Abstract
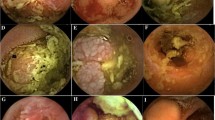
Small bowel tumors are difficult to diagnose because of their endoscopic inaccessibility. This has been overcome by the use of the Pillcam™ SB capsule (Given Imaging, Yoqneam, Israel). The purpose of this report is to describe the largest series of patients with small bowel tumors detected by capsule endoscopy. Eighty six patients were derived from the Given Imaging clinical database on a survey of Pillcam™ SB capsule users who were diagnosed with 87 small bowel tumors, 1 cecal tumor, and 1 gastric tumor. The population consisted of 55 males and 31 females. 69% of patients were referred for capsule endoscopy for obscure gastrointestinal bleeding (59/86 patients) and 31% (27/86 patients) were referred for other indications including anemia, polyposis, and abdominal pain. All patients have histologically confirmed tumors. Eighty six patients reported 395 previous negative procedures (average of 4.6 per patient). Malignant tumors comprised 61% (54/89) and benign 39% (35/89). Of the 87 reported small bowel tumors, 4 were identified in the duodenum, 43 tumors were identified in the jejunum, 18 tumors were identified in the ileum, and 22 tumors were located in the mid to distal small bowel. The most common malignant tumors were adenocarcinoma, carcinoids, melanomas, lymphomas, and sarcomas. The most common benign tumors were GIST, hemangiomas, hamartomas, adenomas, and granulation tissue polyps. Capsule endoscopy is the diagnostic procedure of choice in patients with suspected small bowel tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary neoplasms of the small bowel are an uncommon and heterogeneous group of tumors, comprising approximately 5% of all primary gastrointestinal neoplasms [1]. Although the small bowel represents 75% of the length and over 90% of the mucosal surface area of the alimentary tract, it is the site of only 1–2% of malignant gastrointestinal tumors [2]. Small-bowel tumors have traditionally been difficult to diagnose due to their nonspecific clinical signs and symptoms, combined with radiologic methods that have poor sensitivity and specificity and endoscopic methods which only visualize the proximal small bowel [3]. Endoscopic methods including push enteroscopy and colonoscopy visualize approximately four feet beyond the ligament of Treitz and the distal ileum. The video capsule endoscopy system (Given Imaging Inc., Yoqneam, Israel) has the ability to record photographic images of the entire small bowel during normal peristaltic motion, including those areas not reached by a traditional endoscope.
In order to provide more information for the correct diagnosis and better management of these tumors, we present the largest series of patients with histologically verified small-bowel tumors detected by wireless capsule endoscopy (WCE).
Materials and methods
The clinical records of 86 patients with 87 small-bowel tumors were derived from the Given Imaging clinical database. In total, 125 investigators were contacted regarding submission of tumors detected by WCE. Positive responses were received from 37 investigators. A completed questionnaire was submitted by each contributing investigator and only histologically confirmed small-bowel tumors were included. Five of the 86 patients were involved in clinical trials; the majority were private patients submitted by contributing investigators. Some investigators contributed multiple cases. The indication for capsule endoscopy, location of tumor, number of prior negative procedures per patient, and histological diagnoses for each tumor is reported.
Results
The percentage of benign vs. malignant tumors, as well as age and sex distribution according to tumor type is shown in Table 1. Malignant neoplasms accounted for 52 of the 87 lesions (60%) collected in this study. The youngest patient was 20 years old and the oldest was 86. Malignant neoplasms appeared more frequently in males than in females (63 vs. 37%). The mean ages of the patients were similar for benign and malignant neoplasms (59 and 60 years, respectively), as was the male-to-female ratio (63 vs. 37% for both).
The malignant neoplasms consisted of adenocarcinomas, carcinoid tumors, melanomas, lymphomas, and sarcomas (Table 2). Other lesions included three GIST tumors that were determined to be malignant and one metastatic lung cancer. The most common location of the malignant tumors was in the jejunum (50%). Of note, although the specific location of seven lesions was not specified, all were identified as “mid to distal small bowel.” Therefore, 89% of lesions were located in the jejunum or beyond. Regarding the sarcomas detected, those found in the jejunum and ileum were from the same patient. Benign lesions comprised 40% (35/87) of total lesions detected. GISTs, hemangiomas, and hamartomas were the three most common benign neoplasms (Table 3). The majority of benign lesions with specified location were found in the jejunum (57%). Other lesions identified included: two lipomas, one incarcerated hernia, one focal lymphatic cyst, one site of amyloidosis, and one neurofibroma.
