Abstract
Gluten-sensitive enteropathy is characterized by small intestinal damage. The pathogenic mechanisms involved are not precisely understood. There is recent interest in the possibility that matrix metalloproteinases might play a pathogenic role. Using immunohistochemistry technique, we examined the protein expression of matrix metalloproteinases-1, -3, and -9 and the tissue inhibitor metalloproteinase-1 in duodenal biopsies from 30 patients with celiac disease and dermatitis herpetiformis. We demonstrated that the percentage of cells expressing these enzymes and their inhibitor in all patients was significantly greater than in the normal controls (P < 0.0001). This was evident even in patients with a minimal lesion but was most marked in patients with severe damage, mirroring the degree of inflammation in the small intestinal tissue. The increased expression of these enzymes and their inhibitor in the duodenal mucosa of patients with gluten-sensitive enteropathy suggests a role for these enzymes in the tissue remodeling which is a feature of these disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Celiac disease is a gluten-sensitive disorder characterized by malabsorption and a typical small intestinal histological lesion, marked by considerable tissue remodeling [1]. Similar intestinal lesions are found in dermatitis herpetiformis, another gluten-sensitive enteropathy [2]. The pathogenic mechanisms involved in the tissue damage are not precisely understood, but T-cell reactivity to gluten is likely to play a central role [3]. Increased production of several cytokines, in particular interferon-γ, has been described in the celiac mucosa but it is not clear how this leads to the celiac lesion [4, 5]. There is recent interest in the possibility that matrix metalloproteinases (MMPs) may play a pathogenic role in celiac disease [6–8].
MMPs belong to a major family of neutral proteases capable of degrading extracellular matrix and basement membrane components. They participate in the remodeling of normal tissue components and in inflammatory damage in various pathological conditions [9–13]. To date, 24 different MMPs have been identified: these are divided into four subclasses based on their substrate specificity, including collagenases (MMP-1), interstitial collegenases (MMP-8 and -13), stromelysins (MMP-3, -7, -10, -11, -12, and -26), gelatinases (MMP-2 and -9), and membrane-type MMPs (14–17). The proteolytic activity of MMPs is controlled by specific tissue inhibitors of metalloproteinases (TIMPs) [18] and nonspecific inhibitors, such as α2-macroglobulin [19]. TIMPs can bind to the catalytic domain of MMPs in a 1:1 stochiometry to form complexes, thus inhibiting the enzymatic activity of the MMPs [20]. Currently, four different TIMPs have been identified, revealing different tissue and cell type specific expression and regulation patterns [19, 21]. MMPs and TIMPs are produced by various cell populations, including myofibroblasts, fibroblasts, and inflammatory cells such as macrophages and lymphocytes [6, 18].
Three studies have investigated mRNA expression of MMPs and TIMPs in celiac [6, 8] and dermatitis herpetiformis [7] intestinal mucosa. Employing in situ hybridization, Daum et al. reported increased expression of MMP-1, MMP-3, and the inhibitor TIMP-1 in untreated celiac disease [6]. Using a similar technique, Salmela et al. described elevated expression of MMP-12 in the mucosa of patients with dermatitis herpetiformis, a further gluten-sensitive disorder [7]. In a more recent study, using real-time RT-PCR, increased MMP-1, MMP-12, and TIMP-1 mRNA levels were described in patients with untreated celiac disease [8].
In the current study, using immunohistochemistry, the protein expression of MMP-1, -3, and -9 and TIMP-1 was investigated in patients with both treated and untreated celiac disease and in patients with dermatitis herpetiformis. The patients were grouped according to the degree of tissue damage, and the findings were compared with those of normal control subjects. In addition, protein expression was correlated with the extent of mucosal damage.
Materials and methods
Patients Thirty patients with gluten-sensitive enteropathy were investigated and details of their age, sex, and small intestinal histology grading are presented in Table 1. They included untreated and treated celiac disease patients and patients with dermatitis herpetiformis. Duodenal lesions in these patients were classified based on routine histology, according to the Marsh classification: grade 1, increased intraepithelial lymphocytes counts (n=13); grade 2, increased intraepithelial lymphocytes and villous blunting (n=10); and grade 3, total villous atrophy (n=7). Biopsy sections from 10 individuals with normal intestinal mucosa were used as controls: these patients were classified as having a Marsh grade 0 lesion and all had negative celiac disease antibody serology (Table 1).
