Abstract
Sambucus nigra L. (Elderberry) is widely used as a dietary supplement in functional food and possesses many pharmacological activities to prevent ailments, such as the colds and fever, diabetes and cancer. However, research on its skin anti-aging effect is still limited. Here, we evaluated the recovery effects of elderberry extract (EB) in UVB-irradiated human skin keratinocytes (HaCaTs) and investigated whether EB represents a potential therapeutic agent against skin photoaging and inflammation. In this study, EB showed good efficiency on scavenging free radicals and dose-dependently reduced reactive oxygen species (ROS) generation. EB notably decreased UVB-induced matrix metalloproteinase-1 (MMP-1) expression and inflammatory cytokine secretion through the inhibition of mitogen-activated protein kinases/activator protein 1 (MAPK/AP-1) and nuclear factor-κB (NF-κB) signaling pathways, blocking extracellular matrix (ECM) degradation and inflammation in UVB-irradiated HaCaTs. In addition, EB improved nuclear factor E2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling to increase oxidative defense capacity, and enhanced transforming growth factor beta (TGF-β) signaling activation to promote procollagen type I synthesis, relieving UVB-induced skin cell damage. These results indicated that EB has the potential to ameliorate UVB-induced skin photoaging and inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the largest organ in the human body, skin is the primary barrier against external damage. Extensive epidemiological studies have revealed that ultraviolet (UV) exposure is one of main extrinsic factors in skin damage, leading to dryness, pigmentation, epidermal thickening and wrinkling (Katiyar 2007; Wlaschek et al. 2001). Chronic exposure to UVB disrupts the normal skin structure leading to connective tissue deterioration, DNA damage and immune suppression, contributing to the morbidity or promotion of photoaging (Svobodova et al. 2006).
UVB-induced excess reactive oxygen species (ROS) production destroys cellular oxygen balance and causes oxidative stress, accelerating the process of skin inflammation and photoaging (Dinkova-Kostova 2008; Masaki 2010). Nuclear factor erythroid 2 related factor 2 (Nrf2) is a key component of endogenous antioxidant defense systems that regulates mitochondrial ROS production (Greenwald et al. 2017; Wakabayashi et al. 2010). In order to protect skin cells from oxidative stress, Nrf2 translocates from the cytoplasm to the nucleus, combines with the antioxidant response element (ARE) and activates heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase-1 (NQO1) expression (Saw et al. 2011). Recent study has indicated that HO-1 expression suppresses nuclear factor-κB (NF-κB) activation, leading to inhibited secretion of inflammatory cytokine (Kim et al. 2010). Thus, the Nrf2/ARE signaling activation is a promising approach for relieving UVB-induced oxidative stress and inflammatory responses (Kawachi et al. 2008; Sun et al. 2016).
Oxidative stress and inflammation have been reported to contribute to the progression of skin aging (Pillai et al. 2005). NF-κB complexes are typically retained in the cytoplasm binding with the inhibitory-κB (IκB) protein. Upon exposure to UVB irradiation, IκB is rapidly phosphorylated and degraded via the ubiquitin–proteasome pathway, permitting activation and nuclear import of NF-κB. UVB irradiation activates NF-κB-dependent inflammatory responses in HaCaTs and increases a series of inflammatory cytokine expression such as interleukin-6 (IL-6), causing itchy or painful sensations in skin (Muthusamy and Piva 2010). Meanwhile, NF-κB activation also stimulates the protein release of vascular endothelial growth factor (VEGF) to induce angiogenesis. The overexpression of VEGF increases sensitivity to UVB-induced skin inflammation and photodamage (Kim et al. 2006; Zhang and Ma 2014).
Excess intracellular ROS triggers activation of the mitogen-activated protein kinase (MAPK) signaling pathway through the phosphorylation of Jun-N-terminal kinase (JNK), extracellular-regulated protein kinase (ERK), and p38 kinase (Cargnello and Roux 2012; Luchetti et al. 2009). When the signals translocate to the nucleus, activator protein-1 (AP-1) activation is stimulated, resulting in upregulated expression of matrix metalloproteinases (MMPs), notably MMP-1, which is related to the degradation of collagen (Quan and Fisher 2015; Quan et al. 2009). Collagen, as the primary constituent of the extracellular matrix (ECM), plays a key role in skin strength and resilience (Boland et al. 2004). Transforming growth factor beta 1 (TGF-β1), a fundamental and functional cytokine, is involved in the regulation of many ECM-related genes and controls the synthesis of collagen and elastin (Klingberg et al. 2014; Wells and Discher 2008). Smad proteins, as major downstream targets of TGF-β receptor kinases, exert distinct and opposing functions (Meng et al. 2016). Current studies have reported that the increase in Smad2/3 phosphorylation or the decrease in Smad7 expression can promote collagen synthesis (Moon et al. 2018; Park et al. 2017). Therefore, the decrease in MMP-1 expression, as well as the activation of TGF/Smad signaling pathway, contribute to the prevention of UVB-induced photoaging.
