Abstract
P53 is shown recently to play an important role in the proliferation and differentiation of mesenchymal stem cells. In this study, human umbilical cord derived mesenchymal stem cells (hUCMSCs) were isolated and purified from the umbilical cords of normal or cesarean term deliveries, after treatment with 20 μmol/L PFT-α for 24 h, hUCMSCs were continued to be cultured for 4 weeks, cardiac-specific protein expression of cTnI, Desmin and Nkx2.5 was determined using immunofluorescence assay and RT-PCR. The expression of p53 and p21 was detected by western blot. Results showed that no expression of cTnI, Desmin or Nkx2.5 was observed in the control and the PFT-α group at 1 week after induction. However, after 4 weeks, while control group still had little expression of cTnI, Desmin and Nkx2.5, the PFT-α group demonstrated strong expression of cTnI, Desmin and Nkx2.5 (P < 0.001). At 4 weeks after induction, differentiation rate of cardiomyogenic cells in the PFT-α group (36.98 %) was significantly higher than that in the control group (4.41 %) (P < 0.01). Western blot analysis show that downregulation of p53 and p21 was seen in the PFT-α group at 4 weeks. The difference compared with the control group was statistically significant (P < 0.01). In conclusion, PFT-α can promote the differentiation of hUCMSCs into cardiomyogenic cells by modulating the p53–p21 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, mesenchymal stem cells (MSCs) transplantation has been suggested as a promising approach for the treatment of cardiovascular disease (Cao et al. 2009). MSCs for medical studies are primarily from bone marrow. But bone marrow-derived MSCs (BMMSCs) are easily contaminated by virus and this kind of cells possess markedly decreased capacity for proliferation and differentiation with donor’s aging and challenge a variety of ethical and legal problems, so their application is restrained (Méndez-Ferrer et al. 2010). Human umbilical cord derived mesenchymal stem cells (hUCMSCs) have properties of easy availability, abundance of source and rich content. Accumulating evidence has demonstrated that they can be differentiated into induced cells under appropriate culture conditions (Delalat et al. 2009). Latest research showed that p53 plays an important role in the proliferation and differentiation of BMMSCs (Armesilla et al. 2009). Blocking p53–p21 pathway can significantly increase the differentiation rate of induced pluripotent stem cells (IPS; Hong et al. 2009). However, few studies on the p53–p21 pathway underlying the process of induction and differentiation of hUCMSCs into cardiomyogenic cells are reported. This study aimed to investigate the impacts on the differentiation of hUCMSCs into cardiomyogenically-induced cells by inhibiting the p53–p21 pathway with p53 inhibitor (p-fifty three inhibitor-alpha, PFT-α).
Materials and methods
Materials
DMEM/F12 medium (GIBCO, NVF0274, Carlsbad, CA, USA), fetal bovine serum (Excell, 10057, Shanghai, China), collagenase II (Invitrogen, California, USA), trypase (Shanghai Seebio Biotech. Inc., Shanghai, China), anti-cTnI antibody (Beijing Boaosen Biological Technology Limited Company, bs-0799R, Beijing, China), Nkx2.5 antibody (Beijing Boaosen Biological Technology Limited Company, bs-0799R), normal goat working serum (SIG-31170, Shanghai, China), Desmin antibody (Beijing Boaosen Biological Technology Limited Company, bs-0799R), P53/P21 antibody (Thermo Fisher Scientific, Waltham, MA, USA), mouse anti-human CD44, CD90, CD10, CD34, CD45, HLA-DR antibody (BD Biosciences Company, Franklin Lakes, NJ, USA), flow cytometer (BD Biosciences Company), inverted optical microscope (Olympus, Tokyo, Japan), TRIZol reagent, ReverTra Ace qPCR RT Kit box; general PCR box, SYBR Green Realtime PCR Master Mix box (all from Beijing Boaosen Biological Technology Limited Company), protease inhibitor Cocktail (Sigma-Aldrich P8340, USA), biotinylated goat anti-mouse IgG (Vector labs BA-9200, USA), ECL kit (K820-500, Biovision, USA).
