Abstract
We investigated the wide variability of 18F-2-fluoro-2-deoxy-d-glucose (FDG) uptake, semiquantified as standardized uptake value (SUV), in positron emission tomography (PET) scanning, in 20 patients with colorectal cancer (CRC), including 1 with synchronous hepatic metastasis. The sensitivity of PET in CRC diagnosis was 100%, with a mean SUV of 8.0 (3.1–11.9). Tumor size and depth of invasion were associated with higher SUVs (P=.0004, .042, respectively). Strong glucose transporter-1 (GLUT-1) expression had significantly positive correlation with the SUV (r=.619, P=.003). GLUT-1 expression revealed positive staining in 17 (85%) of the 20 primary lesions. The central part of the tumor, thought to be relatively hypoxic, had stronger GLUT-1 expression and a higher SUV than the periphery, in both the primary tumor and hepatic metastatic foci. Our data suggest that the SUVs of FDG uptake in PET may be a noninvasive biomarker for advanced CRC, indicative of a large hypoxic tumor with deep invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The clinical applications of 18F-2-fluoro-2-deoxy-d-glucose (FDG) positron emission tomography (PET) are continually expanding, especially in the field of oncology. In colorectal cancer (CRC), the diverse uses of PET include initial diagnosis, staging, restaging, and assessment of the therapeutic response [1–3]. PET has also been reported to offer advantages over conventional, anatomically based morphologic modalities for detecting recurrent CRC and metastatic disease, because of its capacity to provide a functional image and evidence of tumor behavior [4, 5]. This is based on the knowledge that enhanced glucose uptake is one of the major metabolic changes characteristic of malignant tumors. Clinically, FDG is the most commonly used positron-emitting radiotracer. It is metabolized similarly to glucose, being transported into the cell, but once enzymatically phosphorylated, FDG-6-phosphate is metabolically trapped in tumor cells. Thus, tumors demonstrate increased emission of positron from FDG and can be distinguished on PET scan images by areas of increased tracer activity [6].
Clinically, variable FDG uptake, semiquantitated as the standardized uptake value (SUV), has been seen on PET scans of tumors from the same origin, including CRC. Much research has been done on the differences in FDG uptake among tumors and the mechanism of this uptake. Emerging evidence indicates that the factors affecting FDG uptake are complicated because the specific biological characteristics of tumors determine the degree of glucose metabolism [7–9]. Most factors affecting FDG uptake, such as hypoxia and cell density, are thought to be associated with changes in glycolysis-related protein expression [10, 11]. The expression of glucose transporter proteins, especially GLUT-1, which is directly involved in FDG uptake, is thought to determine the levels of FDG uptake in cancer cells [12–14].
In the present study, we examined the association between FDG uptake and clinicopathologic features and GLUT-1 expression in patients with CRC. We investigated the factors determining FDG uptake and attempt to explain the SUV variability measured by PET.
Patients and methods
Patients
The subjects of this study were 20 patients with histologically proven colorectal adenocarcinoma treated at the Department of Surgery and Clinical Oncology, Graduate Medical School, Osaka University, between 2000 and 2001. The disease was graded into clinical stages 0–IV, according to the criteria of the International Union Against Cancer. Synchronous liver metastasis was detected in 1 patient. All patients underwent FGD PET before surgery. This study was approved by the institutional review board, and written informed consent was obtained from the patients before inclusion. Patients with underlying inflammatory bowel disease and diabetes were excluded because of the potential diagnostic overlap in PET findings. Patient characteristics and tumor status are shown in Table 1. None of the patients were given preoperative chemotherapy or irradiation.
FDG-PET procedures
After fasting for at least 4 h, the patients had blood collected for serum glucose measurement, and were then given approximately 370 MBq of FDG IV. All patients were normoglycemic (blood glucose level, 101.4±13.6 mg/dL−1). Simultaneous emission–transmission PET scans were acquired 1 h after FDG injection with a dedicated PET scanner (Headtome V/SET 2400W, Shimadzu Corporation, Kyoto, Japan) and a rotating external transmission source (68Ge-68Ga rod source). This system has 32 rings and simultaneously produces 63 slices 3.125 mm thick along a 20-cm longitudinal field. The intrinsic resolution was 3.7 mm full width at half maximum, and the sensitivity of the device was 7.3 cps/Bq cm−3. The bladder was continuously flushed with 1000 mL of saline via a triple-lumen catheter [15, 16].
