Abstract
CagA protein of Helicobacter pylori is injected into epithelial cells, and it undergoes tyrosine phosphorylation, resulting in inducing cytoskeletal rearrangements. A few studies have suggested that the number of CagA tyrosine phosphorylation motifs (EPIYA) and subtypes of CagA were associated with gastric cancer. This study was performed to characterize the 3' variable regions of the cagA gene of H. pylori and to investigate whether or not there is any relationship between the diversities of cagA and the disease outcome in Korea. Seventy-nine patients (chronic gastritis, 15; duodenal ulcer, 27; benign gastric ulcer, 18; gastric cancer, 19) were enrolled. Biopsy specimens were taken from the antrum for H. pylori culture, and genomic DNA was extracted. PCR and DNA sequence analysis was carried out for the 3′ variable region of the cagA gene. Seventy-eight strains (98.8%) contained three EPIYA motifs and one strain (1.2%) isolated from a patient with duodenal ulcer contained four EPIYA motifs. Seventy-six strains (96.2%) were the East Asian type. In conclusion, there was no significant difference between the number of EPIYA motifs or CagA subtypes and various gastroduodenal diseases in Korea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Helicobacter pylori, which infects about 50% of the world population, is associated with the development of gastroduodenal disease such as peptic ulcer disease, mucosa-associated lymphoid tissue (MALT) lymphoma, and distal gastric adenocarcinoma [1, 2]. Epidemiologic studies have suggested that chronic infection of H. pylori is an important risk factor for the development of gastric carcinoma [2, 3]. However, molecular mechanisms by which H. pylori triggers the processes leading to gastric carcinoma remain largely unknown. The fact that only 1%–5% of H. pylori-infected persons develop gastric cancer suggests that virulence factors of H. pylori, the genetic factor of the host, and environmental factors may play important roles in the pathogenesis of gastric cancer.
CagA (cytotoxin-associated gene A) is a part of the cag pathogenic island (PAI), and codes for CagA, a 120- to 140-kDa molecular weight immunodominant antigen [4]. More than 90% of isolated strains from East Asia including Korea, Japan, and China are known to be positive for cagA, whereas 50%–60% of isolated strains from Western countries have cagA [5]. H. pylori strains possessing the cagA gene have been associated with an increased risk of gastric cancer and peptic ulcer [6, 7]. However, some studies fail to show any significant association between cagA status and clinical outcome, especially in studies from East Asia [5, 8–10].
After attachment of cagA + H. pylori to gastric epithelial cells, CagA is directly injected from the bacteria into the cells via the bacterial type IV secretary system and undergoes tyrosine phosphorylation by the Src family of tyrosine kinases [11, 12]. Phosphorylated CagA binds to a cytoplasmic SHP-2 (Src homology 2 domain-containing tyrosine phosphatase), stimulates the phosphatase activity of SHP-2, and induces a cytoskeletal rearrangement known as the hummingbird phenotype. SHP-2 is known to play an important role in mitogenic signal transduction and regulation of migration and adhesion of cells. Derangement of SHP-2 by CagA may induce abnormal proliferation and movement of gastric epithelial cells [11, 13, 14].
The size of the cagA gene and its protein varies in different strains. The structure of the gene reveals a 5′ highly conserved region and the variation in the size of the protein has been correlated with the varying number of repeat sequences located in the 3′ region of the gene [15]. The target of the tyrosine phosphorylation of injected CagA is the EPIYA motif, which consists of a five-amino acid sequence (Glu-Pro-Ile-Tyr-Ala) in the carboxyl-terminal variable region of the protein [16]. Some studies have suggested that the number of EPIYA motifs was associated with different clinical outcomes. H. pylori strains with more EPIYA motifs induce higher levels of CagA phosphorylation and more cytoskeletal rearrangements, which are associated with atrophic gastritis and gastric cancer [17, 18]. Besides the number of EPIYA motifs, subtypes of CagA protein (East Asian type and Western type) may be associated with different disease outcomes. Azuma et al. [19] suggested that infection with East Asian CagA-positive H. pylori from the Japanese is associated with atrophic gastritis and gastric cancer. In contrast, Chinese studies showed no difference between the diversities of CagA protein in patients with different diseases [20, 21]. However, to date there have been no reports about this issue in Korea. The incidence of and death rate from gastric cancer in Korea are high, and the infection rate of H. pylori is also high, compared with those in other developed countries. This study was performed to characterize the 3' variable region of the cagA gene of H. pylori and, furthermore, to investigate whether or not there is any relationship between diversities of cagA and disease outcomes in Korea.