Methods
Isolation and culture of hUCMSCs
Umbilical cords were obtained after normal or cesarean term deliveries from three healthy infants at Taizhou People’s Hospital in September 2013 with informed written consent from the mothers and their family members. The umbilical cords were cannulated and washed three times with PBS to remove blood clots. Then the umbilical cords were cut into 2–3 cm pieces and opened with a scalpel. The Wharton’s Jelly was scratched out and the vessels were removed. Thereafter, the Wharton’s Jelly was incubated in collagenase II/DMEM solution for 24 h. After centrifugation for 10 min, the cell pellet was resuspended and incubated in trypsin at 37 °C for 30 min. Finally, the cell pellet was resuspended with the DMEM/F12 medium containing 10 % FBS and cells were seeded in a T75 culture flask.
Surface markers of hUCMSCs detected by flow cytometry
Following the third passage, the cells were washed twice with PBS, digested with 1:1 mixture of trypsine (2.5 g/L) and EDTA (0.2 g/L), suspension of 1 × 1010 L−1 single cells was obtained by washing with PBS containing bovine serum albumin (20 g/L). Each Eppendorf tube was supplemented with 100 μL cell suspension. Twenty μL of anti-human fluorescent-marked-antibodies of CD44, CD90, CD105, CD34, CD45 and HLA-DR were added, the control group was also treated with the same type of IgG. The cells were incubated for 30 min at 4 °C in dark, washed twice with PBS, then each Eppendof tube was added with 200 μL paraformaldehyde (10 g/L), and flow cytometry was used to detect the surface markers of hUCMSCs.
Cell induction
Following the third passage, the cells were washed twice with PBS, adjusted to a density of 2 × 104/mL, and then seeded in a 24-well plate. After 24 h, 20 μmol/L PFT-α was added (for the control group, only vehicle was added containing no PFT-α), and further incubated for 24 h. Then the culture medium containing PFT-α was removed and it means the DMEM/F12 medium containing 10 % FBS was added. The cells were maintained in culture for 4 weeks after the treatment.
Immunofluorescence staining
After induction for 4 weeks, the third passage of hUCMSCs at a density of 1 × 104/mL in different treatment groups were seeded on glass slides and allowed to adhere overnight. On the following day the cells were washed in PBS and fixed for 15 min with 4 % paraformaldehyde. None-specific binding was avoided by normal goat working serum (SIG-31170, Shanghai, China). Then the cells were incubated for 2–3 h at 37 °C with cTnI, Desmin or Nkx2.5 antibody. After washing with PBS, cells were incubated with biotinylated goat anti-mouse IgG as secondary antibody at 37 °C for 1 h, and washed three times with PBS. Finally, Hoechest 33342 (0.5 l g/mL) was added to 140 μL/well and incubated for 30 min at room temperature in the dark. The cells were examined by fluorescence microscope.
Detection of cTnI, Desmin and Nkx2.5 expression by RT-PCR
Total RNA was extracted using TriZol (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. 5 µL RNA was used for reverse transcription. Detection of the amplified cDNA was performed using Cycler IQ with GAPDH as the reference gene. The fold-change between the induced group and the control group for target genes was calculated with the 2−ΔΔCt method as described previously (Wu et al. 2008). Primers used for RT-PCR are listed in Table 1.
Calculation of cardiomyogenic cells differentiation rate
Following immunocytochemical staining, six fields of view were randomly selected from each well, number of cTnI-positive cells in each field of view was quantified using fluorescence microscope (200×). Cardiomyogenic cells differentiation rate of hUCMSCs was calculated.
Western blot assay
hUCMSCs in different groups were collected and lysed in RIPA buffer (50 mM Tris HCl pH 6.8, 1 % Triton × 100, 0.5 % Sodium deoxycholate, 150 mM NaCl, and 5 mM EDTA and protease inhibitor (Sigma-Aldrich P8340)). Total RNA was extracted from the lysate using TriZol (Invitrogen) following the manufacturer’s instructions. 5 µL RNA was loaded to a 12 % SDS-PAGE and transferred to PVDF membrane. Next, the membrane was incubated with p53, or p21 antibody for 2 h at room temperature. The membrane was washed with TBS/T for three times (8 min each time), then incubated with biotinylated goat anti-mouse IgG (Vector laboratories, BA-9200, Burlingame, CA, USA) as secondary antibody for 1 h at room temperature. The membrane was developed using ECL kit (K820-500, BioVision, Milpitas, CA, USA) and exposed to X-ray film.
Statistical analysis
Data were expressed as mean ± standard deviation of the mean and analyzed using paired Student t test, P < 0.05 was considered statistically significant.