Sambucus nigra L. (Elderberry) belongs to the Adoxaceae family and grows in Asia, Europe, North America, and North Africa. Its fresh fruits are widely used as a natural dietary supplements (Porter and Bode 2017; Veberic et al. 2009). In European folk medicine, elderberry has been used for thousands of years to prevent ailments such as colds and arthritis, and to relieve pain and fever (Sidor and Gramza-Michałowska 2015). Previous studies have demonstrated that elderberry contains large amounts of anthocyanins and polyphenols, and possesses anti-inflammatory, antioxidant, antiviral and anti-diabetes properties (David et al. 2014; Duymuş et al. 2014; Harokopakis et al. 2006; Porter and Bode 2017). Nevertheless, there are few comprehensive studies detailing the effects of elderberry on ameliorating UVB-induced photoaging. In this study, we evaluated the effects of a 70% ethanol extract of elderberry fruits (EB) on UVB-induced keratinocytes (HaCaTs) and investigated whether the extraction is a promising natural product in photoaging therapy.
Materials and methods
Elderberry extract preparation
Sambucus nigra L. (Elderberry) fruits were purchased from Mountain Rose Herbs Company (Eugene, OR, USA). Dried fruits were extracted in 500 mL of 70% ethanol for 3 days at 37 °C in a thermostatic chamber, filtered and concentrated at 40 °C. The extract (EB) was lyophilized and stored at − 20 °C.
Total phenolic content determination
Total phenolics were estimated by optimized for 96-plate version of colorimetric reaction with Folin–Ciocalteu reagent (Sigma, F9252 acidity 2N) (Blainski et al. 2013). Calibration curve was obtained using gallic acid (> 97.5% G7384, Sigma-Aldrich) as a standard. 50 μL of serial dilutions of standard were mixed with 50 μL of 1N Folin–Ciocalteu reagent. After 15 min, 150 μL of 0.7 M Na2CO3/NaOH was added to each well. After 1 h incubation, the optical density was measured using VersaMax™ at 765 nm. Blank wells contained 50 μL of 50% ethanol. Samples were dissolved in 50% ethanol to prepare a final concentration of 5 mg/mL. Colorimetric reactions of the sample were carried out in the same manner. Total phenolic content was calculated using a standard calibration of gallic acid solution and expressed as mg of gallic acid equivalent per g of samples. The experiment was repeated six times, independently.
DPPH radical scavenging activity
2,2-diphenyl-1-picrylhydrazyl (DPPH) was applied to evaluate the antioxidant activity of EB, with arbutin as the positive control. The sample was tested in serial concentrations from 1 to 250 μg/mL. After the sample was incubated with DPPH for 30 min at 37 °C in the dark, the optical density was measured at 520 nm. The experiment was repeated six times, independently.
ABTS radical cation decolorization assay
The ability of EB to scavenge ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) radical cations was compared to that of arbutin. The absorbance of the ABTS+ solution was equilibrated to 0.650 ± 0.02 with PBS at room temperature. 1 mL of ABTS solution was mixed with 10 μL of samples at 37 °C for 10 min, and the absorbance was measured at 734 nm. The experiment was repeated six times, independently.
Cell culture, UVB irradiation and sample treatment
HaCaTs, originating from human keratinocytes, were purchased from the Korea cell line bank (Seoul, Korea). Cells were cultured in DMEM supplemented with 10% heat-inactivated FBS and 1% penicillin–streptomycin (Gibco BRL, Grand Island, NY, USA). Cell cultures were maintained in a humidified incubator with 5% CO2 at 37 °C. The UVB source (Bio-Link BLX-312, Vilber Lourmat, Gmbh, France) had a spectral emission at 312 nm. Cells were subjected to a UVB dose of 125 mJ/cm2, and UVB irradiation lasted 59 s.
Cell viability assay
The MTT assay was used to detect cell viability. HaCaTs in the logarithmic phase were counted and seeded in 96-well plate. After UVB (125 mJ/cm2) radiation and EB (1, 10, and 100 μg/mL) treatment for 24 h, 0.1 mg/mL of MTT was added and incubated for 4 h. DMSO was used to dissolve the formazan crystals. The absorbance was read at 570 nm using a micro plate reader (Molecular Devices E09090; San Francisco, CA, USA). The experiment was repeated six times, independently.
Measurement of ROS generation
ROS production by UVB-induced HaCaT cells exposed to EB was evaluated by the 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Sigma-Aldrich) assay, as previously described (Sun et al. 2018). Following UVB (125 mJ/cm2) irradiation and EB (1, 10, and 100 μg/mL) treatment for 24 h, HaCaTs were stained with 30 μM DCFH-DA for 30 min at 37 °C in the dark and analyzed by a multi-mode microplate reader (Molecular Devices Filter Max F5; Sunnyvale, CA, USA) at excitation wavelength of 485 nm and detection wavelength of 535 nm. The experiment was repeated three times, independently.
