Abstract
The purpose of this study was to compare measurements of hepatic tissue blood flow (TBF) calculated by xenon and perfusion CT. Seven patients with normal liver and eight with chronic liver disease underwent both xenon and perfusion CT. During xenon CT examinations, serial abdominal CT scans were obtained every minute before and during 4 min of nonradioactive 25% (v/v) xenon gas inhalation and 5 min of administration of oxygen-rich air. Hepatic arterial and portal venous TBF were measured separately with a special imaging system using the Kety-Schmidt expression based on the Fick principle (AZ-7000W; Anzai Medical Co.). The hepatic arterial fraction (HAF) was calculated as follows: [hepatic arterial TBF/(hepatic arterial TBF + portal venous TBF)]. During perfusion CT examinations, total hepatic TBF and HAF were also calculated from the enhanced CT cine image data on a workstation using a commercially available software package based on a deconvolution algorithm (CT Perfusion 3 GE Healthcare, USA). Total hepatic TBF measured by xenon and perfusion CT was 82.9±15 and 82.8±18 ml/min/100 g, respectively. The measured values by the two techniques showed a significant correlation (R 2= 0.657, P < 0.05). HAF measured by xenon and perfusion CT was 26.6±11 and 21.8±13%, respectively. The measured values by the two techniques also showed a significant correlation (R 2= 0.869, P < 0.05). We conclude that there was a good correlation between hepatic TBF quantified by xenon CT and perfusion CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In chronic liver disease, high intrahepatic vascular resistance decreases the portal fraction of liver perfusion [1, 2]. This decrease in portal perfusion is partially compensated by an increase in arterial inflow [1, 3, 4]. These hemodynamic changes in cirrhosis have a profound effect on hepatic function and could be useful for a precise and objective diagnosis. Various methods exist for the measurement of hepatic perfusion [5–9], however, the majority of these methods are controversial [5, 10].
Previous reports indicated that xenon computed tomography (CT) could provide quantitative measurement of hepatic tissue blood flow (TBF) [11, 12]. However, the application of xenon CT requires special equipment and materials, such as nonradioactive xenon gas. Recently, some studies have demonstrated the capacity of perfusion CT to evaluate hepatic TBF [13–16]. The application of perfusion CT requires only a workstation with perfusion analyzing software. Therefore, we hypothesized that if a good correlation between xenon CT and perfusion CT, in hepatic TBF, could be demonstrated, xenon CT could be replaced with perfusion CT for quantification of hepatic TBF.
The purpose of this study was to evaluate the correlation between hepatic TBF calculated with xenon CT and perfusion CT, based on a deconvolution algorithm.
Materials and Methods
Patients
Seven patients without liver disease (four females, three males; age range, 24–58 years; mean age, 39.6±12 years [SD]) and eight patients with chronic liver diseases (four females, four males; age range, 48–68 years; mean age, 57.1±8 years) underwent both xenon and perfusion CT examinations from April 2003 to January 2004. Three of the eight patients with chronic liver disease had living donor liver transplantation, and the other five had liver tumors (hepatocellular carcinoma [HCC], three cases; cholangiocarcinoma [CCC], two cases) underwent hepatectomies. With regard to the Child-Pugh classification, five patients were classified as Child A, one as Child B, and two as Child C. The seven patients without liver disease were donor candidates for living liver transplantation; the diagnosis of normal liver was confirmed by needle biopsy history, physical examination, laboratory screening, and Doppler sonography of the liver.
All patients provided informed consent to undergo the enhanced CT, xenon CT, and perfusion CT examinations and to be included in the study, which was conducted in accordance with the principles of the Declaration of Helsinki [17]. Approval from our Ethics Committee was not required for this study.
Xenon CT technique
Xenon CT technique was used to assess regional TBF by using the wash-in/wash-out protocol, used previously for measurement of regional cerebral blood flow [18]. The Kety-Schmidt equation, based on the Fick principle used for cerebral blood flow evaluation, was also used [19, 20]. Xenon gas (Xenon Cold; Anzai Medical Co., Tokyo) was used as the nonradioactive xenon gas, and a xenon gas inhalation system with a closed gas circuit (AZ-725; Anzai Medical Co.) was used. Xenon concentrations in the respiratory circuit (end-tidal peak xenon values) were continuously measured during examination by a xenon monitor incorporated in the AZ-725 circuit. End-tidal peak xenon values were recognized automatically and fitted to a monoexponential curve (end-tidal xenon curve) [21]. Changes in arterial blood xenon concentrations were estimated based on the end-tidal xenon curve [22].