Image analysis
Images were reconstructed with an iterative median root prior to a reconstruction algorithm (mask size 3×3, β 0.3, subsets 24, iteration 1). For visual analysis, 2 experienced nuclear medicine physicians blinded to the clinical data of the patients read the FDG images on a high-resolution computer screen. Areas with focally increased FDG uptake compared with the surrounding tissue were read as positive. The final diagnosis was made by consensus between the 2 observers. Subsequently, all positive sites were compared with the known location of the tumor according to the available morphologic imaging data (endoscopy, computed tomography). For semiquantitative analysis of the FDG uptake, irregular regions of interest (ROIs) were semi-automatically placed over the transaxial slices of the PET images. In each consecutive slice, the ROI was adjusted manually to clearly reveal the boundary enclosing all pixels with an SUV ≥2.0, being the threshold between malignant and benign colorectal tumors in our university. We measured the mean counts per pixel within all the ROIs. SUVmean, normalized for the body weight, was calculated as the formula: SUVmean=(PET counts×calibration factor)/(injected dose/body weight). The effects of partial volume were corrected by recovery coefficients derived from phantom studies simulating lesions of various sizes and uptake values.
Immunohistochemical staining for GLUT-1 expression
The resected surgical specimens, consisting of 20 CRCs and 1 liver metastatic focus, were fixed in formalin, then processed through graded ethanol, and embedded in paraffin. Sections 4 μm thick were mounted on Capillary Gap Plus microscope slides (BioTek Solutions, Santa Barbara, CA), which were deparaffinized in xylene and rehydrated. Immunostaining was done on a TechMate Horizon automated staining system (DAKO, Glostrup, Denmark), as described previously [17]. Briefly, sections were subjected to heat antigen retrieval in 10 mmol citrate buffer (pH 6.0); then, after blocking with 1% bovine serum albumin, they were incubated with anti–GLUT-1 polyclonal antibody (DAKO, Carpinteria, CA) overnight at a dilution of 1:500. After washing, the sections were incubated with biotinylated goat anti-rabbit secondary antibody, and then subjected to the streptavidin-biotin complex method (Histofine SAB-PO kit; Nichirei, Tokyo, Japan). We used 3, 3-diaminobenzidine plus H2O2 for visualization of the signals in brown. Sections were then counterstained with hematoxylin. For a negative control, sections were incubated with normal rabbit IgG (Vector Laboratories, Burlingame, CA) or phosphate-buffered saline instead of the primary antibody, as a substitute for the primary antibody, to exclude false-positive responses from nonspecific binding to IgG or from the secondary antibody.
Intensity of GLUT-1 staining
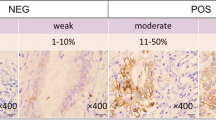
The intensity of GLUT-1 immunoreactivity was categorized as none, faint, or strong by averaging the reaction intensity compared with that of tissue erythrocytes, which were used as an internal control [12]. We randomly selected 10 visual fields and counted the number of strongly immunoreactive tumor cells and all tumor cells per square millimeter under a microscope at 200× magnification. For statistical analysis, we used the number of strongly stained tumor cells, representing the intensity of GLUT-1 expression. Immunohistochemical analysis for anti–GLUT-1 antibody was independently performed 3 times by 2 experienced pathologists who were unaware of the SUVs and clinical data.
Statistical analysis
Statistical analysis was performed using StatView J-5.0 software (Abacus Concepts, Berkeley, CA). Data are expressed as mean values±standard deviations. Associations between discrete variables were assessed using Fisher's exact test. Mean values were compared using the Student's t-test. Bonferroni's correction was applied for multiple comparisons. Correlation significance was assessed using Pearson's correlation coefficient test. P<.05 was considered to indicate that a given correlation was significant.
