Abstract
Carvacrol (CVC) is a phenolic monoterpene present in many essential oils of medicinal and aromatic plants and has attracted attention because of its beneficial biological activities. To date, although various biological activities of CVC have been demonstrated, its neurotoxicity on cultured primary rat neurons and N2a neuroblastoma cells has never been explored. Therefore, in this present study, we aimed to describe in vitro antiproliferative and/or cytotoxic properties (by 3-(4,5 dimetylthiazol -2-yl)-2,5 diphenlytetrazolium bromide (MTT) test), genotoxic damage potentials (by single cell gel electrophoresis (SCGE) or Comet assay) and antioxidant activities (by total antioxidant capacity (TAC) and total oxidative stress (TOS) analysis) of CVC in vitro. Dose (0–400 mg/L) dependent effects of CVC were tested on both cultured primary rat neurons and N2a neuroblastoma cells. Statistical analysis of MTT assay results indicated significant (p < 0.05) decreases of cell proliferation rates in both cell types treated with CVC at 200 and 400 mg/L. On the other hand, the mean values of the total scores of cells showing DNA damage (for comet assay) was not found significantly different from the control values for both cells (p > 0.05). In addition, our results indicated that 10, 25 and 50 mg/L of CVC treatment caused increases of TAC levels in cultured primary rat neurons but not in the N2a cell line. However, CVC treatments led to increases of TOS levels in cultured primary rat neurons at only 400 mg/L while they led to increases of TOS levels in N2a neuroblastoma cells at 200 and 400 mg/L. The present findings demonstrated that CVC could be a source of antioxidant and chemopreventive activities to be studied on cancer diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma (NB), a neoplasm of the sympathetic nervous system, is the second most frequently extra cranial malignant tumor of childhood and the most common solid tumor of infancy and affects approximately 1 in 10,000 individuals (Betts et al. 2005; Park et al. 2008). These tumors account for more than 7 % of malignancies in patients younger than 15 years and around 15 % of all pediatric oncologic deaths (Navarra et al. 2010). Despite of recent advances in multi-modality treatment protocols, the advanced-stage tumors remain aggressive and frequently resistant to chemotherapeutic regimens with an overall 5-year survival rate of only 30–40 % (Kang et al. 2006). The rare adult patient with neuroblastoma may have a very different outcome than pediatric patients. The scattered reports in the medical literature of adults with this disease suggest that, at least in some cases, long term survival may be worse than for younger patients, even in patients with localized disease, although the course may be more prolonged (Matthay 1997; Esiashvili et al. 2007). On the other hand, previous studies revealed that oxidative stress played a key role in neurodegenerative disorders (Cadet and Brannock 1998; Halliwell 2006). Mecocci et al. (1994) reported that oxidative damage to DNA may play a role in neurodegenerative diseases.
Recently, extensive efforts were made to investigate therapeutic substances capable of reducing the genotoxicity or carcinogenicity of various natural and man-made mutagens in human life. These include antibody, fatty acids, minerals, plant extract, lichen, amino acids and vitamins (Turkez and Geyikoglu 2010; Aydin and Turkez 2011, 2012; Turkez and Aydin 2012; Turkez et al. 2012a, b, c, d, e; Dirican et al. 2012). So far, antioxidants have attracted much interest with respect to their protective effect against free radical damage that may be the cause of many diseases including cancer (Shon et al. 2004). Monoterpenes are compounds found in the essential oils of many plants, including fruits, vegetables and herbs (Loza-Tavera 1999). One of which, CVC (1; 5-isopropyl-2-methylphenol) is mainly a naturally occuring compound present in the essential oil fraction of oregano and thyme (Lagouri et al. 1993; Arrebola et al. 1994). CVC has been used as food additive in the food industry for a long time (Yu et al. 2012; Joca et al. 2012). Furthermore, previous reports indicated that CVC has antimicrobial, antibacterial, antioxidant and anticancer potentials (Gany and Mahdi 2008; Giweli et al. 2012; Jaafari et al. 2012). Due to their important biological effects, there is an increasing attention in the evaluation of the antiproliferative effects induced by several monoterpenes such as vallesiachotamine and terpinen-4-ol on human melanoma and non-small cell lung cancer cells (Soares et al. 2012; Wu et al. 2012). To the best of knowledge the neurotoxic and/or anticarcinogenic potentials of CVC have not been investigated yet. It was aimed to determine whether CVC could be a source of natural antioxidants or potential anticancer agents for pharmaceutical application in the present study. Therefore, we evaluated different biological activities including cytotoxic, genotoxic and oxidative effects of CVC on healthy rat neurons and N2a neuroblastoma cells.