Changes in CT values of the liver parenchyma were measured by an eight-channel multidetector-row CT scanner (LightSpeed Ultra; GE Healthcare, USA). Four axial images with a 10-mm slice thickness including the liver parenchyma around the porta hepatis level were obtained incrementally as baseline CT images prior to xenon inhalation. In the next step, patients inhaled 25% (vol/vol) xenon gas for 4 min (wash-in), then breathed room air for 5 min (wash-out) [18]. CT scans were acquired at each level at 1-min intervals. As many as 10 CT images per patient were obtained in total, including the baseline image at each level. Exposure factors were 100 kVp, 200 mA, and 1-sec scans; patients were required to hold their breath for 7 sec during a series of scans at four levels. Smoothing with a 9×9-pixel filter was used to reduce noise contribution on the CT images. Body movement related to respiration was taken into account, and changes in the pancreatic position on each enhanced CT image were digitally corrected relative to the baseline image on the screen of a blood flow imaging analysis computer (AZ-7000W; Anzai Medical Co.) system.
AZ-7000W was also used to create color maps for hepatic arterial and portal venous TBF (ml/min/100 g) from images obtained by xenon CT as well as confidence images, which showed the variance of value for each pixel based on the least squares method. High TBF appeared as red regions and low TBF as blue regions. Confidence images were used to evaluate the reliability of the TBF value for each pixel of the image [11, 12]. TBF was determined by placing regions of interest (ROI) on the noncancerous region of the liver parenchyma on the color maps. The ROI of the noncancerous region was carefully placed away from the tumors without overlapping visible blood vessels.
The percentage hepatic arterial fraction (HAF) was calculated using the following equations.
Perfusion CT technique
Perfusion and xenon CT examinations were performed with the same CT scanner. At first, whole-liver images were obtained with a tube voltage of 120 kV, a tube current of 300 mA, a rotation period of 0.5 sec, a detector collimation of 2.5 mm, and a table increment of 27 mm/rotation. Images with an effective section thickness of 5 mm were reconstructed every 5 mm to provide contiguous sections. Then CT perfusion examinations were performed with a cine mode scan (1 sec/rotation) at the level of the porta hepatis, determined by the whole-liver images. The scan parameters employed for CT perfusion study were 120 kV, 60 mA, 1 sec/rotation, 10-mm slice thickness ×2, cine scan mode, standard reconstruction algorithm, and 35-cm display field of view (DFOV). A dose of 0.5 ml/kg (total dose, 25–40 ml) of 300 mg I/ml of the nonionic contrast medium (Iohexol, Omunipaque; Daiichi Pharmaceutical Co., Tokyo) was injected at a rate of 5 ml/sec through a 20-gauge plastic cannula inserted into the antecubital vein, followed by 30 ml of saline chaser at a rate of 5 ml/sec with a power injector equipped with double syringe drivers. Data acquisition was started 8 sec after the initiation of injection of the contrast medium and the scan duration was 40 sec. Oxygen inhalation (2 L/min) was given to help the patients hold their breath during scanning. Image reconstruction was performed every 0.5 sec, and these images were transferred to the image processing workstation (Advantage Workstation 4.1; GE Healthcare), to calculate color map images of each blood flow parameter in the liver parenchyma by using commercially available software (CT Perfusion 3; GE Healthcare). Blood flow parameters included TBF (ml/min/100 g) and HAF (%). To calculate each functional map image, ROIs (pixel size, 8–20 pixels) were set on the aorta and portal vein trunk as input function. At the time of measuring the parameters, one radiologist with 15 years’ experience in gastrointestinal and hepatobiliary imaging carefully placed 10–20 ROIs (pixel size; ∼400 pixels) in each section of the liver by avoiding intrahepatic large vessels or tumors confirmed on a CT image (Fig. 1). The value of all ROIs was measured and averaged for each case (Fig. 2b). In our study, ROIs of the liver parenchyma in patients with HCC were drawn in a nontumor segment in order to avoid direct mechanical compression of portal radicles and the influence of arteriovenous shunt.