Measurement of MMP-1, IL-6 and VEGF production
HaCaTs in the logarithmic phase were counted and seeded in a 6-well plate following UVB (125 mJ/cm2) irradiation and EB (1, 10, and 100 μg/mL) treatment for 24 h, and the supernatants were collected from each well. MMP-1, IL-6 and VEGF secretions were analyzed by ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA). The experiment was repeated three times, independently.
Reverse transcription-polymerase chain reaction (RT-PCR)
Following UVB (125 mJ/cm2) irradiation and EB (1, 10, and 100 μg/mL) treatment for 24 h, RNA isolation was performed using the TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, CA). PCR amplification was performed by PCR premix (Bioneer CO., Korea). RT-PCR was performed in a Veriti Thermal Cycler (Applied Biosystems, Foster City, CA, USA), and following primer pairs: MMP-1, forward 5′-ATT CTA CTG ATA TCG GGG CTT TGA-3′, reverse 5′-ATG TCC TTG GGG TAT CCG TGT AG-3′; Procollagen type I, forward 5′-CTC GAG GTG GAC ACC ACC CT-3′, reverse 5′-CAG CTG GAT GGC CAC ATC GG-3′; and human β-actin, forward 5′-ATC CAG GCT GTG TTG TCC CTG-3′, reverse 5′-AGG AGC CAG GGC AGT AAT CTC-3′). The experiment was repeated three times, independently.
Western blot analysis
After UVB (125 mJ/cm2) irradiation and EB (1, 10, and 100 μg/mL) treatment, alterations in the MAPK, AP-1, NF-κB, TGF-β, and Nrf2 signaling pathways were investigated by Western blot analysis. Proteins were extracted and analyzed according to the methods described by Hwang et al. (Hwang et al. 2014). The nucleoprotein was separated by a commercial kit (NE-PER nuclear and cytoplasmic extraction reagents; Pierce). Cell lysates were measured and normalized by Bradford reagent (Bio-Rad, Hercules, CA, USA). Proteins were separated on 10% or 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to Immun-Blot® PVDF membrane (Bio-Rad Laboratories, Inc., USA). The antibodies ERK, phospho-ERK, JNK, phospho-JNK, p38, phospho-p38, Nrf2, HO-1, NQO-1, NF-κB, IκBα, phospho-IκBα, Histone, anti-rabbit-HRP, anti-mouse-HRP and anti-goat-HRP were purchased from Cell Signaling Technology (Danvers, USA), and c-fos, phospho-c-fos, c-jun, phospho-c-jun, TGF-β, Smad7, phospho-Smad2/3 and β-actin were purchased from Santa Cruz Biotechnology (Dallas, USA), respectively. The experiment was repeated three times, independently.
Statistical analysis
Value was expressed as mean ± standard deviation (SD) by the GraphPad Prism 5 (GraphPad Software, Inc., CA, USA). Statistical significance among groups was determined by one-way ANOVA analysis followed by Bonferroni’s multiple comparison test. P < 0.05 was considered statistically significant. #P < 0.05, ##P < 0.01, ###P < 0.001 were considered statistically significant compared with the basal cells; *P < 0.05, **P < 0.01, ***P < 0.001 were considered statistically significant compared with only UVB-irradiated cells.
Results
Total phenolic content of EB
Total phenolic content of EB was measured by Folin–Ciocalteu reagent. Total phenolic content was calculated using a standard calibration of gallic acid solution. The result indicated that elderberry contained 4.99% phenolic constituents.
DPPH, ABTS, and ROS scavenging activity
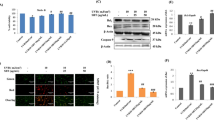
In this study, DPPH and ABTS radical scavenging activity assays were used to evaluate the antioxidative capacity of EB, with arbutin used as a positive control. As shown in Fig. 1a, b, the free-radical-scavenging activity of EB increased in a dose-dependent manner, and the half maximal inhibitory concentration (IC50) value of EB on DPPH and ABTS scavenging were 59.0 µg/mL and 65.9 µg/mL, respectively. At 250 µg/mL, the scavenging activity of EB was better than that of arbutin.
Free-radical-scavenging capacity analysis and intracellular ROS production in EB-treated HaCaTs. a DPPH assay; b ABTS assay; Arbutin was used as the positive control. c Effect of EB on ROS production; following UVB irradiation (125 mJ/cm2), HaCaTs were treated with or without the indicated concentration of EB (1, 10, and 100 µg/mL) for 24 h. Relative ROS generation is shown in each histogram. ###P < 0.001, compared to the Basal; **P < 0.01, ***P < 0.001 compared to the UVB irradiated cells
To evaluate the inhibitory effect of EB on intracellular ROS production, HaCaTs were exposed to UVB irradiation (125 mJ/cm2) and then treated with EB for 24 h. Compared to basal cells, the generation of ROS was significantly increased by UVB radiation in HaCaTs (Fig. 1c), while treatment with EB dose-dependently attenuated UVB-induced ROS production by 19.4%, 29.3% and 40.4%, respectively.