Materials and methods
Experimental design
Cell cultures
This study was conducted at the Medical Experimental Research Center at the Ataturk University (Erzurum, Turkey). The Ethical Committee of Ataturk University approved the study protocol (B.30.2.ATA.0.23.85-73). All procedures were performed in accordance with the National Institute of Health Principles of Laboratory Animal Care (Anonymous 1985). Primary rat neuron cultures were prepared from brains of five newborn Sprague–Dawley rats. The cerebral cortices were dissociated with HBSS (HBSS; Sigma-Aldrich®, Steinheim, Germany) + Trypsin–EDTA (% 0.25 trypsin– %0.02 EDTA; Sigma-Aldrich®), treated with DNAse type 1 (120 U/ml, Sigma®, St. Louis, MO, USA) and centrifuged. After having thrown away the supernatant, Neurobasal Medium (NBM) and fetal calf serum (PAN Biotech GmbH? Aidenbach, Germany) were added to the residue. The cell pellet was resuspended in phosphate buffer solution (PBA), and the single-cell suspension was divided into 3.5 ml samples in each of 10 flasks. The flasks were left in the incubator confirming 5 % CO2 at 37 °C in the ventile position. The flasks were then changed with a fresh medium of half of their volumes every 3 days until the cells were developed (Daikhin and Yudkoff 2000).
We employed a cell line, N2a neuroblastoma, used widely as a model for brain cancer. The rat brain neuroblastoma cell line N2a was obtained from Turkey FMD institute, Ankara, Turkey. Prior to the experiments, the cells were thawed and grown in tissue culture flasks as a monolayer in Dulbecco modified Eagle medium, (DMEM; Sigma-Aldrich®) supplemented with 1 % glutamine, 0·5 % penicillin/streptomycin (PAN Biotech®) and 10 % fetal bovine serum at 37 °C in a humidified (95 %) incubator with CO2 (5 %). The cultured cells were trypsinised with trypsin/EDTA for a maximum of 5 min and seeded with a sub cultivation ratio of 1:3-1:8.
MTT bioassay
The cells were seeded in 48-well plates. Cells were incubated at 37 °C in a humidified 5 % CO2/95 % air mixture and treated with carvacrol (CVC; (CH3)2CHC6H3(CH3)OH, CAS No. 499-75-2; SAFC, Sigma-Aldrich®) at different concentrations (10, 25, 50, 100, 200 and 400 mg/L) for 24 h. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) substrate solution was used according to the manufacturer’s instructions (Cayman Chemical Company®, Ann Arbor, MI, USA). Briefly, MTT was added to the cell cultures for 3 h. Formed formazan crystals were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich®), and the plates were analyzed using a Microquant reader at 570 nm wavelength.
SCGE assay
The comet assay also known as SCGE was performed and scored with slight modifications according to Singh et al. (1998), Kizilian et al. (1999), Tice et al. (2000), Saleha Banu et al. (2000), Heaton et al. (2002), Das et al. (2006) and Turkez (2011). The OxiSelect 96-Well Comet Assay kits (Cell Biolabs®, San Diego, CA, USA) were used to perform this assay. Approximately 5 × 103 of the 6–10 μL aliquots of neuron cells treated as above along with untreated CVC were mixed with 100 μl of comet agarose and layered on the surface on slides. After the application of cover slips, the slides were allowed to gel at 4 °C for 30–60 min. The slides were immersed in freshly prepared cold lysing solution and refrigerated overnight followed by alkali treatment, electrophoresis and neutralization. The dried slides were then stained using vista green after appropriate fixing. The whole procedure was carried out in dim light to minimize artifacts. DNA damage analysis was performed at a magnification of X 100 using a fluorescence microscope (Nicon Eclips E6600, Japan) after coding the slides by one observer (Aydın E). A total of 100 cells were screened per slide. A total damage score for each slide was derived by multiplying the number of cells assigned to each grade of damage by the numeric value of the grade and summing up all grades (giving a maximum possible score of 400, corresponding to 100 cells at grade 4).