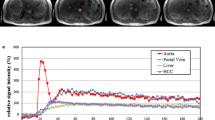
Blood flow maps of a 24 year-old woman free of liver disease (donor candidate of living liver transplantation). (a) Xenon CT images. Left: Hepatic arterial tissue blood flow (TBF) image. Middle: Portal venous TBF image. Right: Confidence image. Total hepatic TBF is 97.0 ml/min/100 g; hepatic arterial TBF, 21.0 ml/min/100 g; portal venous TBF, 76.0 ml/min/100 g; and hepatic arterial fraction (HAF), 22%. (b) Perfusion CT images. Left: Total hepatic TBF image. Right: HAF image. Total hepatic TBF is 110.7 ml/min/100 g and HAF is 21.0%
Statistical analysis
Differences in TBF and HAF calculated by xenon and perfusion CT were analyzed using regression analysis. Data are expressed as mean ± SD. Statistical significance was defined as a P value of < 0.05.
Results
In all cases, total hepatic TBF calculated by xenon and perfusion CT was 82.9±15 and 82.8±18 ml/min/100 g, respectively (Table 1). HAF calculated by xenon and perfusion CT was 26.5±11% and 21.8±13%, respectively (Table 1). Linear regression analysis showed a good correlation in the measurements of both total hepatic TBF (y=0.936x+3.666; R 2=0.657, P < 0.05) and HAF (y=1.056x−6.632; R 2=0.869; P < 0.05) calculated by xenon CT and perfusion CT (Fig. 3).
Total hepatic TBF and HAF measured by xenon CT and perfusion CT in all cases. (a) Correlation between total hepatic TBF perfusion CT and TBF xenon CT. Linear regression analysis shows a good correlation of total hepatic TBF measurements (y=0.936x+3.666; R 2=0.657, P < 0.05). (b) Correlation between HAF perfusion CT and HAF xenon CT. Linear regression analysis shows a good correlation of HAF measurements (y=1.056x−6.632; R 2=0.869, P < 0.05)
In patients without liver disease, total hepatic TBF calculated by xenon CT and perfusion CT was 85.7±10 and 88.7±17 ml/min/100 g, respectively (Table 1). HAF calculated by xenon CT and perfusion CT was 23.1±5 and 20.6±6%, respectively (Table 1). Linear regression analysis showed a good correlation in the measurements of both total hepatic TBF (y=1.411x−32.283; R 2=0.711, P < 0.05) and HAF (y=0.958x−1.502; R 2=0.55, P < 0.05) calculated by xenon CT and perfusion CT (Fig. 4).
Total hepatic TBF and HAF measured by xenon CT and perfusion CT in patients free of liver disease. (a) Linear regression analysis of TBF perfusion CT and TBF xenon CT showing a good correlation of hepatic TBF measurements (y=1.411x−32.283; R 2= 0.711, P < 0.05). (b) Linear regression analysis of HAF perfusion CT and HAF xenon CT. Linear regression analysis also shows a good correlation of HAF measurements (y=0.958x−1.502; R 2=0.55, P < 0.05)
In patients with chronic liver disease, total hepatic TBF calculated by xenon CT and perfusion CT was 80.5±18 and 77.6±18 ml/min/100 g, respectively (Table 1). HAF calculated by xenon CT and perfusion CT was 29.6±14% and 22.9±17 %, respectively (Table 1). Linear regression analysis showed a good correlation in the measurements of both total hepatic TBF (y=0.728x+19.211; R 2=0.566, P < 0.05) and HAF (y=1.17x−12.115; R 2=0.936, P < 0.05) calculated by xenon CT and perfusion CT (Fig. 5).
Total hepatic TBF and HAF measured by xenon CT and perfusion CT in patients with chronic liver disease. (a) Linear regression analysis of TBF perfusion CT and TBF xenon CT showing a good correlation of total hepatic TBF measurements (y=0.728x+19.211; R 2= 0.566, P < 0.05). (b) Linear regression analysis of HAF perfusion CT and HAF xenon CT. Linear regression analysis also shows a good correlation of HAF measurements (y=1.17x−12.115, R 2=0.936, P < 0.05)
Discussion
Quantitative measurement of hepatic TBF, using various methods such as isotope scintigraphy [8], Doppler ultrasound [9], xenon CT [11, 12], and perfusion CT [13–16], has been reported. Among these methods, the nuclear medicine approach has the longest history and is regarded as the gold standard for cerebral blood flow [23]. However, a limitation of this method is that nuclear medicine approaches such as positron emission tomography (PET) and single photon emission tomography (SPECT) require radioisotopes and special equipment that are very expensive. Moreover, the spatial resolution of these imaging modalities is inferior to that of CT. The application of magnetic resonance imaging (MRI), is associated with difficulties in the quantification of TBF, because signal enhancement of vessels and tissue by nonspecific MR contrast medium, such as gadolinium complex, does not show a linear correlation with the concentration of the contrast medium [24]. CT provides much clearer and sharper images than other modalities and can be used to evaluate simultaneously color maps of quantitative TBF and clear anatomical images. In addition, CT is the most practical and universal machine on clinical sites and is used frequently for abdominal scans.