Immunohistochemistry Immunohistochemical analysis was carried out on 5-μm-thick, formalin-fixed, paraffin-embedded tissue sections using the avidin-biotin-peroxidase complex detection method (Vector Labs ABC technique, USA). Tissue sections were deparaffinized and heated in a microwave oven for 20 min in 0.1 M citrate buffer (pH 6.0) to retrieve the antigens. Sections were then immersed in 0.05% hydrogen peroxide in 100% methanol for 20 min to block endogenous peroxidase activity. After incubation in normal horse serum for 20 min, sections were incubated with the anti-MMP-1, MMP-3, and MMP-9 antibodies at a 1/25 dilution and anti-TIMP-1 antibody at a 1/10 dilution in phosphate-buffered saline (PBS; pH 7.6) (Santa Cruz Biotechnology, USA). Sections were then incubated with biotinylated rabbit anti-mouse IgG (Vector Labs ABC technique) for 30 min, followed by peroxidase-conjugated streptavidin for 30 min at room temperature. After each antibody application, sections were washed in PBS. As a negative control, PBS or irrelevant antibodies were used instead of the primary antibody. Color was developed using DAB (Sigma) and slides were counterstained with hematoxylin.
Quantification of MMPs and TIMP-1 Expression Using an eyepiece graticule, five fields at high-power magnification (×40; Olympus Bx41 light microscope) were counted in the lamina propria. The number of stained cells was expressed as the percentage of total cells counted in the five fields examined. The slides were coded and read in a blinded fashion.
Statistical Analysis The mean number of lamina propria cells with 95% confidence intervals was calculated. Results were analyzed using the nonparametric, Mann–Whitney U test. Differences were considered significant at a P value of <0.05.
Results
Expression of MMP -1, -3, and –9
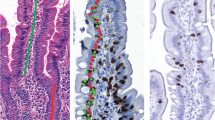
Patients with gluten-sensitive enteropathy (including patients with celiac disease and dermatitis herpetiformis), classified into three groups according to the degree of tissue damage, were compared to normal controls for the level of expression of MMP-1, -3, and -9 (Table 2). Even patients with grade 1 tissue damage, which shows only minimal architectural change but an increase in intraepithelial lymphocyte count, showed a significant elevation in all three MMPs in comparison to normal tissue (P < 0.0001 for each enzyme). This increase was maintained in patients with a grade 2 lesion (Table 2). With additional damage to the intestine seen in grade 3 lesions, a further, significant elevation in the expression of all three MMPs was found (P < 0.004 compared with grade 2 damage; Figures 1A–C). Thus, all patients with gluten-sensitive enteropathy, regardless of the degree of damage, showed a significant increase in the level of expression of these MMPs in the lamina propria, and these levels increased incrementally with the severity of the tissue damage, being most marked in biopsies with a grade 3 lesion. Examples of lamina propria cell expression of MMP-1, -3, and -9 in patients with grade 3 mucosal lesions are shown in Figures 2A–C.
Percentages of lamina propria cells expressing MMP-1, -3, and -9 and TIMP-1 in gluten-sensitive enteropathy patients and normal controls. Grade 0, normal controls; grade 1, patients with increased intraepithelial lymphocytes; grade 2, patients with villous blunting and increased intraepithelial lymphocytes; grade 3, patients with total villous atrophy. Differences between groups were calculated using the Mann–Whitney U test. Bars represent the mean and 95% confidence intervals. Patients with grade 1, 2, and 3 lesions all had significantly elevated cell populations compared with the normal control group (P < 0.0001). Filled circles, patients with celiac disease; open circles, patients with dermatitis herpetiformis
Expression of TIMP-1
The number of cells in the lamina propria expressing TIMP-1 was also increased in all of the gluten-sensitive enteropathy patients compared to the normal controls (P < 0.0001 for each patient group; Figure 1D). As with the MMPs studied, patients with grade 1 tissue damage had a significant elevation in TIMP-1 in comparison with the normal controls (P < 0.0001). TIMP-1 expression increased in accord with the level of tissue damage, and patients with grade 3 lesions showed a significant elevation in TIMP-1 over grade 2 (P < 0.009). Thus, the level in TIMP-1 expression largely mirrored that of the MMPs studied (Table 2, Figure 1D).