Cell viability and MMP-1, IL-6 and VEGF secretion in EB-treated HaCaTs
Following UVB irradiation (125 mJ/cm2), HaCaTs were treated with EB (1, 10, and 100 μg/mL) for 24 h, and the cytotoxicity of EB was determined by the MTT assay. Compared to the basal cells (Fig. 2a), UVB-irradiated cells showed a slight decrease in cell viability. However, after EB treatment, cell proliferation was improved in UVB-irradiated HaCaTs.
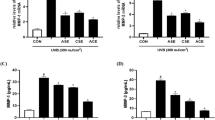
Effects of EB on cell viability and MMP-1, IL-6 and VEGF secretion in UVB-irradiated HaCaTs. Following UVB irradiation (125 mJ/cm2), HaCaTs were treated with or without the indicated concentration of EB (1, 10, and 100 µg/mL) for 24 h. Cell viability was measured by MTT assay (a). MMP-1 secretion (b); IL-6 secretion (c); VEGF secretion (d) in UVB-irradiated HaCaTs were measured by ELISA kits. The results were shown as the mean ± SD of three independent experiments. #P < 0.05, ###P < 0.001, compared to the Basal; *P < 0.05, **P < 0.01 compared to the UVB irradiated cells
The secretion of MMP-1, IL-6 and VEGF was measured by Elisa kits. As shown in Fig. 3b–d, UVB irradiation led to a significant increase of MMP-1, IL-6 and VEGF secretion by 179.7%, 62.4% and 62.8%, respectively. However, the presence of EB significantly reversed these trends. Compared with UVB-irradiated cells, EB inhibited MMP-1 secretion by 37.8%, 40.1% and 48.0%. And EB downregulated the levels of IL-6 and VEGF in a dose-dependent manner, decreasing the release of IL-6 and VEGF by 40.4% and 35.5% at 100 μg/mL, respectively.
Effects of EB on the levels of MMP-1 and procollagen type I mRNA in HaCaTs. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with EB (1, 10 and 100 µg/mL) for 24 h. MMP-1 and procollagen type I mRNA levels were determined by RT-PCR (a). The band intensities were quantified by densitometry and normalized to the basal group (b, c). The results were shown as the mean ± SD of three independent experiments. ##P < 0.01, compared to the Basal; *P < 0.05, **P < 0.01, compared to the UVB irradiated cells
Effects of EB on MMP-1 and procollagen type I mRNA expression
In order to investigate whether EB regulates MMP-1 and procollagen type I in HaCaTs, we detected mRNA levels using RT-PCR. As shown in Fig. 3, UVB radiation upregulated the mRNA level of MMP-1 by 88.6% and reduced procollagen type I mRNA expression by 56.4%. Compared to UVB-irradiated cells, EB effectively decreased the expression of MMP-1 by 28.6% at 100 µg/mL (P < 0.05). The decreased procollagen type I mRNA level was enhanced by EB in a dose-dependent manner, EB increased the expression of procollagen type I by 162.7% at 100 µg/mL (P < 0.01).
Effects of EB on UVB-induced AP-1 signaling pathway
The activation of AP-1 signaling pathway is related to the upregulation of MMP-1 expression (Piao et al. 2012; Quan et al. 2009). In order to evaluate the regulatory mechanism of EB, the phosphorylation of c-Fos and c-Jun were measured by Western blot. HaCaTs were exposed to UVB (125 mJ/cm2) radiation and treated with EB (1, 10, and 100 μg/mL) for 4 h, and the results showed that UVB radiation induced the phosphorylation of c-Fos and c-Jun, increasing p–c–Fos and p–c–Jun expression by 65.8% and 72.3%, respectively. However, these trends were inhibited by the presence of EB (Fig. 4b, c). Compared to the UVB-irradiated cells, EB visibly decreased the expression of p–c–Fos by 49.4% and inhibited the increase of p–c–Jun by 58.8% at 100 µg/mL.