Results
PET findings
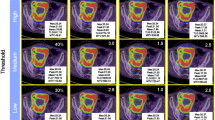
FDG-PET showed increased focal activity in all of the 20 primary CRCs and in the liver metastatic focus. Figure 1 shows the intensive accumulation of FDG in the sigmoid colon in a typical PET scan of a patient with CRC. The central part of the primary CRC tumor (Fig. 2a) and liver metastasis (Fig. 2b) had a higher SUV than the peripheral areas. Regional lymph node involvement in 7 patients was not detected by PET.
The standardized uptake values (SUVs) increased progressively from the periphery to the central part in both the primary colorectal carcinoma (a) and in liver metastatic lesions (b). As the 18F-2-fluoro-2-deoxy-D-glucose (FDG) uptake increased, the color indicating tracer uptake value changed from green (periphery) to yellow (intermediate part), and to red (center of the focus) in the Shimazu color scale system
Intensity of GLUT-1 expression
Intravascular red cells, which were stained strongly in all tissue sections, served as internal controls. Immunoreactivity for GLUT-1 was localized in the cytoplasm of cancer cells and staining intensity was categorized as none, faint, or strong (Fig. 3). GLUT-1 immunostaining revealed positive (faint and strong) staining in 17 (85%) of the 20 primary lesions and in the liver metastasis. The intensity of GLUT-1 staining was stronger in the central part of the metastatic focus (Fig. 4).
SUV and clinicopathologic characteristics
Based on the cutoff value of 8.0 (3.1–11.9), patients were divided into a high SUV group (SUV>8.0; n=8) or a low SUV group (SUV < 8.0, n=12). The SUVs were correlated with tumor size (P=.0004) and invasive grade (P=.042; Table 2).
SUV and GLUT-1 expression
A strong correlation was found between SUV and the cellularity (per mm2) of cancer cells displaying strong GLUT-1 staining (Fig. 5).
Discussion
The use of SUV as a semiquantitative index of regional tracer uptake in PET imaging is practical and widespread. However, SUV measurements are subject to partial-volume effects, duration of tracer uptake, blood glucose level at the time of tracer injection, and ROI effects. To account for these effects, we standardized the measurement of SUVs from 60 min after tracer administration, applied recovery coefficients, and excluded patients with diabetes. All of the patients had a blood glucose level <150 mg/dL−1 before tracer injection. Instead of the SUVmax, we used the SUVmean, which sampled multiple points within the lesion. A threshold method made the ROI selection reliable.
The findings of this study confirmed the high sensitivity of PET scanning in assisting with the detection of CRC. All of the primary carcinomas as well as the liver metastatic focus were seen on PET scans. Previous studies have also shown that PET can accurately identify the site of the primary tumor, with sensitivities ranging from 90–100% [18]. The overall sensitivity for detecting liver metastasis is as high as 99% [19].
A better understanding of the association between FDG uptake and clinicopathologic features will provide more information about the tumor and lead to a clearer interpretation of PET imaging. We found a significant correlation between several tumor characteristics and FDG uptake, which may explain the variability in PET scanning results among patients with CRC. Contrary to reports on gastric cancer [20, 21], we found no association between the SUV and regional lymph nodes metastases or TNM stage, in accordance with previous reports on CRC. In a small series of 48 patients with known or suspected primary CRC, Abdel-Nabi et al. [18] found that FDG-PET imaging identified all primary carcinomas, but it was ineffective for detecting local lymph node involvement. N-staging requires numeric assessment of pericolic and mesenteric nodes; however, the regional lymph nodes in colorectal carcinoma are often small and in close proximity to the primary tumor mass. Furthermore, the pericolic nodes often contain micrometastatic cancer cells, recognized only at the time of histopathologic evaluation. For these reasons, the sensitivity of PET for regional lymph node metastases from CRC was found to be only 29% [18].
