747, Mannheim, Germany).
Liver tissue sampling
The left, middle, and right lobes of each liver were explored. Six different 5 × 5 × 5 mm slices were fixed in 10% buffered formalin, routinely processed, and blocked into paraffin for detecting collagen content by image analysis [14].
Biochemical collagen content determination
The collagen content of the liver was assayed by the colorimetric method described by Lopez de Leon and Rojkind [15]. The principle is the coloring of collagenous protein by Sirius red (36554–8, 2610-10-8; Aldrich Chemical, Deisenhofen, Germany) and noncollagenous proteins by fast green (14280; MERCK, Darmstadt, Germany). Fifteen micrometer-thick liver slices taken from each paraffin block were layered on glass slides. Slices were deparaffinized and assayed as originally described. Collagen content was calculated using the formula described by the authors as microgram collagen per milligram protein [15].
Histopathologic investigations
Five-micrometer liver sections were stained by hematoxylin and eosin and Masson trichrome\Gomory reticulum staining. The grading necroinflammatory activity and the staging fibrosis were set by Knodell’s criteria [16].
Tissue homogenization
Liver samples were weighed and homogenized in 0.15 mol NaCl to determine reactive oxygen species. Homogenates were diluted with 0.15 mol NaCl up to 20%. Tissue homogenates were sonicated 2 times for 30-second intervals at 4°C. After sonication, homogenates were centrifuged at 3000 rpm for 10 min and at 15,000 rpm for 15 min. Aliquots of the supernatants were used for studies.
Oxidative stress parameters
Malondialdehyde measurements
Measurements of thiobarbituric acid reactive species (TBARS) were done according to Yagi [17]. Liver tissues were homogenized in icy trichloroacetic acid (TCA) (10%) solution and then centrifuged. The superficial liquid portion was mixed with equal volume of TBARS (0.67%) and heated at 90°C for 15 min. TBARS were measured in nmol/g tissue according to absorbance at 532 nm.
Chemiluminescence measurements
Reactive oxygen metabolites (ROM) were measured at room temperature via chemiluminescence technique using Mini Lumat LB 9506 Luminometer (EG&G, Berthold, Germany). Samples were placed into a 2-mL 0.02 mol HEPES buffer (pH 7.4) containing 0.5 mol phosphate-buffered saline. For measurement of ROM, 0.2 nmol concentrated lucigenin (specific for superoxide radicals) or luminal (HOCl−, H2O2, OH−) was used. Serial measurements of 15-second intervals for 5 min were done and results were calculated as area under the curve and relative light unit (RLU); correction for fresh tissue weight was done (RLU per milligram of tissue area under the curve) [18, 19].
Glutathione level measurement
GSH levels were measured spectrophotometrically using Ellman’s reagent and method [20]. Results were calculated as μmol GSH/g tissue.
Statistical evaluation
Data were expressed as mean values ± standard deviation or, in case of non-normal distribution, as median and range and were compared using the Kruskal–Wallis test. When significant, subsequent multiple comparison test was performed. P < .05 were considered significant. Comparisons between the groups were tested for significance by Mann–Whitney U and χ2 tests.
Results
Biochemical findings
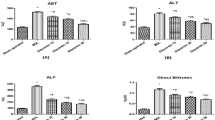
All biochemical parameter levels, including AST, ALT, ALP, GGT, albumin, and total and direct bilirubin, were significantly increased in both of the BDL groups in contrast to the control groups (all P < .001). NAC therapy improved AST and ALP levels in the BDL + NAC group when compared with the BDL + saline group (P < .001); however, no difference was observed in reference to other parameters between the BDL subgroups. There was no significant difference in reference to these biochemical parameters between the sham and the healthy control groups (Table 1).
Oxidative stress parameters
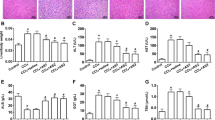
The mean liver malondialdehyde (MDA) levels of the BDL groups were significantly higher than those in the control groups (all P < .001). NAC therapy improved the MDA level between the BDL subgroups (P = .01; Fig. 1).