Results
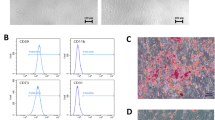
The results of flow cytometry analysis
Flow cytometry analysis revealed that CD44, CD90 and CD105 were highly expressed on the surface of the third passage of hUCMSCs, but they were negative for CD34, CD45 and HLA-DR (Fig. 1).
Immunofluorescence assay for cardiac-specific protein expression
hUCMSCs of passage three were induced for 24 h, followed by normal culture condition for 4 weeks. Cardiac-specific protein expression, cTnI, Desmin and Nkx2.5 was determined using an immunofluorescence assay to identify the induced cells. Results showed that, at 1 week after induction, no expression of cTnI, Desmin or Nkx2.5 was observed in the control and the PFT-α group. However, after 4 weeks, while the control group still had little expression of cTnI, Desmin and Nkx2.5, the PFT-α group demonstrated strong expression of cTnI, Desmin and Nkx2.5 (Figs. 2, 3). RT-PCR analysis showed that the level of cTnI, Desmin and Nkx2.5 expression was higher in the PFT-α group compared with the control group (P < 0.001; Table 2).
cTnI, Desmin and Nkx2.5 expression in the PFT-α group 4 weeks after induction assayed by the immunofluorescence method (×400). A, C, E, the cell morphology before immunofluorescence staining of cTnI, Desmin and Nkx2.5, respectively. B, D, F, the immunofluorescence staining of cTnI, Desmin and Nkx2.5, respectively. Red indicates positive staining. (Color figure online)
cTnI, Desmin and Nkx2.5 expression in the control group 4 weeks after induction assayed by the immunofluorescence method (×400). A, C, E, the cell morphology before immunofluorescence staining of cTnI, Desmin and Nkx2.5, respectively. B, D, F, the absence of expression of cTnI, Desmin and Nkx2.5, respectively, by immunofluorescence. (Color figure online)
Cardiomyogenic cells differentiation rate of hUCMSCs in different groups
Flow cytometry was employed to determine the differentiation rate of cardiomyogenic cells in the different groups. At 4 weeks after induction, differentiation rate of cardiomyogenic cells in the PFT-α group (36.98 %) was significant higher than in the control group (4.41 %; P < 0.01; Fig. 4).
Expression of p53 and p21 in the different groups of hUCMSCs
Western blot analysis showed that downregulation of p53 and p21 was seen in the PFT-α group at 4 weeks after treatment. The difference compared with the control group was statistically significant (P < 0.01; Figs. 5, 6).
Discussion
MSCs are stem cells that possess capacity for self-renewal and multi-directional differentiation. Recent evidences have suggested that MSCs can differentiate into cardiomyogenically-induced cells in vivo or in vitro and MSCs transplantation is a promising treatment modality to improve heart function in acute myocardial infarction (Janssens et al. 2006; Wollert et al. 2004; Turan et al. 2011). MSCs can be isolated from different tissues. Among them, BMMSCs were the first described and are the most frequently used. However, BMMSCs are easily contaminated by virus and possess markedly decreased capacity for proliferation and differentiation with donor’s aging. At the same time, the requirement of invasive methods to obtain BMMSCs and the challenge of ethical and legal problems also restrained their application. Therefore, they hold potential to become the donor source of myocardial cells for cell therapy in clinical treatment of myocardial infarction, and provide an optimal replacement of BMMSCs for clinical use and become the hotspot in the fields of biology medicine.
Umbilical cord is promising to serve as the first choice for harvesting MSCs owing to its wide source and no ethical concerns. Compared with BMMSCs, hUCMSCs are easier to isolate and expand, which makes it become a novel source of adult MSCs. More and more studies show that hUCMSCs may play an important role in applications and experimental research of adult MSCs.
In this study, we used collagenase and harvested a large number of adherent cells within a short time period. Results showed that, after thawing, these cells maintain high cell viability, and can be cultured for long time to obstain more passages of hUCMSCs. Flow cytometry results demonstrated that hUCMSCs harvested in this study could maintain their phenotypes after long-term in vitro proliferation, and passage 3 cells were positive for CD44, CD90 and CD105, but were negative for CD34, CD45 and HLA-DR, which is consistent with a previous report (Chabannes et al. 2007).