Discussion
In this study, the protein expression of the matrix metalloproteinases MMP-1, MMP-3, and MMP-9 and the tissue inhibitor of metalloproteinases-1 (TIMP-1) was investigated in mucosal biopsies from patients with celiac disease and dermatitis herpetiformis. The patients were grouped according to their degree of mucosal damage; however, the small intestinal expression of these three metalloproteinases, and of TIMP-1, was significantly increased in all of these patients compared with the normal control group. Furthermore, the expression of these enzymes and the inhibitor mirrored the degree of mucosal damage, with the most marked increase in staining for each of the four proteins observed in patients with Marsh grade 3 lesions. However, it was of interest that the expression of all four proteins was also significantly increased in patients with a Marsh 1 lesion, in which an increase in intraepithelial lymphocytes is the principal histological abnormality. The majority of the latter group of patients were taking a gluten-free diet.
These observations imply that metalloproteinases may be involved in the tissue remodeling seen in the intestinal lesion in gluten-sensitive enteropathy. Moreover, the finding of increased expression of TIMP-1 as the severity of the lesion increased could represent an involvement of the normal inhibitory mechanism in an attempt to limit the extent of metalloproteinase-mediated damage.
This is the first study to investigate protein expression of metalloproteinases in the intestinal lesion in celiac and dermatitis herpetiformis tissue. Interestingly, the findings are in broad agreement with three earlier studies that investigated mRNA expression of MMPs and TIMP-1 in celiac [6, 8] and dermatitis herpetiformis [7] intestinal mucosa. Using in situ hybridization, Daum et al. (1999) reported increased mRNA expression of MMP-1, MMP-3, and TIMP-1 in untreated celiac disease [6]. Likewise, Salmela et al. (2004) described elevated expression of MMP-12 mRNA in the mucosa of patients with dermatitis herpetiformis [7]. Finally, in a recent study, using real-time RT-PCR, increased mRNA levels of MMP-1, MMP-12, and TIMP-1 were described in patients with untreated celiac disease [8]. However, whereas in these reports mRNA expression of MMP-3 was either absent [7] or less prominent [6], in this study equal protein expression of this enzyme was observed. This highlights the need to examine protein, as well as mRNA, expression of given cell products in order to understand their role in disease processes.
The metalloproteinases, including MMP-1, MMP-3, and MMP-9, can cause degradation of the extracellular matrix and basement membranes and could promote the influx of cells of both the innate (e.g., dendritic cells) and the acquired (e.g., T cells) immune system into the lamina propria and epithelial departments [22, 23]. These events may contribute to the celiac lesion including the marked mucosal damage that can develop within hours of in vivo gliadin challenge of celiac patients [24]. Evidence in support of this concept is found in a fetal gut model of small intestinal damage, where tissue cultured in the presence of pokeweed mitogen [22] or Staphylococcus aureus [25] showed increased expression of MMP-1 and MMP-3. Moreover, in these models, morphological changes similar to the celiac lesion developed.
Increased metalloproteinase activity has been implicated in several other inflammatory disorders, such as inflammatory bowel disease [19, 26] and rheumatoid arthritis [27, 28], and may also play a role in malignancy [29, 30]. In many of these studies, the findings were based on estimation of mRNA transcripts, using techniques such as in situ hybridization or PCR [19]. Elevated mRNA levels for the three metalloproteinases investigated in this study have been reported in inflammatory bowel disease [19, 26] and transcript levels correlated positively with the degree of inflammation in some studies [31]. Furthermore, MMP protein expression examined by immunohistochemistry was shown to be present extracellularly in areas of mucosal damage in both Crohn’s disease and ulcerative colitis [13, 19]. In rheumatoid arthritis, raised levels of metalloproteinase protein were described in both serum and synovial fluid and found to correlate with clinical indicators of disease activity [27, 28].