There are two methods available for calculation of TBF with CT: xenon and perfusion CT. Xenon CT has been in clinical use for approximately 30 years [25], and perfusion CT was first described by Miles in 1991 [26]. Patients have to hold their breath for a relatively long time at the same level of respiratory cycle in order to have useful CT data for precise calculation of hepatic TBF. However, recent technical innovations in CT, such as fast imaging ability, have overcome this limitation by reducing the breath-holding duration. However, xenon CT might not be practical, given its requirement for excellent collaboration from patients, in addition to special materials such as xenon gas and specialized equipment. Xenon gas may occasionally be responsible for a decrease in respiratory rate, headaches, nausea, vomiting, and convulsions [27]. However, there have been no reports of respiratory failure. A large study involving 1839 patients reported that 3.6%, 0.4%, 0.2%, and 0.2% of patients experienced xenon side effects of decreased respiratory rate, headaches, nausea, vomiting, and convulsions, respectively [27].
Compared with the complexity of xenon CT, perfusion CT requires only universal materials to measure blood flow, such as small amounts of iodine contrast material, except for a workstation with the CT perfusion analyzing software. Moreover, perfusion CT can be performed in the same clinical session, immediately before the usual dynamic CT with iodine contrast medium. In this study, the fast imaging ability of multidetector CT provided high-quality images that also contributed to precise measurement of blood flow with decreasing noise. For these reasons, we considered perfusion CT to be significantly more practical than xenon CT. This study demonstrated that total hepatic TBF and HAF measured by perfusion CT show a good correlation with data from xenon CT.
In the procedure of perfusion CT, the past method using the Fick principle requires injection rates, as high as 10–20 ml/sec, of contrast material via a peripheral vein and, thus, can be very dangerous in patients with fragile or thin vessels [13]. However, the new perfusion technique using the central volume principle with a deconvolution algorithm, employed in this study, needs an injection rate of only 3–5 ml/sec, which is the normal rate in routine work and is much safer than previous methods [15].
Under the present conditions, there were some limitations of this study. Because the aorta and the portal vein should always be included in the scan section for the calculation process and a maximum width of 20 mm could be scanned in cine mode, the examination range was limited to the porta hepatis. However, wider detectors such as those of 32 to 40 mm that are available in the newest multidetector-row CT model with a greater number of detector rows can overcome this limitation. Although we used low-dose x-rays for the perfusion CT study, doses of radiation exposure to patients can increase. It is expected that this can be reduced by new techniques such as the quantum noise filter [28].
In conclusion, xenon CT and perfusion CT showed a good correlation for measurements of total hepatic TBF and HAF. We expect that perfusion CT, based on the deconvolution algorithm, could replace xenon CT for measurement of hepatic TBF.