To identify whether hUCMSCs can be induced to differentiate into cardiomyogenic cells, the present study detected the expression of cTnI, Desmin and Nkx2.5 in induced cells. cTnI is highly specific for the heart, therefore, cTnI is a highly specific protein for identifying cardiomyocyte (Pereira et al. 2008). Desmin is expressed in the early stage of cardiogenesis and the main muscle intermediate filament protein. It is highly expressed in cardiomyocyte (2 %) (Taylor et al. 2007). Nkx2.5 is a key transcription factor in myocardial development. Nkx2.5 activation represents triggering of myocardial differentiation. It is expressed in the early stage of myocardial differentiation (Gandia et al. 2009). In this study, the expression of cTnI, Desmin and Nkx2.5 was determined using immunofluorescence assay. Results showed that there was no expression of cTnI, Desmin or Nkx2.5 in the control and the PFT-α group after 1 week of induction. However, after 4 weeks, while the control group still had little expression of cTnI, Desmin and Nkx2.5, the PFT-α group demonstrated strong expression of cTnI, Desmin and Nkx2.5. The level of cTnI, Desmin and Nkx2.5 expression was higher in the PFT-α group compared with control group. The above results indicated that PFT-α plays a role in inducing hUCMSCs differentiation into cardiomyogenic cells.
As the best known tumor suppressor, p53 is a transcription factor with sequence-specific DNA-binding activity that plays an important role in regulating cell cycle progression and cellular growth, which is involved in cell cycle regulation, DNA damage repair, apoptosis, and inhibition of tumor proliferation (Armesilla et al. 2009; Hong et al. 2009). Much of the function of p53 derives from its role as a transcription factor capable of turning on the expression of cell cycle regulatory proteins such as p21 (Waf1/Cip1) and apoptosis-promoting proteins such as bax. Evidence from chromatin immunoprecipitation studies suggests that the transactivating activity of p53 may control the expression of more than a hundred different genes (Gary and Jensen 2005). P53 protein can induce and activate downstream p21 protein and p53–p21 pathway has been shown to play a role in the modulation of the differentiation and proliferation of stem cells (Hong et al. 2009; Inoue et al. 2013). The mitogen-activated protein kinase (MAPK)/extracellular related kinase (ERK) signaling pathway is involved in activating the early stage of the differentiation of bone marrow-derived mesenchymal stem cells into osteoblasts, signal transduction of p38 is another important pathway in osteoblast differentiation and stimulated by the bone morphogenetic protein (BMP) family (Nishikawa et al. 2014). As a downstream target, p53 protein is phosphorylated and activated by a number of protein kinases in response to stressful stimuli. As an upstream activator, activated p53 acts as transcription factor to induce and/or suppress a number of genes whose expression leads to the activation of diverse signaling pathways. It has become clear that p53 protein can functionally interact with the MAPK pathways, including the stress-activated protein kinase [SAPK/c-Jun N-terminal protein kinase (JNK)], the p38 MAPK, and the extracellular signal related kinase (ERK) (Gen 2004). As a small molecule inhibitor of p53 activity, PFT-α is a reversible inhibitor of p53-mediated apoptosis, p53-dependent gene transcription, as well as down stream responsive gene function, it inhibits p53 apoptotic signaling by interfering with its nuclear translocation and transcriptional activation of p53 inducible genes. PFT-α is considered a useful tool to characterize p53-mediated events in a variety of cell types with various apoptosis-inducing agents (Walton et al. 2005; Misra and Pizzo 2010). Begum et al (2002) reported that PFT-α did not show significant difference in protecting HepG2 cells against cadmium or arsenic, in contrast, there was significant difference in the protection of rat liver cells upon exposure to arsenic or cadmium, which indicated that PFT-α exhibits protection to normal cells and can play an important role in cancer chemotherapy.
In this study, by inhibiting p53–p21 pathway with the p53 inhibitor PFT-α, the differentiation rate of cardiomyogenic cells in the PFT-α group was significantly higher than that in the control group. Western blot analysis confirmed that PFT-α led to a downregulation of p53 and p21 expression. However, the underlying mechanisms by which p53–p21 pathway inhibition promotes hUCMSCs differentiation need further studies in the future.
In summary, our study was the first to show that PFT-α could significantly promote hUCMSCs proliferation, and induce hUCMSCs to differentiate into cardiomyogenic cells. PFT-α may promote the process through modulating p53–p21 pathway for directed differentiation of hUCMSCs into cardiomyogenic cells, which will be valuable for cardiovascular regenerative medicine.