In the present study, SUV was significantly related to tumor size (P=.0004) and depth of invasion (P=.042), which is in agreement with most clinical studies on various malignancies [7, 12, 20–22]. We hypothesize that this is because the number of tumor cells in the mass augments in parallel with an increase in the tumor size. According to a study on breast cancer, FDG uptake was affected by cellularity [9], although in general, the number of viable tumor cells per gram of tissue correlates linearly with the mass size. Therefore, the need for glucose, which determines FDG uptake or SUV, correlates with the tumor size; however, there is 1 exception. In the present study, a mucinous adenocarcinoma had the lowest SUV (3.1), even though its diameter was not the smallest. It has been suggested that the sensitivity of FDG-PET for detecting mucinous adenocarcinoma is lower than that for detecting nonmucinous adenocarcinoma [20, 21]. In a retrospective review of 22 patients with mucinous carcinoma, FDG-PET showed the mucinous carcinoma in only 13 (59%). The authors of that study found that the tumor cellularity and the amount of mucin within the tumor mass were predictive of FDG-PET results [23]. The lack of expression of GLUT-1 may also account for the low sensitivity, as indicated in our study. According to another study, the sensitivity of FDG-PET for detecting mucinous colorectal adenocarcinomas ranged from 41–58% versus 92% for nonmucinous CRCs, probably because of their relative hypocellularity [24]. Moreover, because clinical studies have shown that the presence of mucin in CRCs is associated with poorer survival rates [25], PET might be of limited value in monitoring recurrence and metastasis in these patients.
FDG uptake is positively correlated with tumor invasion in gastrointestinal carcinoma, including esophageal and gastric cancers [20, 21, 26]. Mochiki et al. [20] found that the size and depth of invasion of the primary tumor were significantly correlated with the SUV in gastric cancer, and that T3 and T4 tumors were detected more frequently than T1 tumors. Furthermore, PET scans did not show the primary tumor in 21 of 85 patients with gastric cancer, 15 (71.4%) of whom had T1 tumors that invaded the submucosa [20]. Furudoi et al. [27] found that depth of invasion was also associated with GLUT-1 in CRC. This suggests that as the invasion becomes deeper, the resulting GLUT-1 overexpression causes the SUV to increase.
In the present study, SUV was significantly related to the intensity of GLUT-1 expression (r=0.619, P=.003) and there was a strong relationship between GLUT-1 intensity and the SUV. It is reasonable to assume that glucose consumption, as calculated by the SUV in FDG-PET, predicts the level of GLUT-1 transporters in CRC. Like other cancers, CRC usually expresses higher levels of glucose transporter proteins to satisfy the demand for energy for rapidly increasing proliferation. Among the GLUT isoforms, the basic glucose transporter (GLUT-1) is thought to play a major role in glucose uptake by many tumors [27]. The positive relationship between SUV and GLUT-1 expression has been confirmed in vitro by PET previously [28, 29], and the same correlation has been proven by PET clinically in many malignancies [7, 9, 12, 13, 22].
We noticed that more FDG accumulated in the intermediate and central part of the lesions, in parallel with higher GLUT-1 expression. Studies have shown that hypoxia upregulates GLUT-1 expression in vitro [29]. In the expanding tumor mass, which is generally characterized by a limited O2 supply and a high glucose consumption rate, a series of hypoxia-induced proteomic and genomic changes activate angiogenesis, anaerobic metabolism, and other processes that enable tumor cells to survive or escape their oxygen-deficient environment [30]. The hypoxia-inducible factor 1 (HIF-1), which is overexpressed in response to hypoxia, is the primary transcription factor mediating several physiologic and biological changes, including GLUT-1 overexpression [31]. An aggravated hypoxic state increases GLUT-1 expression, which would result in increased FDG uptake, seen as the SUV from the periphery to the intermediate and central part of the lesion in the present study. We must bear in mind that the microenvironment around the tumor is complicated and that GLUT-1 is not the only hypoxia-associated factor that influences FDG uptake. Other factors, such as hexokinase and various isoforms of the GLUT family also play important roles in determining FDG uptake and SUVs [22, 29].