TAC and TOS analysis
The automated total antioxidant capacity (TAC) and total oxidant status (TOS) assays were carried out in the culture medium using commercially available kits (Rel Assay Diagnostics®, Gaziantep, Turkey) on primary rat neurons and N2a neuroblastoma cell cultures for 24 h. The major advantage of the TAC assay is that it measures the antioxidant capacity of all antioxidants in a biological sample and not just of a single compound (Kusano and Ferrari 2008). In this test, antioxidants in the sample reduce dark blue-green colored 2,2′- azinobis-(3- ethylbenzothiazoline-6-sulfonate (ABTS) radical to its colorless form. The change in absorbance at 660 nm corresponds to the total antioxidant level in a sample. The assay is calibrated with a stable antioxidant standard solution of vitamin E analog, (Trolox-equivalent) (Erel 2004). The TOS assay used here is based on the oxidation of the ferrous ion-chelator complex to ferric ion (Fe3+), which is mediated by oxidants contained in the tested sample. The reaction is further enhanced by other molecules from the reaction medium. The reaction of Fe3+ with chromogen in an acidic medium produces a colored complex. Its intensity corresponds to the total amount of oxidants in the sample and can be measured spectrophotometrically. The TOS assay is calibrated with hydrogen peroxide and the results are expressed in terms of micro molar hydrogen peroxide equivalent per liter (Erel 2005).
Statistical analysis
Statistical analysis was performed using SPSS Software (version 18.0, SPSS, Chicago, IL, USA). For statistical analysis of obtained data Duncan’s test was used. Statistical decisions were made with a significance level of 0.05.
Results
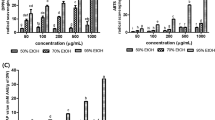
Anti-proliferative activity
Anti-proliferative activity was detected by the MTT assay, a colorimetric method for determining the number of viable cells in proliferation (Fig. 1). The addition of CVC at concentrations below 200 mg/L for 24 h did not cause any change in the cell viability of both cell cultures. However, the high doses of CVC (200 and 400 mg/L) exhibited an highly cytotoxic effect on cultured primary rat neurons and an anti-proliferative effect on N2a neuroblastoma cells.
Genotoxicity assay
Comet assay was performed on cultured primary rat neurons and N2a neuroblastoma cell line to measure the genotoxic potential of CVC. As shown in Fig. 2, the mean values of the total scores of cells showing DNA damage (for SCGE assay) was not found significantly different from the control values in both cell cultures.
Antioxidant activity
Table 1 shows the biochemical data obtained with various concentrations of CVC, on cultured primary rat neurons and N2a neuroblastoma cells. As shown from the results presented in Table 1, 25, 50 and 100 mg/L doses of CVC treatments did not lead to any alterations in TAC levels while 10 mg/L dose of CVC treatment caused significant increases of the TAC levels in cultured primary rat neuron cells as compared to control value. In contrast, CVC (at doses of 200 and 400 mg/L) caused significant decreases of TAC levels. Similarly, CVC (at concentrations below 100 mg/L) did not lead to any alterations in the TAC levels in rat N2a neuroblastoma cell line. However, 100, 200 and 400 mg/L of CVC caused significant increases of TAC levels when compared to controls. On the other hand, the TOS levels increased only at 400 mg/L doses of CVC in cultured primary rat neurons. However, CVC did not change the TOS levels in cultured primary rat neuron cells at doses lower than 400 mg/L. In addition, CVC caused statistically important (p < 0.05) increases in TOS levels at concentrations higher than 100 mg/L in comparison with control values for rat N2a neuroblastoma cell line.