The MMPs are regulated by specific tissue inhibitors of metalloproteinases, or TIMPs [21]. An imbalance of this system can adversely affect the composition of the intercellular matrix and functions of immunocompetent cells including their adhesion, migration, and differentiation [21, 30]. TIMP-1 is the first member of the TIMP family and is known to form a stable complex with MMP-1, MMP-3, and MMP-9 [20], thereby inhibiting their activity. In this study, although the expression of TIMP-1 mirrored the severity of the histological lesion, it is possible that levels of this inhibitor were insufficient to negate the increased activity of MMPs in celiac and dermatitis herpetiformis tissue with consequent tissue damage. In support of this hypothesis, in patients with grade 3 mucosal lesions it was noted that the number of cells expressing MMP-1 significantly exceeded that of cells expressing TIMP-1 (P < 0.0002).
As is the case with metalloproteinases, increased expression of TIMP-1 is reported in various inflammatory conditions, including inflammatory bowel disease [19, 26] and rheumatoid arthritis [27, 28] and, also, in malignancy [29, 30]. In inflammatory bowel disease tissue, both mRNA transcripts and protein levels of this inhibitor were increased [13, 19]. Raised levels of soluble TIMP-1 have been found in culture supernatants of inflamed biopsies from ulcerative colitis and Crohn`s disease patients [32, 33]. Increased soluble TIMP-1 levels were found in synovial fluid [27] and serum samples of rheumatoid arthritis patients [28]. Likewise, increased tissue transcripts and plasma protein levels of TIMP-1 have been described in patients with gastrointestinal malignancy [29, 30].
The increased protein expression of MMP-1, MMP-3, MMP-9, and TIMP-1 described in this study was localized to cells widely distributed throughout the lamina propria in patients’ biopsies. In the case of MMP-1 and MMP-3, prominent collections of cells in the subepithelial region were noted. The morphological appearance of these cells was in keeping with cells of both macrophage and lymphocyte lineages. However, the specific identity of cell types was not investigated. In earlier studies of gluten-sensitive enteropathy, RNA transcripts for these proteins were particularly located in fibroblasts, myofibroblasts, and macrophages [6–8] and these cells were principally located in the subepithelial region [6, 7]. Cytokines produced by macrophages and T cells including TNF-α, IFN-γ, and IL-1 stimulate cellular production of these proteins [12]. The involvement of TNF was supported by the finding that a p55 TNF receptor fusion protein inhibited MMP-3 production [25]. Overproduction of these cytokines has been reported in celiac mucosa [4, 5]. A further potential T-cell cytokine candidate is IL-17, shown to be upregulated in inflammatory bowel disease and to cause increased secretion of MMP-3 [34].
In conclusion, this is the first study to demonstrate increased protein expression of MMP-1, -3, and -9 and the inhibitory molecule TIMP-1 in the intestinal mucosa of patients with celiac disease and dermatitis herpetiformis. Moreover, the level of expression of these enzymes and their inhibitor, although significantly increased even in grade 1 biopsies, was most significantly elevated in biopsies with the most severe lesion (grade 3), suggesting that these enzymes play a role in the pathological lesion. These findings advance the information reported in earlier studies, in which mRNA transcripts for some these molecules were shown to be increased in gluten-sensitive disorders.
References
Maki M, Collin P (1979) Coeliac disease. Lancet 349:1755–1759
Savilahti E, Reunala T, Maki M (1992) Increase of lymphocytes bearing the gamma/delta T cell receptor in the jejunum of patients with dermatitis herpetiformis. Gut 33:206–211
Sollid LM (2000) Molecular basis of celiac disease. Annu Rev Immunol 18:53–81
Kilmartin C, Lynch S, Abuzakouk M, et al. (2003) Avenin fails to induce a Th1 response in coeliac tissue following in vitro culture. Gut 52:47–52
Forsberg G, Hernell O, Melgar S, Israelsson A, Hammarstrom S, Hammarstrom ML (2002) Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology 123:667–678
Daum S, Bauer U, Foss HD, et al. (1999) Increased expression of mRNA for matrix metalloproteinases-1 and -3 and tissue inhibitor of metalloproteinases-1 in intestinal biopsy specimens from patients with coeliac disease. Gut 44:17–25
Salmela MT, Pender SL, Reunala T, MacDonald TT, Saarialho-Kere U (2001) Parallel expression of macrophage metalloelastase (MMP-12) in duodenal and skin lesions of patients with dermatitis herpetiformis. Gut 48:496–502