The tissue luminal level of the BDL + NAC group was significantly lower than the BDL group (P = .002). The tissue luminol level in the BDL group was higher than the control groups (P < .001). NAC improved the luminol level of the BDL + NAC group, statistically similar to the control groups (Fig. 2). The tissue lucigenin levels of the groups were not statistically different (Fig. 2).
The tissue GSH levels of the BDL groups were significantly lower than the control groups (all P < .001). The tissue GSH level in the BDL + NAC group was higher than the BDL group (P < .001; Fig. 3). No difference in the oxidative stress parameters was observed among the control groups.
Biochemical collagen content measurement
Hepatic collagen content in both BDL rat groups were significantly higher than the control groups (P < .01). However, the hepatic collagen levels were not found to be significantly affected by NAC administration. Similarly, no significant statistical difference was found between the control groups (P > .05; Fig. 4).
Histopathologic findings
In the histopathologic examination of the liver sections, prominent bile duct proliferation and stage 3 fibrosis were demonstrated in all BDL groups. The histologic activity and fibrosis observed in the BDL groups by Knodell scoring did not change with NAC therapy (Fig. 5).
(A) A sham-operated group rat liver section with normal finding (HE, original magnification ×100). (B) A bile duct ligation (BDL) + N-acetylcysteine group liver slide with cellular loss and fibrous septa (HE, original magnification ×100). (C) A BDL group liver slide with prominent bile duct proliferation, portal–portal fibrous bridging with bile duct proliferation and fibrous septa, connecting portal areas to each others and lobule centers (Trichrome-stained original magnification ×100)
Discussion
Liver fibrosis is the pathologic result of ongoing chronic inflammatory liver diseases. Lipid peroxidation is not only a marker of tissue damage, but is involved in the pathogenesis as well as an activator of collagen production which mediates the development of fibrosis of the tissues [21]. The reduction of liver fibrosis, accompanied by a decrease in oxidative stress, suggests a common mechanism of protection against fibrogenesis, most likely attributed to the inhibition of HSC activation [13]. If the main factor of lipid peroxidation is not removed, this process results in liver cirrhosis.
Oxidative stress has an important role in the etiopathogenesis of liver fibrosis by aggravating it via stellate cell activation and lipid peroxidation which stimulates the collagen gene transcription in cell culture [22–24].
NAC is commonly used as an antioxidant in vivo and in vitro. In addition, it can be used in acetaminophen intoxication, CCI4, chloroform, and carbon monoxide intoxication [25]. It has a well-known mucolytic effect in chronic obstructive lung diseases. NAC was shown to be effective in the reversal of ischemic and reperfusion damage [26], valuable in the treatment of HIV infections [27] and in adult respiratory distress syndrome [28].
The antifibrotic effects of NAC were shown in 3 studies of bleomycin-induced liver fibrosis models by inhalation [10], intratracheal [11], or intraperitoneal [12] administration. Kawada et al. [29] studied the effects of antioxidants, resveratrol, quercetin, and NAC on the functions of cultured rat HSC and Kupffer cells. NAC had therapeutic potential against liver injury via regulating functions of HSC and Kupffer cells. These effects may be related to the antioxidant potential of the agents.
Bataller et al. [30] studied a pro-oxidant and fibrogenic cytokine, angiotensin II–induced effects in HSC after BDL. Angiotensin II stimulated DNA synthesis, cell migration, procollagen alpha1(I) mRNA expression, and secretion of transforming growth factor-β1 and inflammatory cytokines. These effects were attenuated by NAC and diphenylene iodonium. Dogru-Abbasoglu et al. [31] showed in their study that NAC treatment was able to reduce lipopolysaccharide-enhanced hepatotoxicity without making any changes in oxidative stress in the liver of rats with tioacetamide-induced cirrhosis. Similarly, in the study by Liu et al. [32], NAC pretreatment significantly attenuated endotoxin induced biochemical changes in CCI4-induced cirrhosis in rats.