References
Armesilla DA, Elvira G, Silva A (2009) P53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp Cell Res 315:3598–3610
Begum RA, Farah IO, Ishaque AB (2002) Pifithrin-alpha (PFT-alpha) caused differential protection of rat liver cells and HepG2 cell line in response to the selective cytotoxicity of arsenic and cadmium. Biomed Sci Instrum 38:41–46
Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X, Feng X, Zhang J, Duan Y, Wang J, Liu D, Wang H (2009) Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J 30:1986–1994
Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC (2007) A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 110:3691–3694
Delalat B, Pourfathollah AA, Soleimani M, Mozdarani H, Ghaemi SR, Movassaghpour AA, Kaviani S (2009) Isolation and exvivo expansion of human umbilical cord blood-derived CD34 + stem cells and their cotransplantation with or without mesenchymal stem cells. Hematology 14:125–132
Gandia C, Bartual M, Garcia-Verdugo JM (2009) Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev 18:907–918
Gary RK, Jensen DA (2005) The p53 inhibitor pifithrin-α forms a sparingly soluble derivative via intramolecular cyclization under physiological conditions. Mol Pharm 6:462–474
Gen SW (2004) The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol Ther 32:156–161
Hong H, Takahashi K, Ichisaka T (2009) Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature 460:1132–1136
Inoue Y, Tomiya T, Nishikawa T, Ohtomo N, Tanoue Y, Ikeda H, Koike K (2013) Induction of p53-dependent p21 limits proliferative activity of rat hepatocytes in the presence of hepatocyte growth factor. PLoS ONE 8:e78346
Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F (2006) Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367:113–121
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834
Misra UK, Pizzo SV (2010) PFT-α inhibits antibody-induced activation of p53 and pro-apoptotic signaling in 1-LN prostate cancer cells. Biochem Biophys Res Commun 391:272–276
Nishikawa R, Anada T, Ishiko-Uzuka R, Suzuki O (2014) Osteoblastic differentiation of stromal ST-2 cells from octacalcium phosphate exposure via p38 signaling pathway. Dent Mater J 33:242–251
Pereira WC, Khushnooma I, Madkaikar M, Ghosh K (2008) Reproducible methodology for the isolation of mesenchymal stem cells from human umbilical cord and its potential for cardiomyocyte generation. J Tissue Eng Regen Med 2:394–399
Taylor MR, Slavov D, Ku L, Di Lenarda A, Sinagra G, Carniel E, Haubold K, Boucek MM, Ferguson D, Graw SL, Zhu X, Cavanaugh J, Sucharov CC, Long CS, Bristow MR, Lavori P, Mestroni L, Familial Cardiomyopathy Registry, BEST (Beta-Blocker Evaluation of Survival Trial) DNA Bank (2007) Prevalence of Desmin mutations in dilated cardiomyopathy. Circulation 115:1244–1251
Turan RG, Bozdag-T I, Ortak J, Kische S, Akin I, Schneider H, Turan CH, Rehders TC, Rauchhaus M, Kleinfeldt T, Belu C, Brehm M, Yokus S, Steiner S, Sahin K, Nienaber CA, Ince H (2011) Improved functional activity of bone marrow derived circulating progenitor cells after intra coronary freshly isolated bone marrow cells transplantation in patients with ischemic heart disease. Stem Cell Rev Rep 7:646–656
Walton MI, Wilson SC, Hardcastle IR, Mirza AR, Workman P (2005) An evaluation of the ability of pifithrin-α and -β to inhibit p53 function in two wild-type p53 human tumor cell lines. Mol Cancer Ther 9:1369–1377
Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H (2004) Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364:141–148
Wu CX, Zhang EY, Yang SY, Ma JY, An Q, Shi YK (2008) The expression of GATA-4 and Nkx2. 5 gene in the transformation of rattus mesenchymal stem cells into cardiomyocytes [In Chinese]. Sichuan Da Xue Xue Bao Yi Xue Ban 39:882–885
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruan, ZB., Zhu, L., Yin, YG. et al. Inhibitor of p53–p21 pathway induces the differentiation of human umbilical cord derived mesenchymal stem cells into cardiomyogenic cells. Cytotechnology 68, 1257–1265 (2016). https://doi.org/10.1007/s10616-015-9886-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-015-9886-5