Discussion
CVC is the major component of the essential oil fraction of oregano and thyme (Ultee et al. 1999). At this point, the anti-proliferative effects of CVC on both cultured primary rat neurons and N2a neuroblastoma cells were evaluated by MTT assay in this study. We found that 200 and 400 mg/L of CVC significantly reduced the cell viability in both cell cultures. Our findings are in accordance with published reports. In fact, the results of previous investigations revealed that several monoterpenes such as d-limonene (on human normal epithelial prostate PZ-HPV-7 cells), α-pinene, myrcene and linalool (on Vero monkey kidney cells), stylosin (on human fetal fibroblast cells) could have cytotoxic effects (Silva et al. 2007; Rabi and Bishayee 2009; Rassouli et al. 2011). Similarly, Aristatile et al. (2011) reported that CVC (at concentrations above 100 μg/mL) exposure caused increases of cell death rates in human lymphocytes. Slamenová et al. (2007) suggested that CVC exhibited cytotoxicity in different mammalian cells (Caco-2, HepG2 and V79 cells). A recent study reported that CVC exerts an anti-proliferative activity on MDA-MB 231 human metastatic breast cancer cells (Arunasree 2010). Again, Yin et al. (2012) revealed that CVC inhibited cell growth in A549 lung carcinoma cells in a dose-dependent manner and decreased cell viability was monitored at 24 h. Again, it was determined that CVC suppressed in vitro growth of mouse melanomas (He et al. 1997), human larynx carcinoma (Stammati et al. 1999), Hep-G2 (Sivas and Tomsuk 2011) and non-small cell lung cancer cells (Koparal and Zeytinoglu 2003). The exact mechanisms of the cytotoxic action of CVC are not known, but oxidative stress is thought to be the main responsible mechanism in its cellular toxicity. In addition to oxidative stress, previous studies reported that different mechanisms have been linked to plant products cytotoxicity, (I) including proteasome inhibition, (II) topoisomerase inhibition, (III) inhibition of fatty acid synthesis, (IV) accumulation of p53, (V) induction of cell cycle arrest, (VI) inhibition of phosphatidyl-inositol 3-kinase or (VII) enhanced expression of c-fos and c-myc (Constantinou et al. 1995; Lepley et al. 1996; Plaumann et al. 1996; Agullo et al. 1997; Chen et al. 1998; Kazi et al. 2004; Chen et al. 2005; Brusselmans et al. 2005).
Our findings also indicate CVC is neither genotoxic nor mutagenic on healthy neurons and N2a neuroblastoma cells using the comet assay since the observed mean values of the total scores of cells showing DNA damage (for SCGE assay) were not found significantly different from the control values for both cells. In parallel to this finding, Slamenova et al. (2011) reported that chromosomal aberration assay (CA) in rat primary hepatocytes did not prove any genotoxic activity of CVC. CVC concentrations up to 25 μM did not induce significant DNA strand breakage in V79 Chinese hamster lung fibroblast cells (Undeger et al. 2009). Furthermore, a recent study demonstrated that CVC did not lead to DNA damage in rat lymphocytes and hepatocytes (Aristatile et al. 2011). And, CVC did not increase the formation of sister chromatid exchange (SCE) rates, whereas it inhibited the rate of SCEs induced by MMC for human peripheral blood lymphocytes (Ipek et al. 2003). Ipek et al. (2005) also found that CVC strongly inhibited mutagenicity induced by 4-nitro-o-phenylenediamine and 2-aminofluorene in Salmonella typhimurium TA98 and TA100 strains with or without S9. Similarly, it was suggested that CVC significantly reduced the level of DNA damage induced by the strong oxidant hydrogen peroxide (H2O2) in K562 cells demonstrated via comet assay (Horvathova et al. 2007). In another study, the incubation of Hep G2 and Caco-2 cells in the presence of the whole scale of concentrations of CVC led to a significant protection of the cells studied from DNA strand breaks induced by the potent oxidant H2O2 (Slamenová et al. (2007)). In contrast to our findings, a previous study (Azirak and Rencuzogullari 2008) reported that CVC induced the numerical CA in bone marrow cells of rats.
Some dietary antioxidants may have the potential as adjuvant in cancer therapy by their ability to induce programmed cell death (apoptosis) (Zimmermann et al. 2001). Recent studies in cell cultures showed that some antioxidants, including vitamin E, vitamin C, selenium, flavonoids, carotenoids, and terpenoids, had anticancer effect (Sigounas et al. 1997; Borek and Pardo 2002; Taper et al. 2004; Borek 2004; Salminen et al. 2008). Previous investigations showed that monoterpenes such as myrtenal, vallesiachotamine, geraniol, terpinen-4-ol, linalool exhibited anticancerogenic properties in different experimental (liver, melanoma, prostate, nonsmall cell lung and breast cancers) models (Ravizza et al. 2008; Hari Babu et al. 2012; Soares et al. 2012; Kim et al. 2012; Wu et al. 2012). CVC was suggested to have an important in vitro antitumor effect against tumor cell lines, like Hep-2 (Stammati et al. 1999), B16 (He et al. 1997) and A-549 (Zeytinoglu et al. 2003; Koparal and Zeytinoglu 2003).