References
Leen E, Goldberg JA, Anderson JR, Robertson J, Moule B, Cook TG, Mcardle CS (1993) Hepatic perfusion changes in patients with liver metastases: comparison with those patients with cirrhosis. Gut 34:554–557
Tsushima Y, Blomley MJ, Kusano S, Endo K (1999) The portal component of hepatic perfusion measured by dynamic CT: an indicator of hepatic parenchymal damage. Dig Dis Sci 44:1632–1638
Lautt WW (1985) Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. Am J Physiol 249:G549–G556
Kleber G, Steudel N, Behrmann C, Zipprich A, Hubner G, Lotterer E, Fleig WE (1999) Hepatic arterial flow volume and reserve in patients with cirrhosis: use of intra-arterial Doppler and adenosine infusion. Gastroenterology 116:906–914
Johnson DJ, Muhlbacher F, Wilmore DW (1985) Measurement of hepatic blood flow. J Surg Res 39:470–481
Zeeh J, Lange H, Bosch J, Pohl S, Loesgen H, Eggers R, Navasa M, Chesta J, Bircher J (1988) Steady-state extrarenal sorbitol clearance as a measure of hepatic plasma flow. Gastroenterology 95:749–759
Miles KA, Hayball MP, Dixon AK (1993) Functional images of hepatic perfusion obtained with dynamic CT. Radiology 188:405–411
Ziegler SI, Haberkorn U, Byrne H, Tong C, Kaja S, Richolt JA, Byrne H, Tong C, Schosser R, Krieter H, Kaja S, Richolt JA, Lammertsma AA, Price P (1996) Measurement of liver blood flow using oxygen-15 labelled water and dynamic positron emission tomography: limitations of model description. Eur J Nucl Med 23:169–177
Taourel P, Blanc P, Dauzat M, Chabre M, Pradel J, Gallix B, Larrey D, Bruel JM (1998) Doppler study of mesenteric, hepatic, and portal circulation in alcoholic cirrhosis: relationship between quantitative Doppler measurements and the severity of portal hypertension and hepatic failure. Hepatology 28:932–936
Reichen J (1995) Assessment of hepatic function with xenobiotics. Semin Liver Dis 15:189–201
Gur D, Good WF, Herbert DL, Yonas H, Wozney P, Van Thiel DH, Wolfson SK Jr (1985) Blood flow mapping in the human liver by the xenon/CT method. J Comput Assist Tomogr 9:447–450
Sase S, Monden M, Oka H, Dono K, Fukuta T, Shibata I (2002) Hepatic blood flow measurements with arterial and portal blood flow mapping in human liver by means of xenon CT. J Comput Assist Tomogr 26:243–249
Bader TR, Herneth AM, Blaicher W, Steininger R, Mühlbacher F, Lechner G, Grabenwoger F (1998) Hepatic perfusion after liver transplantation: noninvasive measurement with dynamic single-section CT. Radiology 209:129–134
Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y (2001) Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol 176:667–673
Lee TY (2002) Functional CT: physiological models. Trends Biotechnol 20:S3–S10
Blomley MJ, Lim AK, Harvey CJ, Patel N, Eckersley RJ, Basilico R, Heckemann R, Urbank A, Cosgrove DO, Taylor-Robinson SD (2003) Liver microbubble transit time compared with histology and Child-Pugh score in diffuse liver disease: A cross sectional study. Gut 52:1188–1193
World Medical Association (2000) Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 284:3043–3045
Kalender WA, Polacin A, Eidloth H, Kashiwagi S, Yamashita T, Nakano S (1991) Brain perfusion studies by xenon-enhanced CT using washin/washout study protocols. J Comput Assist Tomogr 15:816–822
Kety S (1951) The theory and applications of the exchange of inert gas at the lungs and tissue. Pharmacol Rev 3:1–41
Fick A (1870) Uber die Messung des Blutquantums in den Herzventrikeln Verhandl dphys-med Ges zu Wurzburg 2:16–28
Sase S (1996) Correction method for end-tidal xenon concentration in CBF measurements with xenon-enhanced CT. J Comput Assist Tomogr 20:688–692
Meyer JS, Hayman LA, Yamamoto M, Sakai F, Nakajima S (1980) Local cerebral blood flow measured by CT after stable xenon inhalation. AJR Am J Roentgenol 135:239–251
Frackowiak RS, Lenzi GL, Jones T, Heather JD (1980) Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr 4:727–736
Brasch RC, Weinmann HJ, Wesbey GE (1984) Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex. AJR Am J Roentgenol 142:625–630
Winkler SS, Holden JE, Sackett JF, Flemming DC, Alexander SC (1977) Xenon and krypton as radiographic inhalation contrast media with computerized tomography: preliminary note. Invest Radiol 12:19–20
Miles KA (1991) Measurement of tissue perfusion by dynamic computed tomography. Br J Radiol 64:409–412
Latchaw RE, Yonas H, Pentheny SL, Gur D (1987) Adverse reactions to xenon-enhanced CT cerebral blood flow determination. Radiology 163:251–254
Okumura M, Toyoshima N, Katada K, Sasaki M (2003) Performance evolution for image filters used for CT images. Radiological Society of North America. 89th Scientific assembly and annual meeting program: 720
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashimoto, K., Murakami, T., Dono, K. et al. Quantitative Tissue Blood Flow Measurement of the Liver Parenchyma: Comparison Between Xenon CT and Perfusion CT. Dig Dis Sci 52, 943–949 (2007). https://doi.org/10.1007/s10620-006-9327-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9327-6