The indications for capsule endoscopy included (1) 69% (59/86) for investigation of obscure GI bleeding (2) 21% (18/86) for anemia (3) 8% (7/86) for abdominal pain and (4) 2% (2/86) for history of polyposis (Table 4). Malignant neoplasms were found in 72% of patients referred for anemia, 58% of patients referred for OGIB, and 43% of patients referred for abdominal pain. Both patients referred for history of polyposis were found to have benign neoplasms.
The 86 patients had undergone 395 previous negative procedures prior to WCE (average of 4.6 per patient). This included 137 colonoscopies, 131 upper endoscopies, 40 small-bowel follow-through radiological studies, 26 enteroscopies, 24 CT scans, 16 enteroclysis, six nuclear medicine bleeding scans, six angiographies, five plain abdominal x-rays, two Meckel's scans, one abdominal ultrasound, and one laparoscopy (Table 5). Patients found by WCE to have duodenal tumors underwent an average of two prior negative procedures, while those with jejunal and ileal tumors underwent an average of 5.3 and 4.1 negative procedures, respectively. While 22 tumors were classified as “not specified” because data regarding specific anatomic location was not available, the majority of these lesions were identified as being in the “mid to distal” small bowel.
Discussion
Primary neoplasms of the small bowel are a rare group of tumors with a reported incidence of 3–6% among all tumors of the gastrointestinal tract and 1–3% among malignant lesions [4]. This low incidence is accompanied by a lack of accuracy of radiographic investigations and relative inaccessibility of the distal small bowel for endoscopic investigations. The result is a significant delay in diagnosis. In fact, the mean symptom to diagnosis interval has been reported to be more than 6 months [5].
Small-bowel disorders are among the most difficult to evaluate endoscopically because of the long length and multiple complex looped configuration of the small bowel. Although endoscopy has replaced barium studies as the primary means of assessing the upper GI tract and the colonic mucosa, radiographic studies have remained the primary method of evaluating the small bowel, especially the distal small bowel. Enteroclysis has been reported to be more accurate than barium small-bowel follow-through examinations and is reported to have a diagnostic yield in patients with small-bowel tumors (around 90%). However, this figure is limited to centers of excellence and is not applicable to the usual exam. Furthermore, enteroclysis requires a skilled radiologist, causes patient discomfort, necessitates light sedation, involves more patient radiation, and is expensive and time-consuming [6].
Endoscopy has the advantage of visualizing subtle mucosal changes such as vascular abnormalities that do not alter the mucosal surface and are thus undetectable on contrast studies. There have been two non-surgical endoscopic evaluations of the small bowel available, push enteroscopy and sonde-type enteroscopy. Push enteroscopy is a procedure in which a long endoscope is passed orally and pushed beyond the ligament of Treitz. With standard enteroscopes it is possible to intubate the jejunum approximately 60 cm beyond the ligament of Treitz, whereas we have shown that with dedicated small-bowel enteroscopy, we averaged 120 cm beyond the ligament of Treitz [7]. Push-type enteroscopy, unlike radiological procedures, permits the operator to collect biopsy specimens and perform endotherapy. Sonde enteroscopy is performed by placing an enteroscope transnasally into the stomach, advancing it through the pylorus with a gastroscope passed through the mouth, and allowing peristalsis to carry the endoscope distally. Passage time averages 8 h, and endoscopic examination of the small intestine is performed on instrument withdrawal. Although sonde enteroscopy has the potential to examine the entire small bowel, in up to 75% of patients the distal ileum is not reached. It is also uncomfortable, time consuming, has only diagnostic potential, and does not allow for collection of biopsy specimens [8]. For these reasons, sonde-type enteroscopy is currently rarely performed. Intraoperative endoscopy, although often successful, is associated with a higher complication rate and longer hospital stay for patients with a positive or negative examination [9].