Effects of EB on UVB-induced AP-1 signaling activation. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with EB (1, 10, and 100 µg/mL) for 4 h. Phosphorylated and non-phosphorylated forms of c-Fos and c-Jun were detected by Western blot (a). The band intensities were quantified by densitometry, normalized to the level of β-actin, and calculated as the percentage of the basal response (b, c). The results were shown as the mean ± SD of three independent experiments. ##P < 0.01 compared to the Basal; *P < 0.05, ***P < 0.001, compared to the UVB irradiated cells
Effects of EB on UVB-induced MAPK signaling pathway
The activation of AP-1 is regulated by the MAPK signaling pathway. We measured the expression of the phosphorylated forms of the MAPKs family, including p-JNK, p-p38 and p-ERK, in order to further investigate the effects of EB on UVB-induced photoaging. As shown in Fig. 5, UVB irradiation significantly increased the expression of p-JNK, p-p38 and p-ERK in HaCaTs, whereas EB diminished the activation of MAPK signaling pathway. Compared with UVB-induced HaCaTs, EB dose-dependently decreased the phosphorylation of JNK and p38 expression. At 100 μg/mL, EB reduced the expression of p-JNK and p-p38 by 47.4% and 63.4% (Fig. 5c, d).
Effects of EB on UVB-induced MAPK signaling activation. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with EB (1, 10, and 100 µg/mL) for 0.5 h. Phosphorylated and non-phosphorylated forms of ERK, JNK and P38 were detected by Western blot (a). The band intensities were quantified by densitometry, normalized to the level of β-actin, and calculated as the percentage of the basal response (b–d). The results were shown as the mean ± SD of three independent experiments. ##P < 0.01, ###P < 0.001, compared to the Basal; **P < 0.01, ***P < 0.001, compared to the UVB irradiated cells
Effects of EB on NF-κB signaling pathway
The activation of MAPK signaling caused by UVB radiation leads to NF-κB-dependent inflammatory responses in HaCaTs. We measured the protein levels of NF-κB, p-IκB-α and IκB-α by Western blot. As shown in Fig. 6, compared with the basal cells, UVB irradiation significantly increased the expression of NF-κB by 99.3%. However, this trend was remarkably controlled by EB treatment. EB improved IκB-α protein expression to the normal level and dose-dependently inhibited IκB-α phosphorylation. Compared with UVB-irradiated cells, EB at 100 µg/mL suppressed NF-κB protein expression by 45.9%, relieving UVB-induced inflammation.
Effects of EB on the UVB-induced NF-κB signaling activation. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with EB (1, 10, and 100 µg/mL) for 4 h. NF-κB, p-IκB-α and IκB-α protein expression were detected by Western blot (a). The band intensities were quantified by densitometry, normalized to the level of β-actin, and calculated as the percentage of the basal response (b–d). The results were shown as the mean ± SD of three independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001, compared to the Basal; *P < 0.05, **P < 0.01, ***P < 0.001, compared to the UVB irradiated cells
Effects of EB on promoting TGF-β/Smad signaling pathway
Smad proteins play important roles in TGF-β-dependent collagen regulation and ECM production. To investigate the positive effect of EB on increasing procollagen type I mRNA expression, we measured the protein levels of the TGF-β/Smad signaling pathway in UVB-irradiated HaCaTs (Fig. 7). In this study, UVB irradiation significantly decreased the levels of TGF-β1 and p-Smad2/3 by 41.8% and 51.3% and enhanced the expression of Smad7 by 37.4% in HaCaTs. As expected, the activation of TGF-β/Smad signaling pathway was promoted by the treatment of EB. EB increased TGF-β1 expression in a dose-dependent manner and enhanced the level of p-Smad2/3 by 41.7% at 100 µg/mL. Meanwhile, EB dose-dependently decreased the expression of Smad7, with the trend being in line with the increase in TGF-β1 expression.
Effects of EB on TGF-β/Smad pathway in UVB-irradiated HaCaTs. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with EB (1, 10, and 100 µg/mL) for 1.5 h. TGF-β1, Smad7, and p-Smad2/3 protein expression was detected by Western blot (a). The band intensities were quantified by densitometry, normalized to the level of β-actin, and calculated as the percentage of the basal response (b-d). The results were shown as the mean ± SD of three independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001, compared to the Basal; *P < 0.05, **P < 0.01, ***P < 0.001, compared to the UVB irradiated cells
Effects of EB on Nrf2/ARE signaling pathway
To determine the effects of EB on Nrf2 signaling, total Nrf2, HO-1, and NQO-1 protein expression were determined in UVB-irradiated HaCaTs (Fig. 8a). Compared with UVB-irradiated HaCaTs, EB effectively improved the expression of Nrf2 and dose-dependently enhanced HO-1 expression by 16.9%, 47.1% and 61.2% at 1, 10 and 100 µg/mL, respectively (Fig. 8b, c). At 10 µg/mL, EB also increased the level of NQO-1 by 21.5% (Fig. 8d). To the further study, nucleus and cytoplasm Nrf2 expression were separated and detected by Western blot (Fig. 8e), As shown in Fig. 8f, g, EB promoted Nrf2 transfer from the cytoplasm to the nucleus, dose-dependently increased the ratio of nucleus/cytoplasm Nrf2 expression to maintain proper redox balance.