Epidemiological studies have shown that antioxidant compounds possessed anticarcinogenic effects to a greater or lesser extent. And the intake of natural antioxidants has been associated with reduced risks of cancer (Cai et al. 2004). To evaluate the antioxidative activity of CVC, TAC and TOS assays were performed in this study. CVC applications at its low concentration (10 mg/L) caused increases of TAC levels in cultured primary rat neurons. Similar to our finding, Undeger et al. (2009) found that 1, 2.5, 5, 10 and 15 mg/L of CVC showed a higher antioxidant capacity by using Trolox equivalent antioxidant capacity (TEAC) assay in V79 Chinese hamster lung fibroblast cells. Recent studies demonstrated that CVC had antioxidant properties by employing oxygen radical absorbance capacity (ORAC), TEAC, TRAP/TAR and 2,2-diphenyl-2-picryl-hydrazil (DPPH) methods (Miguel et al. 2009; Guimarães et al. 2010; Amiri et al. 2011). On the contrary, CVC (at 200 and 400 mg/L) caused significant decreases in TAC levels and high concentrations of CVC (only at 400 mg/L) caused increases in TOS levels in healthy neurons. In addition, high concentrations of CVC (higher than 50 mg/L) caused significant decreases in TAC levels and high concentrations of CVC (at 200 and 400 mg/L) caused increases in TOS levels in cancer cells in vitro. Our findings support the results of the study by Lima et al. (2004), which revealed that due to increasing concentrations monoterpenoid compounds could lead to neurotoxicity by reducing of total glutathione levels (TGSH) and leaking lactate dehydrogenase (LDH) in primary rat hepatocytes.
In conclusion, our results showed differences between rat N2a neuroblastoma cells and cultured primary rat neuron cells. This paper demonstrates that the CVC possesses weak antioxidant and cytotoxic activity in cultured primary rat neurons. In addition, CVC has weak antioxidant properties and little anticancer potentials in rat N2a neuroblastoma cell line. The efficacy of anti-cancer chemotherapy is limited by the cytotoxic effect on healthy cells due to a lack of selectivity of CVC and poor uptake of the therapeutics by N2a neuroblastoma cells.
References
Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, Payrastre B (1997) Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmaco 53:1649–1657
Amiri H, Yazdi HL, Dehshiri M, Eghbali D, Mohammadi A, Zarei A (2011) Essential oils composition and antioxidant properties of three thymus species. Planta Med 77:1294
Anonymous (1985) National Institute of Health, Principles of Laboratory Animal Care, USA 85:1–112
Aristatile B, Al-Numair KS, Al-Assaf AH, Pugalendi KV (2011) Pharmacological effect of carvacrol on D: -galactosamine-induced mitochondrial enzymes and DNA damage by single-cell gel electrophoresis. J Nat Med 65:568–577
Arrebola ML, Navarro MC, Jimenez J, Ocana FA (1994) Yield and composition of the essential oil of Thymus serpylloides subsp. serpylloides. Phytochemistry 36:67–72
Arunasree KM (2010) Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 17:581–588
Aydin E, Turkez H (2011) Antioxidant and genotoxicity screening of aqueous extracts of four lichens collected from North East Anatolia. Fresen Environ Bull 20:2085–2091
Aydin E, Turkez H (2012) Effects of lichenic extracts (Bryoria capillaris, Peltigera rufescens and Xanthoria elegans) on human blood cells: a cytogenetic and biochemical study. Fresen Environ Bull 20:2992–2998
Azirak S, Rencuzogullari E (2008) The in vivo genotoxic effects of carvacrol and thymol in rat bone marrow cells. Environ Toxicol 23:728–735
Betts DR, Cohen N, Leibundgut KE, Kühne T, Caflisch U, Greiner J, Traktenbrot L, Niggli FK (2005) Characterization of karyotypic events and evolution in neuroblastoma. Pediatr Blood Cancer 44:147–157
Borek C (2004) Dietary antioxidants and human cancer. Integrative Cancer Ther 3:333–341