The only therapeutic trial of NAC in liver fibrosis was performed in the DMN-induced liver fibrosis model by Vendemiale et al. [13], where they evaluated the role of oxidative processes in rat hepatic fibrogenesis induced by DMN and assessed the effect of NAC against the development of hepatic fibrosis. NAC administration resulted in a reduction of lipid peroxidation and replacement of GSH stores, suggesting an effective role of this agent against oxidative stress. NAC significantly reduced ALT elevation and liver fibronectin deposition in this model. They explained that these effects were related to 3 mechanisms: (1) the restoration of the total intracellular sulfhydryl pool; (2) the maintenance of intracellular GSH concentration, a fundamental detoxifying system against toxic metabolites; and (3) the potential antioxidant action exerted by NAC itself.
Alcoholic liver fibrogenesis was shown to be closely associated with enhanced hepatic lipid peroxidation as demonstrated by significant correlation between the degree of liver fibrosis and hepatic levels of MDA [33, 34]. MDA level was high in BDL-induced liver fibrosis [35].
Shiesh et al. [36] studied the BDL model in guinea pigs and revealed increased pH, bile salts, and MDA compared with sham controls. Pretreatment of guinea pigs with melatonin at a dose of 1 μg/kg significantly decreased the incidence of pigment gallstone formation at day 14 after ligation as compared with controls. Melatonin also improved the ligation-induced changes in biliary bile salts, pH, and MDA to control levels. These in vivo findings support a causative role of oxidative stress in the BDL-induced pigment gallstone formation.
In our study, liver and plasma MDA levels in the BDL group were significantly higher than the other 3 groups. In accordance with the previous studies, NAC improved the lipid peroxidation and lipid peroxidation end product, MDA to a similar degree with the control groups.
Pastor et al. [37] induced secondary biliary cirrhosis in rats via 28 days of bile duct obstruction, which resulted in decreased liver GSH, TBARS, catalase, superoxide dismutase (SOD) and glutathione peroxidase (GPx) levels. NAC corrected the reduction in GSH and TBARS concentrations. In addition, NAC treatment resulted in significant preservation of membrane fluidity and of the activities of catalase, mitochondrial SOD, and different forms of GPx. Their data indicated that NAC maintains antioxidant defenses in biliary obstructed rats and NAC may be a useful agent to preserve liver function in patients with biliary obstruction.
Cabre et al. [38] studied the relationships between hepatic lipid peroxidation, GSH antioxidant system and development of cirrhosis in CCI4-treated rats. Induction of cirrhosis produced a decrease in the components of the hepatic GSH antioxidant system. This impairment was related to increase in free radical generation. Hepatic lipid peroxidation was correlated with GPx activity (r = −0.47; P < .001) in CCl4-treated rats. Lopez et al. [39] showed decreases of both liver and erythrocyte GSH levels in BDL induced cholestasis model in another study.
In the present study, liver GSH levels in the BDL group were higher than the 3 other groups (P < .001). NAC administration improved the GSH level similar to the control groups. In addition, the other free radical parameter—luminal level—was lower in the BDL + NAC group than the BDL group (P = .002). NAC improved the luminol level similar to the control groups.
AST and ALP levels in the BDL + NAC group were lower than the BDL group (both P < .001). NAC was ineffective on either GGT or bilirubin levels of the groups. Likewise, the ALT improvement was not statistically significant either. The AST improvement might be associated with a possible antifibrotic effect of NAC; however, the antifibrotic effect of the drug was not prominent in the 1-month study period. The failure of an antifibrotic effect of NAC on BDL rats in our study may be attributed to the short duration of the treatment period.
In conclusion, daily intraperitoneal NAC administration in the BDL model for 28 days was found to have a beneficial effect on liver enzymes (AST, ALP) and oxidative stress parameters (MDA, luminol, GSH). Whether NAC can be used in cholestatic jaundice against hepatotoxicity and as a possible antifibrotic agent remains to be proved in long-term studies.