Effects of EB on Nrf2 signaling in UVB-irradiated HaCaTs. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with EB (1, 10, and 100 µg/mL) for 3 h. Total Nrf2, HO-1 and NQO-1 expression were detected by Western blot (a). The band intensities of total Nrf2, HO-1 and NQO-1 were quantified by densitometry, normalized to the level of β-actin, and calculated as the percentage of the basal response (b–d). Nucleus and cytoplasm Nrf2 expression were detected by Western blot (e). The band intensities were calculated as the percentage of the basal response (f), and the fold increase of Nucleus/cytoplasm Nrf2 was normalized to the basal group and presented as column chart (g). The results were shown as the mean ± SD of three independent experiments. ##P < 0.01, compared to the Basal; *P < 0.05, **P < 0.01, compared to the UVB irradiated cells
Discussion
Research on botanical medicine with potential antioxidant and anti-inflammatory properties is popular in the search for a means of preventing and treating UV mediated dermatological diseases (Radice et al. 2016). Elderberry is a common supplement in a variety of processed foods and beverages possessing a high commercial value. In Europe and parts of Asia, elderberry has been used for thousands of years in folk medicine to prevent ailments such as the colds and arthritis and to relieve pain and fever (Porter and Bode 2017; Sidor and Gramza-Michałowska 2015). However, comprehensive studies detailing the effects of elderberry on ameliorating UVB-induced photodamage are still needed. In this study, we evaluated the recovery effects of a 70% ethanol elderberry extract (EB) in UVB-irradiated keratinocytes (HaCaTs). The phytochemical profile study indicated ethanol extraction of elderberry contain large amounts of anthocyanins, flavonoids, organic acids, and other polyphenols, which exhibits antioxidant, anti-inflammatory, antibacterial, antiallergic and antiviral properties (Dawidowicz et al. 2006; Veberic et al. 2009; Christensen et al. 2008). Their consumption may contribute to prevention of EB on skin aging and inflammatory disease. As expected, the free-radical-scavenging activity of EB increased in a dose-dependent manner, at 250 µg/mL, the scavenging activity of EB was better than that of arbutin. Meanwhile, EB attenuated UVB-induced ROS production in HaCaTs, effectively suppressed the expression of MMP-1 to block the degradation of collagen and promoted the TGF-β/Smad and Nrf2/HO-1 signaling pathways to increase procollagen type I synthesis. These results indicated that EB has the potential to alleviate UVB-induced photoaging.
Skin photoaging is a complex biological process induced by oxidative stress. Chronic UVB irradiation accelerates the process of photoaging mainly due to excessive ROS generation. Nrf2, as an important component of endogenous antioxidant defense systems, affects mitochondrial ROS production and acts as a key regulator against oxidative stress (Saw et al. 2011; Sun et al. 2016). Recent studies have indicated that some natural products, such as fennel extract and sulforaphane, alleviated UVB-induced skin damage via activation of the Nrf2-ARE antioxidant system (Kawachi et al. 2008; Sun et al. 2016). Thus, pharmacological activation of Nrf2 is a promising approach for the prevention of skin photodamage. In our study, UVB irradiation initiated excessive ROS generation in HaCaTs. The presence of EB accelerated Nrf2 signaling transfer to the nucleus, dose-dependently increased the ratio of nucleus/cytoplasm Nrf2 expression and effectively enhanced HO-1 expression, decreasing the production of ROS and maintaining the balance of cellular oxidation.
In the photoaging process, UVB-induced ROS production upregulates the expression of MMPs, which degrades collagen and other ECM proteins and UVB-induced MMP-1 overexpression initiates collagen breakdown by cleaving type I and type III collagen, plays an important role in the physiological mechanisms of skin photoaging (Brennan et al. 2003). Thus, the development of the MMP-1 inhibitor is an area of interest in anti-aging research. Fortunately, EB significantly decreased MMP-1 protein secretion and reduced the level of MMP-1 mRNA in UVB-irradiated HaCaTs. Previous studies found that UVB-induced ROS production activates MAPK signaling pathway through the phosphorylation of JNK, p38 kinase and ERK, and then stimulates MMPs gene transcription by the activation of AP-1 transcriptional factor, the MAPK/AP-1 signaling activation has been thought to play a dominant role in inducing the release of MMP-1 (Piao et al. 2012; Quan et al. 2009). In order to understand the possible inhibitory mechanism of EB on MMP-1 secretion, this study investigated the effects of EB on MAPK/AP-1 signaling phosphorylation. The results indicated that EB showed better effects on the decrease of JNK and p38 phosphorylation, and dose-dependently attenuated the expression of p–c–Fos and p–c–Jun. Here, the ameliorative effect of EB on MMP-1 overexpression was likely to be regulated by the inhibition of MAPK/AP-1 signaling activation.