Borek C, Pardo F (2002) Vitamin E and apoptosis: a dual role. In: Pasquier C (ed) Biennial Meeting of the Society for Free Radicals Research International. Paris, France, Bologna, Italy; Monduzzi Editore; 1994:327-331
Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV (2005) Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem 280:5636–5645
Cadet JL, Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32:117–131
Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184
Chen ZP, Schell JB, Ho CT, Chen KY (1998) Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett 129:173–179
Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP (2005) Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol 69:1421–1432
Constantinou A, Mehta R, Runyan C, Rao K, Vaughan A, Moon R (1995) Flavonoids as DNA topoisomerase antagonists and poisons: structure-activity relationships. J Nat Prod 58:217–225
Daikhin Y, Yudkoff M (2000) Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr 130:1026–1031
Das GP, Shaik AP, Jamil K (2006) Cytotoxicity and genotoxicity induced by the pesticide profenofos on cultured human peripheral blood lymphocytes. Drug Chem Toxicol 29:313–322
Dirican E, Turkez H, Toğar B (2012) Modulatory effects of Thymbra spicata L. different extracts against the mercury induced genotoxicity in human lymphocytes in vitro. Cytotechnology 64:181–186
Erel O (2004) A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 37:112–119
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Esiashvili N, Goodman M, Ward K, Marcus RB Jr, Johnstone PA (2007) Neuroblastoma in adults: incidence and survival analysis based on SEER data. Pediatr Blood Cancer 49:41–46
Gany ZSA, Mahdi MF (2008) Cytotoxic assay of Nigella sativa leaf callus extract (thymol) on hep-2 cell line using ELISA assay. Iraqi J Pharm Sci 17:63–67
Giweli A, Džamić AM, Soković M, Ristić MS, Marin PD (2012) Antimicrobial and antioxidant activities of essential oils of Satureja thymbra growing wild in Libya. Molecules 17:4836–4850
Guimarães AG, Oliveira GF, Melo MS, Cavalcanti SC, Antoniolli AR, Bonjardim LR, Silva FA, Santos JP, Rocha RF, Moreira JC, Araújo AA, Gelain DP, Quintans-Júnior LJ (2010) Bioassay guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin Pharmacol Toxicol 107:949–957
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Hari Babu L, Perumal S, Balasubramanian MP (2012) Myrtenal, a natural monoterpene, down-regulates TNF-α expression and suppresses carcinogen-induced hepatocellular carcinoma in rats. Mol Cell Biochem 369:183–193
He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE (1997) Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr 127:668–674
Heaton PR, Ransley R, Charlton CJ (2002) Application of single-cell gel electrophoresis (comet) assay for assessing levels of DNA damage in canine and feline leukocytes. J Nutr 132:1598S–1603S
Horvathova E, Turcaniova V, Slamenova D (2007) Comparative study of DNA-damaging and DNA-protective effects of selected components of essential plant oils in human leukemic cells K562. Neoplasma 54:478–483
Ipek E, Tüylü BA, Zeytinoğlu H (2003) Effects of carvacrol on sister chromatid exchanges in human lymphocyte cultures. Cytotechnology 43:145–148
Ipek E, Zeytinoglu H, Okay S, Tuylu BA, Kurkcuoglu M, Baser KHC (2005) Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem 93:551–556
Jaafari A, Tilaoui M, Mouse HA, Mbark LA, Aboufatima R, Chait A, Lepoivre M, Zyad A (2012) Comparative study of the antitumor effect of natural monoterpenes: relationship to cell cycle analysis. Rev Bras Farmacogn (in press) (doi:10.1590/S0102-695X2012005000021)
Joca HC, Cruz-Mendes Y, Oliveira-Abreu K, Maia-Joca RP, Barbosa R, Lemos TL, Lacerda Beirão PS, Leal-Cardoso JH (2012) Carvacrol decreases neuronal excitability by inhibition of voltage-gated sodium channels. J Nat Prod 75:1511–1517
Kang J, Kamal A, Burrows FJ, Evers BM, Chung DH (2006) Inhibition of neuroblastoma xenograft growth by Hsp90 inhibitors. Anticancer Res 26:1903–1908
Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP (2004) Structure activity relationships of synthetic analogs of (−)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res 24:943–954
Kim SH, Park EJ, Lee CR, Chun JN, Cho NH, Kim IG, Lee S, Kim TW, Park HH, So I, Jeon JH (2012) Geraniol induces cooperative interaction of apoptosis and autophagy to elicit cell death in PC-3 prostate cancer cells. Int J Oncol 40:1683–1690
Kizilian N, Wilkins RC, Reinhardt P (1999) Silverstained comet assay for detection of apoptosis. Biotechniques 27:926–930
Koparal AT, Zeytinoglu M (2003) Effects of carvacrol on a human non-small cell lung cancer (NSCLC) cell line, A549. Cytotechnology 43:149–154
Kusano C, Ferrari B (2008) Total antioxidant capacity: a biomarker in biomedical and nutritional studies. J Cell Mol Biol 7:1–15
Lagouri V, Blekas G, Tsimidou M, Kokkini S, Boskou D (1993) Composition and antioxidant activity of essential oils from oregano plants grown wild in Greece. Z. Lebensm-Unters-Forsch 197:20–23
Lepley DM, Li B, Birt DF, Pelling JC (1996) The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis 17:2367–2375
Lima CF, Carvalho F, Fernandes E, Bastos ML, Santos - Gomes PC, Fernandes-Ferreira M, Pereira-Wilson C (2004) Evaluation of toxic/protective effects of the essential oil of Salvia officinalis on freshly isolated rat hepatocytes. Toxicol In Vitro 18:457–465
Loza-Tavera H (1999) Monoterpenes in essential oils. Biosynthesis and properties. Adv Exp Med Biol 464:49–62
Matthay KK (1997) Neuroblastoma: biology and therapy. Oncology 11:1857–1866
Mecocci P, Mac Garvey U, Beal MF (1994) Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol 36:747–751
Miguel MG, Dandlen SA, Figueiredo AC, Pedro LG, Barroso JG, Marques MH (2009) Comparative evaluation of the antioxidant activities of thymol and carvacrol and the corresponding β-cyclodextrin complexes. ISHS Acta Hortic 853:363–368
Navarra M, Celano M, Maiuolo J, Schenone S, Botta M, Angelucci A, Bramanti P, Russo D (2010) Antiproliferative and pro-apoptotic effects afforded by novel Src-kinase inhibitors in human neuroblastoma cells. BMC Cancer 10:602
Park JR, Eggert A, Caron H (2008) Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am 55:97–120
Plaumann B, Fritsche M, Rimpler H, Brandner G, Hess RD (1996) Flavonoids activate wild-type p53. Oncogene 13:1605–1614
Rabi T, Bishayee A (2009) d -Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: generation of reactive oxygen species and induction of apoptosis. J Carcinog 8:1–9
Rassouli FB, Matin MM, Iranshahi M, Bahrami AR (2011) Investigating the cytotoxic and apoptosis inducing effects of monoterpenoid stylosin in vitro. Fitoterapia 82:742–749
Ravizza R, Gariboldi MB, Molteni R, Monti E (2008) Linalool, a plant-derived monoterpene alcohol, reverses doxorubicin resistance in human breast adenocarcinoma cells. Oncol Rep 20:625–630
Saleha Banu B, Dana Devi K, Mahboob M (2000) In vivo genotoxic effect of zinc sulfate in mouse peripheral blood leukocytes using comet assay. Drug Chem Toxicol 24:63–73
Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J (2008) Terpenoids: natural inhibitors of NF-Kappa B signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci 65:2979–2999
Shon MY, Choi SD, Kahng GG, Nam SH, Sung NJ (2004) Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem Toxicol 42:659–666
Sigounas G, Anagnostu A, Steiner M (1997) Dl-alpha tocopherol induces apoptosis in erythroleukemia, prostate and breast cancer cells. Nutr Cancer 28:30–35
Silva SL, Figueiredo PM, Yano T (2007) Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amaz 37:281–286
Singh NP, McCoy MT, Tice RR (1998) A simple technique for quantitation of low level of DNA damage in individual cells. Exp Cell Res 17:184–191
Sivas H, Tomsuk O (2011) Antiproliferative and apoptotic effects of the essential oil of Origanum onites and carvacrol on Hep-G2 cells. Anadolu Univ J Sci Technol C Life Sci Biotechnol 1:171–180