References
Svegilati Baroni G, D’Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A (1998) Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology 27:720–726
Parola M, Leonarduzzi G, Robino G, Albano E, Poli G (1996) On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radical Biol Med 20:351–359
Tahan V, Ozaras R, Canbakan B, Uzun H, Aydin S, Yildirim B, Aytekin H, Ozbay G, Mert A, Senturk H (2004) Melatonin reduces dimethylnitrosamine-induced liver fibrosis in rats. J Pineal Res 37:78–84
Tasci I, Mas MR, Vural SA, Comert B, Alcigir G, Serdar M, Mas N, Isik AT, Ates Y (2006) Rat liver fibrosis regresses better with pegylated interferon alpha2b and ursodeoxycholic acid treatments than spontaneous recovery. Liver Int 26:261–268
Ryhanen L, Stenback F, Ala-Kokko L, Savolainen ER (1996) The effect of malotilate on type III and type IV collagen, laminin and fibronectin metabolism in dimethylnitrosamine-induced liver fibrosis in the rat. J Hepatol 24:238–245
Pines M, Knopov V, Genina O, Lavelin I, Nagler A (1997) Halofuginone, a specific inhibitor of collagen type I synthesis, prevents dimethylnitrosamine-induced liver cirrhosis. J Hepatol 27:391–398
Chen MH, Chen JC, Tsai CC, Wang WC, Chang DC, Tu DG, Hsieh HY (2005) The role of TGF-beta 1 and cytokines in the modulation of liver fibrosis by Sho-saiko-to in rat’s bile duct ligated model. J Ethnopharmacol 97:7–13
Houglum K, Lee KS, Chojkier M (1997) Proliferation of hepatic stellate cells is inhibited by phosphorylation of CREB on serine 133. J Clin Invest 99:1322–1328
Kawada N, Seki S, Inoue M, Kuroki T (1998) Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology 27:1265–1274
Hagiwara SI, Ishii Y, Kitamura S (2000) Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 162:225–231
Pardo A, Ruiz, Arreola JL, Ramirez R, Cisneros-Lira J, Gaxiola M, Barrios R, Kala SV, Lieberman MW, Selman M (2003) Bleomycin-induced pulmonary fibrosis is attenuated in -glutamyl transpeptidase–deficient mice. Am J Respir Crit Care Med 167:925–932
Serrano-Mollar A, Closa D, Prats N, Blesa S, Martinez-Losa M, Cortijo J, Estrela JM, Morcillo EJ, Bulbena O (2003) In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. Br J Pharmacol 138:1037–1048
Vendemiale G, Grattagliano I, Caruso ML, Serviddio G, Valentini AM, Pirrelli M, Altomare E (2001) Increased oxidative stress in dimethylnitrosamine-induced liver fibrosis in the rat: effect of N-acetylcysteine and interferon-alpha. Toxicol Appl Pharmacol 175:130–139
Tarçın O, Avşar K, Demirtürk L, Gültepe M, Oktar BK, Özdoğan OC, Tarçın Ö, Baloğlu HN, Gürbüz AK (2003) In vivo inefficiency of pentoxifylline and interferon-alpha on hepatic fibrosis in biliary-obstructed rats: assessment by tissue collagen content and prolidase activity. J Gastroenterol Hepatol 18:437–444
Lopez de Leon A, Rojkind M (1985) A simple micromethod for collagen and total protein determination in formalin-fixed and paraffin-embedded sections. J Histochem Cytochem 33:737–743
Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J (1981) Formulation and application of numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431–435
Yagi K (1984) Assay for blood plasma or serum. Methods Enzymol 105:328–331
Davies GR, Simmonds NJ, Stevens TR, Grandison A, Blake DR, Rampton DS (1992) Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut 33:1467–1472
Haklar G, Sayın-Özveri E, Yüksel M, Aktan AÖ, Yalçın AS (2001) Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett 165:219–224
Boyne AF, Ellman GL (1972) A methodology for analysis of tissue sulfhydryl components. Anal Biochem 46:639–653