Methods
H. pylori strains
The cagA + H. pylori strains used in this study were isolated from human gastric biopsy samples obtained from 79 Koreans at Seoul National University Hospital, Seoul, and Seoul National University Bundang Hospital, Sungnam-si, Gyeonggi-do, which is located near Seoul, between January 1993 and October 2004. They were 45 males and 34 females, with their ages ranging between 23 and 80 years (mean age, 53.5 years) (Table 1). The diagnoses of the 79 patients were as follows: 15 chronic gastritis, 27 duodenal ulcer, 18 benign gastric ulcer, and 19 gastric cancer. Informed consent was obtained from all the patients, according to the World Medical Association Helsinki Declaration. For comparison with Korean strains, Western standard strains ATCC 43054 and G27 were used.
Isolation and culture of H. pylori
For H. pylori isolation, biopsy specimens were taken from the antrum at the time of endoscopy, an area of endoscopically intact mucosa distant from any focal lesions such as erosions. Gastric biopsy specimens from each patient were inoculated onto H. pylori isolation medium (GC medium base, 0.024% yeast extract, 1% hemoglobin, 1% isoVitalex, 5 mg/L vancomycin, 1 mg/L mycostatin, 5% sheep blood) and were cultured under microaerophilic conditions (5% O2, 10% CO2, 85% N2). After initial isolation, a single colony from each biopsy specimen was subcultured, and the isolate was identified as H. pylori on the basis of colony morphology, Gram's staining, and urease reaction. The strains were preserved at –70°C in Brucella broth (Difco Laboratories, Detroit, MI, USA) supplemented with 10% fetal bovine serum and 15% glycerol, then thawed and subcultured for use in subsequent experiments [5].
H. pylori DNA extraction and PCR analysis
The bacteria were harvested and suspended in 600 μL TE buffer (10 mM Tris–HCl, pH 8.0, 1 mM EDTA). DNA from H. pylori was extracted by the protease/phenol–chloroform method and stored at –70°C until PCR amplification [22]. Bacterial genomic DNA was amplified in 50 μL of 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and a 200 mM concentration each of deoxyadenosine 5′-triphosphate (dATP), deoxycytidine 5′-triphosphate (dCTP), deoxyguanosine 5′-triphosphate (dGTP), and deoxythymidine 5′-triphosphate (dTTP), and in the presence of 500 pmol each of 5′ and 3′ primer [5]. The primers used to amplify the 3′ region of the cagA gene were 5′-ACCCTAGTCGGTAATGGGTTA-3′ for sense and 5′-GTAATTGTCTAGTTTCGC-3′ for antisense [18]. PCR was performed for 35 cycles, each cycle consisting of 1 min at 95°C, 1 min at 50°C, and 1 min at 72°C. The final cycle included a 7-min extension step to ensure full extension of the PCR product [18]. After confirming the existence of the cagA gene in the 2% agarose gel electrophoresis containing ethidium bromide, the PCR products were purified using a PCR purification kit (Quiagen, Hilden, Germany).
Analysis of the 3' region of the cagA gene by PCR. Strains S5, S179, and S378 show sizes ranging from 640 to 670 bp. In contrast, strain B78 appears to be approximately 720 bp, and H. pylori strain G27, ATCC 43504, isolated from Western patients, about 820 bp. Strains S5, S179, S378, and B78 were isolated from patients with chronic gastritis, gastric cancer, benign gastric ulcer, and duodenal ulcer, respectively. NC, negative control; M, molecular size marker
DNA sequence analysis
DNA sequencing was performed using an ABI PRISM 377 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The primers used for sequencing analysis were 5′-ACCCTAGTCGGTAATGGGTTA-3′ for sense and 5′-GTAATTGTCTAGTTTCGC-3′ for antisense [18].