A significant advantage of wireless capsule endoscopy is its ability to detect small-bowel abnormalities not reached by traditional endoscopy. Indeed, the overall sensitivity of WCE in detecting small-bowel lesions has been found to be significantly superior compared with push enteroscopy [10]. In our series, 89% of lesions were located in the mid to distal small bowel, areas beyond the reach of an enteroscope. The fact that only four of 87 tumors detected by WCE were located in the duodenum likely represents the high yield of traditional endoscopic methods for tumors located within reach of a conventional endoscope. Furthermore and of note, wireless capsule endoscopy has also been found to have diagnostic superiority over barium follow-through, which has traditionally been the primary examination for evaluation of small-bowel disease [11]. Double-balloon enteroscopy (DBE) represents a new technology that is currently in its infancy. In theory, it enables total bowel visualization and may be a confirmatory test of a non-diagnostic or negative WCE. In a recent comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding, Nakamura and colleagues found that WCE yielded superior access to the entire small intestine (90.6 vs. 62.5%, P < 0.05). However, the diagnostic rate between the two modalities was not significantly different (59.4 vs. 42.9%, P=0.30) [12]. Furthermore, DBE allowed for histological diagnosis and/or treatment in some of the lesions. Their research suggests that WCE and DBE should be employed as complimentary procedures, with WCE used for initial diagnosis and DBE for histo-pathological diagnosis or treatment after detection of the bleeding site by WCE.
The majority (69%) of patients in our series were referred for capsule endoscopy for investigation of obscure gastrointestinal bleeding. Although the diagnostic yield of enteroclysis in patients with small-bowel tumors and Crohn's disease is around 90%, in unexplained GI bleeding it is poor, ranging from 10 to 20% [6]. Angiography and radionuclide scanning are sensitive only in the presence of active bleeding [13]. Investigation of obscure GI bleeding therefore represents an area where WCE has clearly demonstrated its benefits over other available methods.
Other indications for capsule endoscopy in our sample included investigation of anemia (21%), abdominal pain (8%), and history of polyposis (2%). That small-bowel tumors were discovered in patients referred for chronic abdominal pain suggests that capsule endoscopy should also be considered in these patients, irrespective of normal findings on conventional endoscopic and radiographic investigations.
The percentage of benign to malignant tumors in our sample detected by WCE (39.3 vs. 60.7%) correlates with published surgical series of small-bowel tumors in which malignant tumors predominate in 60–75% of cases [1]. As described above, the most common type of malignant tumor was adenocarcinoma (35%), followed by carcinoid (31%), metastatic melanoma (9%), lymphoma (9%), and sarcoma (7%). This distribution correlates well with other series of malignant small-bowel tumors [14]. Benign lesions included GIST (51%), hemangioma and hamartoma (11% each), and adenomas (6%).
Other series of patients with small-bowel tumors found by WCE have been described. Bailey, Debinski, and Appleyard, et al. reported on the diagnosis and outcomes in 26 patients with SBTs detected by capsule endoscopy in three Australian centers [15]. In their series, 26 patients were diagnosed with 27 tumors. Prior radiologic investigations including small-bowel series and CT scans were performed and interpreted as normal in 23 out of 26 patients. The mean age (57 years), the predominant indication of obscure GI bleeding (21/26 patients or 80%), and the percentage of malignant tumors (18/27 tumors or 66%) correlate well with our data. Furthermore, malignant tumors included adenocarcinomas, carcinoids, metastatic melanomas, and GISTs, similar to the tumor types in our series.
To our knowledge, this report represents the largest series of small-bowel tumors detected by capsule endoscopy. Standard endoscopic and radiologic methods failed to suggest the correct diagnosis in all of the 86 patients. The average number of negative procedures prior to WCE was 4.6 per patient. Possible explanations for this phenomenon include radiologic misinterpretation and/or false negative examinations. Furthermore, four tumors detected by WCE were actually located in the duodenum and therefore may have been within reach of an upper intestinal endoscope. That capsule endoscopy is helpful in locating lesions likely missed by traditional upper endoscopy has been documented in prior studies of capsule endoscopy [10].