Ciccocioppo R, Sabatino AD, Bauer M, et al. (2005) Matrix metalloproteinase pattern in celiac duodenal mucosa. Lab Invest 85:397–407
Khasigov PZ, Podobed OV, Ktzoeva SA, et al. (2001) Matrix metalloproteinases of normal human tissues. Biochemistry (Mosc) 66:130–140
Nagase H, Woessner JFJr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Goetzl EJ, Banda MJ, Leppert D (1996) Matrix metalloproteinases in immunity. J Immunol 156:1–4
Salmela MT, Pender SL, Karjalainen-Lindsberg ML, Puolakkainen P, Macdonald TT, Saarialho-Kere U (2004) Collagenase-1 (MMP-1), matrilysin-1 (MMP-7), and stromelysin-2 (MMP-10) are expressed by migrating enterocytes during intestinal wound healing. Scand J Gastroenterol 39:1095–1104
Kirkegaard T, Hansen A, Bruun E, Brynskov J (2004) Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut 53:701–709
de Coignac AB, Elson G, Delneste Y, et al. (2000) Cloning of MMP-26, a novel matrilysin-like proteinase. Eur J Biochem 267:3323–3329
Grant GM, Giambernardi TA, Grant AM, et al. (1999) Overview of expression of matrix metalloproteinases (MMP-17, MMP-18, and MMP-20) in cultured human cells. Matrix Biol 18:145–148
Welgus HG, Fliszar CJ, Seltzer JL, et al. (1990) Differential susceptibility of type X collagen to cleavage by two mammalian interstitial collagenases and 72-kDa type IV collagenase. J Biol Chem 265:13521–13527
Welgus HG, Jeffrey JJ, Eisen AZ (1981) The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem 256:9511–9515
Salmela MT, MacDonald TT, Black D, et al. (2002) Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut 51:540–547
von Lampe B, Barthel B, Coupland SE, et al. (2000) Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 47:63–73
Brew K, Dinakarpandian D, Nagase H (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477:267–283
Gomez DE, Alonso DF, Yoshiji H, et al. (1997) Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 74:111–122
Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT (1997) A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol 158:1582–1590
Bailey CJ, Hembry RM, Alexander A, et al. (1994) Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. J Clin Pathol 47:113–116
Kontakou M, Przemioslo RT, Sturgess RP, et al. (1995) Cytokine mRNA expression in the mucosa of treated coeliac patients after wheat peptide challenge. Gut 37:52–57
Pender SL, MacDonald TT (1998) Regulation of matrix metalloproteinase production in human fetal intestinal mesenchymal cells by cytokines and the bacterial superantigen Staphylococcus aureus enterotoxin B. Ann NY Acad Sci 859:188–191
Heuschkel RB, MacDonald TT, Monteleone G, et al. (2000) Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut 47:57–62
Yoshihara Y, Nakamura H, Obata K, et al. (2000) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 59:455–461
Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik B, Skowronski J, Chwiecko J (2000) Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in different histological variants of rheumatoid synovitis. Rheumatology (Oxford) 41:78–87
Zhang S, Li L, Lin JY, Lin H (2003) Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol 9:899–904
Murray GI, Duncan ME, Arbuckle E, et al. (1998) Matrix metalloproteinases and their inhibitors in gastric cancer. Gut 43:791–797
Stallmach A, Chan CC, Ecker KW, et al. (2000) Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut 47:415–422
Louis E, Ribbens C, Godon A, et al. (2000) Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin Exp Immunol 120:241–246
Wiercinska-Drapalo A, Jaroszewicz J, Flisiak R, Prokopowicz D (2003) Plasma matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 as biomarkers of ulcerative colitis activity. World J Gastroenterol 9:2843–2845
Bamba S, Andoh A, Yasui H, Araki Y, Bamba T, Fujiyama Y (2003) Matrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17. J Gastroenterol 38:548–554
Acknowledgments
The authors would like to thank Dr. J. Kearney for advice on statistical analysis and Dr. J. Jackson and Ms. Caroline Liddy for general support. We would also like to acknowledge the Department of Histopathology, St. James’s Hospital, Dublin, Ireland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamed, B.M., Feighery, C., Kelly, J. et al. Increased Protein Expression of Matrix Metalloproteinases -1, -3, and -9 and TIMP-1 in Patients with Gluten-Sensitive Enteropathy. Dig Dis Sci 51, 1862–1868 (2006). https://doi.org/10.1007/s10620-005-9038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-9038-4