Based on our findings of the association of FDG uptake with tumor size, depth of invasion, and GLUT-1 overexpression, we propose the following biological sequence: As the tumor grows larger and invades deeper, the intermediate and central part of the tumor, which contains more viable tumor cells, becomes more hypoxic. The hypoxic condition stimulates GLUT-1 overexpression and induces high FDG uptake. Because depth of invasion and GLUT-1 are prognostic markers of CRC, the SUVs determined by PET scans may offer important biological information about CRC.
References
Truant S, Huglo D, Hebbar M, Ernst O, Steinling M, Pruvot FR (2005) Prospective evaluation of the impact of [18F]fluoro-2-deoxy-d-glucose positron emission tomography of resectable colorectal liver metastases. Br J Surg 92:362–369
Delbeke D, Martin WH (2004) PET and PET-CT for evaluation of colorectal carcinoma. Semin Nucl Med 34:209–223
Dimitrakopoulou-Strauss A, Strauss LG, Burger C, Ruhl A, Irngartinger G, Stremmel W, Rudi J (2004) Prognostic aspects of 18F-FDG PET kinetics in patients with metastatic colorectal carcinoma receiving FOLFOX chemotherapy. J Nucl Med 45: 1480–1487
Wong CY, Salem R, Qing F, Wong KT, Barker D, Gates V, Lewandowski R, Hill EA, Dworkin HJ, Nagle C (2004) Metabolic response after intraarterial 90Y-glass microsphere treatment for colorectal liver metastases: comparison of quantitative and visual analyses by 18F-FDG PET. J Nucl Med 45:1892–1897
Rohren EM, Paulson EK, Hagge RH, Wong TZ, Killius J, Clavien PA, Nelson RC (2002) The role of F-18-FDG PET in preoperative assessment of the liver in patients being considered for curative resection of hepatic metastases from colorectal cancer. Clin Nucl Med 27:550–555
Rohren EM, Turkington TG, Coleman RE (2004) Clinical applications of PET in oncology. Radiology 231:305–332
Kurokawa T, Yoshida Y, Kawahara K, Tsuchida T, Okazawa H, Fujibayashi Y, Yonekura Y, Kotsuji F (2004) Expression of GLUT-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [F-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int J Cancer 109:926–932
Chung JK, Lee YJ, Kim SK, Jeong JM, Lee DS, Lee MC (2004) Comparison of [18F]fluorodeoxyglucose uptake with glucose transporter-1 expression and proliferation rate in human glioma and non-small-cell lung cancer. Nucl Med Commun 25:11–17
Bos R, Van Der Hoeven JJ, Van Der Wall E, Van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA, Molthoff CF (2002) Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 20:379–387
Rajendran JG, Mankoff DA, O'Sullivan F, Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG, Krohn KA (2004) Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F] fluoromisonidazole and [18F] fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 10:2245–2252
Pugachev A, Ruan S, Carlin S, Larson SM, Campa J, Ling CC, Humm JL (2005) Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys 62:545–553
Yen TC, See LC, Lai CH, Yah-Huei CW, Ng KK, Ma SY, Lin WJ, Chen JT, Chen WJ, Lai CR, Hsueh S (2004) 18F-FDG uptake in squamous cell carcinoma of the cervix is correlated with glucose transporter 1 expression. J Nucl Med 45:22–29
Kato H, Takita J, Miyazaki T, Nakajima M, Fukai Y, Masuda N, Fukuchi M, Manda R, Ojima H, Tsukada K, Kuwano H, Oriuchi N, Endo K (2003) Correlation of 18-F-fluorodeoxyglucose (FDG) accumulation with glucose transporter (Glut-1) expression in esophageal squamous cell carcinoma. Anticancer Res 23:3263–3272
Koga H, Matsuo Y, Sasaki M, Nakagawa M, Kaneko K, Hayashi K, Kuwabara Y, Honda H (2003) Differential FDG accumulation associated with GLUT-1 expression in a patient with lymphoma. Ann Nucl Med 17:327–331
Paul AK, Tatsumi M, Higuchi I, Fukunaga H, Yasuda T, Nishimura T (2003) Gamma camera coincidence imaging with [18F]fluorodeoxyglucose in the pretreatment evaluation of patients with oesophageal cancer. Nucl Med Commun 24:963–970