Statistics
Fisher's exact test was employed for analysis of categorical data. The result was considered to be significant at a P value of <0.05.
Results
Characteristics of the 3′ variable region of cagA and relation to disease outcome
The size of PCR products of all strains ranged from 640 to 670 bp, except for one strain (strain B78) with a size of about 720 bp (Fig. 1). DNA sequence analysis revealed that 78 of 79 strains (98.8%) had three EPIYA motifs, and 1 strain (1.2%; strain B78) from a 64 year-old man with duodenal ulcer had four EPIYA motifs. As defined by Higashi et al. [16], we designated the first and second EPIYA motifs “EPIYA-A” and “EPIYA-B,” respectively, and the remaining EPIYA motifs were designated “EPIYA-C,” which was placed in a 34-amino acid sequence known as the Western CagA specific SHP-2 binding sequence (WSS). The distinct amino acid sequence of CagA from H. pylori isolated in East Asian countries was designated East Asian CagA specific SHP-2 binding sequence (ESS) in the corresponding region. ESS contains an EPIYA motif, which was designated “EPIYA-D” (Fig. 2) [16].
Amino acid sequence characteristics in the 3′ region of the cagA gene. S5 and S179, isolated from patients with chronic gastritis and gastric cancer, respectively, show cagA with three EPIYAs and the “A-B-D” pattern. In contrast, S378, isolated from a patient with benign gastric ulcer, shows cagA with three EPIYAs and the “A-B-C” pattern. B78, isolated from a patient with duodenal ulcer, shows cagA with four EPIYAs and the “A-B-C-C” pattern, similar to ATTC 43504, a Western standard strain. ESS, East Asian CagA specific SHP-2 binding sequence; WSS, Western CagA specific SHP-2 binding sequence
Among our 79 strains, 1 (strain B78; isolated from a duodenal ulcer patient) was “A-B-C-C” type, with four EPIYA motifs, and another strain (strain S378; isolated from a benign gastric ulcer patient) was “A-B-C” type. So these two strains (2.5%) had a Western-type CagA. Seventy-six strains (96.2%) were “A-B-D” type with East Asian-type CagA. One strain (strain B200; isolated from a gastric cancer patient), which was neither ESS nor WSS, was classified as “A-B-B” type (Table 2). The number of EPIYA motifs, whether East Asian-type or Western-type CagA, were not associated with different disease outcomes (P = 0.83).
To determine whether or not there is any sequence difference other than EPIYA motif among strains isolated from different clinical outcomes, we analyzed multiple sequence alignments of the 3' variable region of cagA sequences of 79 strains according to disease outcome using strain F32, which was isolated from a Japanese cancer patient, as a standard (Fig. 3). Strain S380, isolated from a duodenal ulcer patient, had an ESS sequence by definition. However, it had peculiar intervening amino acid sequences (Q-I[A]-A-S-G-L[F]-G- D[G/N]-V-G-Q-A-T[A]-G-F-P-L-K-K[G/R]-H-D[T]-K-V-G[D]-D-L-S-K-V-G-L[R]) between the second and the third EPIYA, similarly to two other Western-type strains (B78, S378), suggesting that strain S380 is an intermediate type. Otherwise there was no significant sequence difference found in this analysis among strains isolated from different outcomes.