Increasing evidence has shown that oxidative stress and inflammation are involved in multiple processes and play crucial roles in skin photoaging. UVB-induced excessive ROS generation causes the activation of MAPK kinases, leading to NF-κB-dependent inflammatory responses in HaCaTs. Thus, the crosstalk between NF-κB and MAPKs under oxidative stress has recently garnered interest (Piao et al. 2012; Schulze-Osthoff et al. 1997). In this study, UVB irradiation stimulated IκB phosphorylation and degradation, promoting NF-κB transfer to the nucleus. The expressions of various inflammatory genes were subsequently induced. As expected, EB prevented UVB-induced IκB-α degradation and suppressed NF-κB expression, effectively reducing protein secretion and mRNA level of IL-6. It has been reported that NF-κB activation inhibits the transcription of collagen type I gene (Rippe et al. 1999), and IL-6 could directly repress human collagen gene expression (Porée et al. 2008). UVB-induced inflammatory response also accelerate aging by degrading the collagen and elastin proteins in the skin. Therefore, EB attenuated UVB-induced inflammatory responses, and decreased inflammation-induced ECM breakdown.
VEGF, an important factor in the pathogenesis of psoriasis and skin carcinogenesis, its excess expression accelerates inflammation and results in more serious skin damage (Xia et al. 2003). Similar to previous studies, the overexpression of VEGF is related to NF-κB activation and MAPK/ERK signaling in UVB-induced HaCaTs (Kim et al. 2006; Zhang and Ma 2014). These findings support the idea that VEGF is both directly and indirectly involved in various steps in the UVB-induced photoaging process. Thus, inhibition of VEGF expression should be effective in preventing the process of UVB-induced cutaneous alterations and photoaging. It is worth mentioning that EB dose-dependently decreased UVB-induced overexpression of VEGF, however, the mechanism on ameliorating photodamage in HaCaTs needs further investigation.
Collagens provide resiliency and strength to the skin; therefore, promoting collagen synthesis is an important and necessary factor in repair skin aging. TGF-β1 is an important multifunctional cytokine involved in the regulation of many ECM-related genes that controls the synthesis of collagen and elastin (Klingberg et al. 2014; Wells and Discher 2008). Our results showed that UVB irradiation inhibited the synthesis of procollagen type I by downregulating the level of TGF-β1 and upregulating Smad7 expression. The presence of EB concentration-dependently decreased the expression of Smad 7. As a negative feedback inhibitor, the downregulation of Smad 7 promoted the binding of Smad 2 and Smad 3 proteins to the TGF-β1 gene promoter. EB activated TGF-β/Smad signaling, accelerating the synthesis of procollagen type I. The positive regulation of EB on TGF-β/Smad signaling pathway indicated the effect of EB on improving procollagen expression.
Conclusions
This study revealed that EB possesses the ability to alleviate UVB-induced skin damage. The proposed mechanism can be concluded as following (Fig. 9): EB promoted Nrf2/HO-1 signaling to enhance resistance to oxidative damage and suppressed NF-κB protein expression to control the release of IL-6, preventing UVB-induced inflammatory responses. EB blocked the overexpression of MMP-1, a major collagenase, by inhibiting the MAPK/AP-1 signaling pathway. And the synthesis of collagen was increased by EB-induced TGF-β/Smad signaling activation. Elderberry, a natural product, represents a complementary and alternative medicine for skin photoaging therapy.
References
Blainski A, Lopes GC, De Mello JCP (2013) Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules 18:6852–6865
Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL (2004) Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci 9:e32
Brennan M et al (2003) Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol 78:43–48
Cargnello M, Roux PP (2012) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 76:496
Christensen LP, Kaack K, Fretté XC (2008) Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur Food Res Technol 227:293–305
David L et al (2014) Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf B 122:767–777
Dawidowicz AL, Wianowska D, Baraniak B (2006) The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT-Food Sci Technol 39:308–315
Dinkova-Kostova AT (2008) Phytochemicals as protectors against ultraviolet radiation: versatility of effects and mechanisms. Planta Med 74:1548–1559
Duymuş HG, Göger F, Başer KHC (2014) In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem 155:112–119
Greenwald MBY, Frušić Zlotkin M, Soroka Y, Ben Sasson S, Bianco Peled H, Kohen R (2017) A novel role of topical iodine in skin: activation of the Nrf2 pathway. Free Radical Biol Med 104:238–248
Harokopakis E, Albzreh MH, Haase EM, Scannapieco FA, Hajishengallis G (2006) Inhibition of proinflammatory activities of major periodontal pathogens by aqueous extracts from elder flower (Sambucus nigra). J Periodontol 77:271–279
Hwang E, Park SY, Sun ZW, Shin HS, Lee DG, Yi TH (2014) The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar Biotechnol 16:361–370