Geesin JC, Hendricks LJ, Falkenstein PA, Gordon JS, Berg RA (1991) Regulation of collagen synthesis by ascorbic acid: characterization of the role of ascorbate-stimulated lipid peroxidation. Arch Biochem Biophys 290:127–132
Svegliati Baroni G, D’Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A (1998) Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology 27:720–726
Bedossa P, Houglum K, Trautwein C, Holstege A, Chojkier M (1994) Stimulation of collagen alpha 1(I) gene expression is associated with lipid peroxidation in hepatocellular injury: a link to tissue fibrosis? Hepatology 19:1262–1271
Lee KS, Buck M, Houglum K, Chojkier M (1995) Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 96:2461–2468
Howard RJMW, Blake DR, Pall H, Williams A, Green ID (1987) Allopurinol/N-acetylcysteine for carbon monoxide poisoning. Lancet 2:628–629
Ceconi C, Curello S, Cargnoni A, Ferrari R, Albertini A, Visioli O (1988) The role of glutathione status in the protection against ischemic and reperfusion damage: effects of N-acetylcysteine. J Mol Cell Cardiol 20:5–13
Kalebic T, Kinter A, Poli G, Anderson ME, Meister A, Fauci AS (1991) Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proc Natl Acad Sci USA 88:986–990
Bernard GR (1990) Potential of N-acetylcysteine as treatment for the adult respiratory distress syndrome. Eur Respir J 3(supp1):496–498
Kawada N, Seki S, Inoue M, Kuroki T (1998) Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology 27:1265–1274
Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA (2003) NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 112:1383–1394
Dogru-Abbasoglu S, Balkan J, Kanbagli O, Cevikbas U, Aykac-Toker G, Uysal M (2002) Aminoguanidine, an inducible nitric oxide synthase inhibitor, plus N-acetylcysteine treatment reduce the lipopolysaccharide-augmented hepatotoxicity in rats with cirrhosis. Hum Exp Toxicol 21:359–364
Liu JJ, Wang JY, Zhang C, Nilsson A, Duan RD (2002) Hepatic cirrhosis increases sensitivity of kidney to endotoxin in rats. Med Sci Monit 8:BR56–60
Tsukamoto H, Horne W, Kamimura S, Niemela O, Parkkila S, Yla-Herttuala S, Brittenham GM (1995) Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest 96:620–630
Tsukamoto H (1993) Oxidative stress, antioxidants, and alcoholic liver fibrogenesis. Alcohol 10:465–467
Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, Cales P (1998) Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. J Hepatol 29:263–270
Shiesh SC, Chen CY, Lin XZ, Liu ZA, Tsao HC (2000) Melatonin prevents pigment gallstone formation induced by bile duct ligation in guinea pigs. Hepatology 32:455–460
Pastor A, Collado PS, Almar M, Gonzalez-Gallego J (1997) Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol 27:363–370
Cabre M, Camps J, Paternain JL, Ferre N, Joven J (2000) Time-course of changes in hepatic lipid peroxidation and glutathione metabolism in rats with carbon tetrachloride-induced cirrhosis. Clin Exp Pharmacol Physiol 27:694–699
Lopez PM, Finana IT, De Agueda MC, Sanchez EC, Munoz MC, Alvarez JP, De La Torre Lozano EJ (2000) Protective effect of melatonin against oxidative stress induced by ligature of extra-hepatic biliary duct in rats: comparison with the effect of S-adenosyl-L-methionine. J Pineal Res 28:143–149
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tahan, G., Tarcin, O., Tahan, V. et al. The Effects of N-Acetylcysteine on Bile Duct Ligation–Induced Liver Fibrosis in Rats. Dig Dis Sci 52, 3348–3354 (2007). https://doi.org/10.1007/s10620-006-9717-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9717-9