Discussion
As H. pylori causes different gastroduodenal diseases among infected individuals, differences in virulence factors of H. pylori strains are regarded as important in causing various diseases. The CagA protein is one of the most studied virulence factors of H. pylori. Recent studies have revealed the mechanisms by which CagA is directly injected into host cells. The injected CagA, after tyrosine phosphorylation, induces cytoskeletal rearrangement and may causes derangement in mitogenic signal transduction. Therefore, CagA protein attracts attention as an important factor in relation to atrophic gastritis and gastric cancer [6, 13, 14]. Furthermore, the variable region of CagA in the C-terminal possesses a varying number of tyrosine phophorylation sites, and CagA from H. pylori isolated in East Asia, where the incidence of gastric cancer is high, has a distinct amino acid sequence. Therefore, the association between the diversity of CagA and the outcome of different diseases has been studied in several countries. Azuma et al. [23] reported that one-third of strains with more than four EPIYA motifs were gastric cancer-causing strains, and strains with more than four EPIYA motifs were more frequently isolated in atrophic gastritis patients than duodenal ulcer patients. CagA proteins with more EPIYA motifs showed increased tyrosine phosphorylation and increased SHP-2 binding as well, and induced greater morphological change [16, 17]. In addition to the number of EPIYA motifs, East Asian-type CagA led to stronger SHP-2 binding and morphologically transforming activities than Western-type CagA [16]. The CagA/SHP-2 interaction requires the SH2 domains of SHP-2. De Souza et al. [24] reported that the two SH2 domains from SHP-2 bind to highly related sequences, and the consensus ligand-binding motif for the N- and C-terminal SH2 domains of SHP-2 is pY-(S/T/A/V/I)-X-(V/I/L)-X-(W/F), which perfectly matches the SHP-2 binding sites of East Asian CagA, pY-A-T-I-D-F [25]. Furthermore, Azuma et al. [19] reported that almost all strains isolated in the Fukui area, where the incidence of gastric cancer is high, were the East Asian type, but 16% of Okinawa strains, where the incidence of gastric cancer is low, were the Western type. This suggested that infection with East Asian CagA-positive H. pylori is associated with atrophic gastritis and gastric cancer.
However, in our study, almost all strains from different diseases had three EPIYA motifs and there was no association between the number of EPIYA motifs and the disease outcomes. One strain with four EPIYA motifs was isolated from a duodenal ulcer patient. These findings correspond to the Chinese study which showed no differences in the number of EPIYA motifs in H. pylori from patients with different diseases [20]. One Japanese study reported that among 30 strains isolated from gastric cancer patients, 24 strains (80%) had three EPIYA motifs, 6 strains (20%) had four EPIYA motifs, and no strains had more than five EPIYA motifs. Therefore there seemed to be no definite positive correlation between the number of EPIYA motifs and gastric cancer development [18], and our data also support this. Argent et al. [17] reported that among 22 strains from South African patients, 6 strains (27%) had more than three EPIYA motifs, and 5 of 6 strains with more than three EIPYA motifs were isolated from gastric cancer patients. Therefore, the relationship between gastric cancer and the number of EPIYA motifs of H. pylori isolated in Koreans could be different from that for Western countries, and East Asian type, which is different from Western type, could be another determining factor for gastric cancer instead of the number of EPIYA motifs. However, in our study, only two strains isolated from peptic ulcer patients had the Western-type CagA, and all strains from gastric cancer patients had the East Asian-type CagA, without statistical significance.
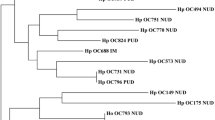
To clarify the phylogenetic relationships among Korean, Japanese, and Western strains, the nucleotide sequences of the 3′ variable region of cagA were aligned and a phylogenetic tree was constructed. The tree comprised the 3′ variable region of cagA among 79 strains from Korea, 4 Japanese strains (F32 from a patient with gastric cancer; F28, OK112, and OK129 from patients with gastritis), and 2 Western strains (26695 from a patient with gastritis, J99 from a patient with duodenal ulcer). Most Korean strains that have East Asian-type CagA were included in the same cluster as the Japanese strains, and Korean strains that have Western-type CagA were included in the same cluster as Western strains and one Japanese strain which possesses Western-type CagA (OK112). However, there was no significant cluster according to the disease outcome.
These findings could suggest that other factors such as a genetic factor of the host, environmental factors, and another virulence factor of H. pylori, not yet identified, could be more important in gastric cancer development than CagA subtype in the region where the majority of strains had East Asian-type CagA.
In conclusion, the majority of H. pylori isolated in Korean patients had three EPIYA motifs and the East Asian-type CagA, with no significant correlation between the number of EPIYA motifs or CagA subtypes and different gastroduodenal diseases. Thus the number of EPIYA motifs and CagA subtype cannot be used as a marker to discern the risk of developing different gastroduodenal diseases in the host.