Slamenová D, Horváthová E, Sramková M, Marsálková L (2007) DNA-protective effects of two components of essential plant oils carvacrol and thymol on mammalian cells cultured in vitro. Neoplasma 54:108–112
Slamenova D, Horvathova E, Chalupa I, Wsolova L, Navarova J (2011) Ex vivo assessment of protective effects of carvacrol against DNA lesions induced in primary rat cells by visible light excited methylene blue (VL+MB). Neoplasma 58:14–19
Soares PR, de Oliveira PL, de Oliveira CM, Kato L, Guillo LA (2012) In vitro antiproliferative effects of the indole alkaloid vallesiachotamine on human melanoma cells. Arch Pharm Res 35:565–571
Stammati A, Bonsi P, Zucco F, Moezelaar R, Alakomi HL, Von Wright A (1999) Toxicity of selected plant volatiles in microbial and mammalian short-term assays. Food Chem Toxicol 37:813–823
Taper HS, Jamison JM, Gilloteax J, Summers JL, Calderon PB (2004) Inhibition of the development of metastases by dietary vitamin C:K3 combination. Life Sci 75:955–967
Tice RR, Agurell E, Anderson D (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Turkez H (2011) The role of ascorbic acid on titanium dioxide-induced genetic damage assessed by the comet assay and cytogenetic tests. Exp Toxicol Pathol 63:453–457
Turkez H, Aydin E (2012) Anti-genotoxic role of eicosapentaenoic acid against imazalil-induced DNA damage in vitro. Toxicol Ind Health (in press) (10.1177/0748233711433943)
Turkez H, Geyikoglu F (2010) Boric acid: a potential chemoprotective agent against aflatoxin b(1) toxicity in human blood. Cytotechnology 62:157–165
Turkez H, Aydin E, Aslan A (2012a) Xanthoria elegans (Link) (lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology 64:679–686
Turkez H, Geyikoğlu F, Dirican E, Tatar A (2012b) In vitro studies on chemoprotective effect of borax against aflatoxin B1-induced genetic damage in human lymphocytes. Cytotechnology 64:607–612
Turkez H, Geyikoglu F, Mokhtar YI, Togar B (2012c) Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology 64:15–25
Turkez H, Geyikoglu F, Yousef MI, Celik K, Bakir TO (2012d) Ameliorative effect of supplementation with l-glutamine on oxidative stress, DNA damage, cell viability and hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat hepatocyte cultures. Cytotechnology 64:687–699
Turkez H, Togar B, Polat E (2012e) Olive leaf extract modulates permethrin induced genetic and oxidative damage in rats. Cytotechnology 64:459–464
Ultee A, Kets EP, Smid EJ (1999) Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 65:4606–4610
Undeger U, Başaran A, Degen GH, Başaran N (2009) Antioxidant activities of major thyme ingredients and lack of (oxidative) DNA damage in V79 Chinese hamster lung fibroblast cells at low levels of carvacrol and thymol. Food Chem Toxicol 47:2037–2043
Wu CS, Chen YJ, Chen JJ, Shieh JJ, Huang CH, Lin PS, Chang GC, Chang JT, Lin CC (2012) Terpinen-4-ol induces apoptosis in human nonsmall cell lung cancer in vitro and in vivo. Evid Based Complement Alternat Med 2012:818261
Yin QH, Yan FX, Zu XY, Wu YH, Wu XP, Liao MC, Deng SW, Yin LL, Zhuang YZ (2012) Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology 64:43–51
Yu H, Zhang ZL, Chen J, Pei A, Hua F, Qian X, He J, Liu CF, Xu X (2012) Carvacrol, a food-additive, provides neuroprotection on focal cerebral ischemia/reperfusion injury in mice. PLoS ONE 7:e33584
Zeytinoğlu H, Incesu Z, Baser KH (2003) Inhibition of DNA synthesis by carvacrol in mouse myoblast cells bearing a human N-RAS oncogene. Phytomedicine 10:292–299
Zimmermann KC, Bonzon C, Green DR (2001) The machinery of programmed cell death. Pharmacol Ther 92:57–70
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aydın, E., Türkez, H. & Keleş, M.S. The effect of carvacrol on healthy neurons and N2a cancer cells: some biochemical, anticancerogenicity and genotoxicity studies. Cytotechnology 66, 149–157 (2014). https://doi.org/10.1007/s10616-013-9547-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-013-9547-5