Fukunaga H, Sekimoto M, Ikeda M, Higuchi I, Yasui M, Seshimo I, Takayama O, Yamamoto H, Ohue M, Tatsumi M, Hatazawa J, Ikenaga M, Nishimura T, Monden M (2005) Fusion image of positron emission tomography and computed tomography for the diagnosis of local recurrence of rectal cancer. Ann Surg Oncol 12:561–569
Hayashi N, Yamamoto H, Hiraoka N, Dono K, Ito Y, Okami J, Kondo M, Nagano H, Umeshita K, Sakon M, Matsuura N, Nakamori S, Monden M (2001) Differential expression of cyclooxygenase-2 (COX-2) in human bile duct epithelial cells and bile duct neoplasm. Hepatology 34:638–650
Abdel-Nabi H, Doerr RJ, Lamonica DM, Cronin VR, Galantowicz PJ, Carbone GM, Spaulding MB (1998) Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology 206:755–760
Topal B, Flamen P, Aerts R, D'Hoore A, Filez L, Van Cutsem E, Mortelmans L, Penninckx F (2001) Clinical value of whole-body emission tomography in potentially curable colorectal liver metastases. Eur J Surg Oncol 27:175–179
Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K (2004) Evaluation of 18F-2-deoxy-2-fluoro-d-glucose positron emission tomography for gastric cancer. World J Surg 28:247–253
Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, Noh SH (2005) Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 103:2383–2390
Tian M, Zhang H, Nakasone Y, Mogi K, Endo K (2004) Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging 31:5–12
Berger KL, Nicholson SA, Dehdashti F, Siegel BA (2000) FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol 174:1005–1008
Whiteford MH, Whiteford HM, Yee LF, Ogunbiyi OA, Dehdashti F, Siegel BA, Birnbaum EH, Fleshman JW, Kodner IJ, Read TE (2000) Usefulness of FDG-PET scan in the assessment of suspected metastatic or recurrent adenocarcinoma of the colon and rectum. Dis Colon Rectum 43:759–767
Kubota K, Akasu T, Fujita S, Sugihara K, Moriya Y, Yamamoto S (2004) Clinical and pathological prognostic indicators with colorectal mucinous carcinomas. Hepatogastroenterology 51:142–146
Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Ojima H, Tsukada K, Oriuchi N, Inoue T, Endo K (2002) Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 94:921–928
Furudoi A, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F (2001) Clinical significance of human erythrocyte glucose transporter 1 expression at the deepest invasive site of advanced colorectal carcinoma. Oncology 60:162–169
Burt BM, Humm JL, Kooby DA, Squire OD, Mastorides S, Larson SM, Fong Y (2001) Using positron emission tomography with [(18)F]FDG to predict tumor behavior in experimental colorectal cancer. Neoplasia 3:189–195
Aloj L, Caraco C, Jagoda E, Eckelman WC, Neumann RD (1999) Glut-1 and hexokinase expression: relationship with 2-fluoro-2-deoxy-D-glucose uptake in A431 and T47D cells in culture. Cancer Res 59:4709–4714
Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE (2003) Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol 163:1233–1243
Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL (2003) Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1.Cancer Res 63:1138–1143
Acknowledgments
We thank Dr. Ichiro Higuchi of the Department of Nuclear Medicine and Tracer Kinetics, Osaka University, for technical assistance with PET imaging, and Dr. Chew Yee Ngan, Dr. Hideyuki Hemmi, Dr. Kyo Naganori, for his help in preparing this manuscript. This work was supported by a Grant-in Aid for Cancer Research from the Ministry of Education, Science, Sports, and Culture Technology, Japan (to H.Y.). This study was supported in part by the Japan-China Sasakawa Medical Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, J., Yamamoto, H., Fukunaga, H. et al. Correlation of GLUT-1 Overexpression, Tumor Size, and Depth of Invasion with 18F-2-fluoro-2-deoxy-d-glucose Uptake by Positron Emission Tomography in Colorectal Cancer. Dig Dis Sci 51, 2198–2205 (2006). https://doi.org/10.1007/s10620-006-9428-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9428-2