This report is not without its limitations. Specifically, while we have information regarding the tumors detected by WCE, we do not have any follow-up data at this time and are therefore unable to draw any conclusions regarding the importance of early versus late detection. The available literature regarding small-bowel tumors suggests that the failure to obtain a proper diagnostic test and/or misinterpretation of results contributes to an average delay in diagnosis of 6–8 months [16]. For patients with malignant small-bowel tumors, late detection and inaccurate diagnosis not only contribute to the advanced stage at the time of surgery, but also to a 50% rate of metastasis at presentation, and thus to an overall poor prognosis [17–19]. At this time, we can only speculate that routine use of wireless capsule endoscopy in the diagnostic algorithm for obscure GI bleeding, iron deficiency anemia, and abdominal pain will lead to earlier diagnosis, and therefore improvement in the overall prognosis for malignant small-bowel tumors. However, prospective trials with long-term follow-up are necessary to validate this theory.
In summary, wireless capsule endoscopy is a new, non-invasive diagnostic technique that visualizes the small intestine and identifies lesions in parts of the small bowel not reached by traditional endoscopy. The sensitivity of WCE compares favorably with both push enteroscopy and small-bowel follow-through, especially with regard to obscure GI bleeding, the most common indication for capsule endoscopy in our series of patients. Thus we conclude that capsule endoscopy is the diagnostic procedure of choice in patients with suspected small-bowel tumors who may present with occult GI bleeding, anemia, or abdominal pain.
References
Giuliani A, Caporale A, Teneriello F, et al. (1985) Primary tumors of the small intestine. Int Surg 70(4):331–334
Rochlin DB, Longmire WP Jr (1961) Primary tumors of the small intestine. Surgery 50:568–592
North JH, Pack MS (2000) Malignant tumors of the small intestine: a review of 144 cases. Am Surg 66:46–51
Coutsoftides T, Shibata HR (1979) Primary malignant tumors of the small intestine. Dis Colon Rectum 22:24–26
O’Riordan BG, Vilor M, Herrera L (1996) Small bowel tumors: an overview. Dig Dis 14:245–257
Maglinte DD, Kelvin FM, O’Connor K, Lappas JC, Chernish SM (1996) Current status of small bowel radiography. Abdom Imaging 21:247–257
Barkin JS, Reiner DK (1992) Diagnostic and therapeutic jejunoscopy with the SIF-10.5L new enteroscope. Am J Gastroenterol 97(9):1311
Seensalu R (1999) The sonde exam. Gastrointest Endosc Clin N Am 9:37–59
Zaman A, Sheppard B, Katon RM (1999) Total peroral intraoperative enteroscopy for obscure GI bleeding using a dedicated push enteroscope: diagnostic yield and patient outcome. Gastrointest Endosc 50:506–510
Appleyard M, Fireman Z, Glukhovsky A, et al. (2000) A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small bowel lesions. Gastroenterology 119:1431–1438
Costamagna G, Shah S, Riccioni M, et al. (2002) A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology 123:999–1005
Nakamura M, Niwa Y, Ohmiya N, et al. (2006) Preliminary comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding. Endoscopy 38:59–66
Deschamps C, Schmit A, Van Gossum A (1999) “Missed” upper gastrointestinal tract lesions may explain “occult” GI bleeding. Endoscopy 31:452–455
Marcilla J, Bueno F, Aguilar J, et al. (1994) Primary small bowel malignant tumors. Eur J Surg Oncol 20:630–634
Bailey AA, Debinski H, Appleyard M, et al. (2005) Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three center Australian experience. Gastrointest Endosc 61:AB159
Ciresi DL, Scholten DJ (1995) The continuing clinical dilemma of primary tumors of the small intestine. Am Surg 61:698
Maglinte DDT, O’Connor K, Bessette J, et al. (1991) The role of the physician in the late diagnosis of primary malignant tumors of the small intestine. Am J Gastroenterol 86:304
Cunningham JD, Aleali R, Aleali M, et al. (1997) Malignant small bowel neoplasms: histopathologic determinants of recurrence and survival. Ann Surg 225:300
North JH, Pack MS (2000) Malignant tumors of the small intestine: a review of 144 cases. Am Surg 66:46
Acknowledgments
The authors are grateful to Melissa Cohen from Given Imaging for her help with this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwartz, G.D., Barkin, J.S. Small-Bowel Tumors Detected by Wireless Capsule Endoscopy. Dig Dis Sci 52, 1026–1030 (2007). https://doi.org/10.1007/s10620-006-9483-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9483-8