References
Peek RM Jr, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Natl Rev Cancer 2:28–37
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345:784–789
Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK (2004) Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291:187–194
Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A (1996) cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA 93:14648–14653
Kim JM, Kim JS, Jung HC, Song IS, Kim CY (2000) Virulence factors of Helicobacter pylori in Korean isolates do not influence proinflammatory cytokine gene expression and apoptosis in human gastric epithelial cells, nor do these factors influence the clinical outcome. J Gastroenterol 35:898–906
Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A (1995) Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 55:2111–2115
Figueiredo C, Van Doorn LJ, Nogueira C, Soares JM, Pinho C, Figueira P, Quint WG, Carneiro F (2001) Helicobacter pylori genotypes are associated with clinical outcome in Portuguese patients and show a high prevalence of infections with multiple strains. Scand J Gastroenterol 36:128–135
Jenks PJ, Megraud F, Labigne A (1998) Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut 43:752–758
Maeda S, Ogura K, Yoshida H, Kanai F, Ikenoue T, Kato N, Shiratori Y, Omata M (1998) Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut 42:338–343
Park SM, Park J, Kim JG, Cho HD, Cho JH, Lee DH, Cha YJ (1998) Infection with Helicobacter pylori expressing the cagA gene is not associated with an increased risk of developing peptic ulcer diseases in Korean patients. Scand J Gastroenterol 33:923–927
Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M, Suzuki T, Sasakawa C (2000) Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med 191:593–602
Selbach M, Moese S, Hauck CR, Meyer TF, Backert S (2002) Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J Biol Chem 277:6775–6778
Backert S, Moese S, Selbach M, Brinkmann V, Meyer TF (2001) Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol Microbiol 42:631–644
Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M (2002) SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683–686
Covacci A, Censini S, Bugnoli M, et al. (1993) Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA 90:5791–5795
Higashi H, Tsutsumi R, Fujita A, Yamazaki S, Asaka M, Azuma T, Hatakeyama M (2002) Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA 99:14428–14433
Argent RH, Kidd M, Owen RJ, Thomas RJ, Limb MC, Atherton JC (2004) Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori. Gastroenterology 127:514–523
Yamaoka Y, Kodama T, Kashima K, Graham DY, Sepulveda AR (1998) Variants of the 3' region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol 36:2258–2263
Azuma T, Yamazaki S, Yamakawa A, Ohtani M, Muramatsu A, Suto H, Ito Y, Dojo M, Yamazaki Y, Kuriyama M, Keida Y, Higashi H, Hatakeyama M (2004) Association between diversity in the Src homology 2 domain-containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J Infect Dis 189:820–827
Zhu YL, Zheng S, Du Q, Qian KD, Fang PC (2005) Characterization of CagA variable region of Helicobacter pylori isolates from Chinese patients. World J Gastroenterol 11:880–884
Lai YP, Yang JC, Lin TZ, Wang JT, Lin JT (2003) CagA tyrosine phosphorylation in gastric epithelial cells caused by Helicobacter pylori in patients with gastric adenocarcinoma. Helicobacter 8:235–243
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Azuma T, Yamakawa A, Yamazaki S, Fukuta K, Ohtani M, Ito Y, Dojo M, Yamazaki Y, Kuriyama M (2002) Correlation between variation of the 3' region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J Infect Dis 186:1621–1630
De Souza D, Fabri LJ, Nash A, Hilton DJ, Nicola NA, Baca M: SH2 domains from suppressor of cytokine signaling-3 and protein tyrosine phosphatase SHP-2 have similar binding specificities. Biochemistry 41:9229–9236
Azuma T (2004) Helicobacter pylori CagA protein variation associated with gastric cancer in Asia. J Gastroenterol 39:97–103
Acknowledgments
This work was supported by Grant 02-03-001 from the SNUBH Research fund. We appreciate the skillful assistance of Mi Soon Kim.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, K.D., Kim, N., Lee, D.H. et al. Analysis of the 3′ Variable Region of the cagA Gene of Helicobacter pylori Isolated in Koreans. Dig Dis Sci 52, 960–966 (2007). https://doi.org/10.1007/s10620-005-9030-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-9030-z