Katiyar SK (2007) UV-induced immune suppression and photocarcinogenesis: chemoprevention by dietary botanical agents. Cancer Lett 255:1–11
Kawachi Y et al (2008) Attenuation of UVB-induced sunburn reaction and oxidative DNA damage with no alterations in UVB-induced skin carcinogenesis in Nrf2 gene-deficient mice. J Invest Dermatol 128:1773–1779
Kim MS, Kim YK, Eun HC, Cho KH, Chung JH (2006) All-trans retinoic acid antagonizes UV-induced VEGF production and angiogenesis via the inhibition of ERK activation in human skin keratinocytes. J Invest Dermatol 126:2697–2706
Kim J, Cha YN, Surh YJ (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res Fundam Mol Mech Mutagen 690:12–23
Klingberg F et al (2014) Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol 207:283–297
Luchetti F, Betti M, Canonico B, Arcangeletti M, Ferri P, Galli F, Papa S (2009) ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radic Biol Med 46:339–351
Masaki H (2010) Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci 58:85–90
Meng XM, Nikolic Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12:325
Moon NR, Kang SN, Park SM (2018) Consumption of ellagic acid and dihydromyricetin synergistically protects against UV-B induced photoaging, possibly by activating both TGF-β1 and wnt signaling pathways. J Photochem Photobiol, B 178:92–100
Muthusamy V, Piva TJ (2010) The UV response of the skin: a review of the MAPK, NFκB and TNFα signal transduction pathways. Arch Dermatol Res 302:5
Park B, Hwang E, Seo SA, Zhang MY, Park SY, Yi TH (2017) Dietary Rosa damascena protects against UVB-induced skin aging by improving collagen synthesis via MMPs reduction through alterations of c-Jun and c-Fos and TGF-β1 stimulation mediated smad2/3 and smad7. J Funct Foods 36:480–489
Piao MJ, Zhang R, Lee NH, Hyun JW (2012) Phloroglucinol attenuates ultraviolet B radiation-induced matrix metalloproteinase-1 production in human keratinocytes via inhibitory actions against mitogen-activated protein kinases and activator protein-1. Photochem Photobiol 88:381–388
Pillai S, Oresajo C, Hayward J (2005) Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—a review. Int J Cosmet Sci 27:17–34
Porée B et al (2008) Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1· Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem 283:4850–4865
Porter RS, Bode RF (2017) A review of the antiviral properties of black elder (Sambucus nigra L.) products. Phytother Res 31:533–554
Quan TH, Fisher GJ (2015) Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology 61:427–434
Quan TH, Qin ZP, Xia W, Shao Y, Voorhees JJ, Fisher GJ (2009) Matrix-degrading metalloproteinases in photoaging. In: Journal of investigation dermatology symposium proceedings. pp 20–24
Radice M, Manfredini S, Ziosi P, Dissette V, Buso P, Fallacara A, Vertuani S (2016) Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia 114:144–162
Rippe RA, Schrum LW, Stefanovic B, Solis-Herruzo JA, Brenner DA (1999) NF-kappaB inhibits expression of the alpha1 (I) collagen gene. DNA Cell Biol 18:751–761
Saw CL, Huang MT, Liu Y, Khor TO, Conney AH, Kong AN (2011) Impact of Nrf2 on UVB-induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol Carcinog 50:479–486
Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S (1997) Regulation of NF-κB activation by MAP kinase cascades. Immunobiology 198:35–49
Sidor A, Gramza-Michałowska A (2015) Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food-a review. J Funct Foods 18:941–958
Sun ZW et al (2016) Dietary Foeniculum vulgare Mill extract attenuated UVB irradiation-induced skin photoaging by activating of Nrf2 and inhibiting MAPK pathways. Phytomedicine 23:1273–1284
Sun ZW, Du J, Hwang E, Yi TH (2018) Paeonol extracted from Paeonia suffruticosa Andr. ameliorated UVB-induced skin photoaging via DLD/Nrf2/ARE and MAPK/AP-1 pathway. Phytother Res 32:1741–1749
Svobodova A, Walterova D, Vostalova J (2006) Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Olomouc 150:25–38
Veberic R, Jakopic J, Stampar F, Schmitzer V (2009) European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem 114:511–515
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who’s listening? Antioxid Redox Signaling 13:1649–1663
Wells RG, Discher DE (2008) Matrix elasticity, cytoskeletal tension, and TGF-β: the insoluble and soluble meet. Sci Signal 1:13
Wlaschek M et al (2001) Solar UV irradiation and dermal photoaging. J Photochem Photobiol B 63:41–51
Xia YP, Li BS, Hylton D, Detmar M, Yancopoulos GD, Rudge JS (2003) Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood 102:161–168
Zhang J, Ma WY (2014) Nerve growth factor regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes via PI3K/mTOR pathway. Genet Mol Res 13:9324–9335
Acknowledgements
This work was supported by the Snow White Factory Inc., Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, P., Hwang, E., Ngo, H.T.T. et al. Sambucus nigra L. ameliorates UVB-induced photoaging and inflammatory response in human skin keratinocytes. Cytotechnology 71, 1003–1017 (2019). https://doi.org/10.1007/s10616-019-00342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-019-00342-1