Abstract
A recent report showed that reversine treatment could induce murine myoblasts dedifferentiation into multipotent progenitor cells and inhibit proliferation of some tumors, and other reports showed that apoptosis of lung adenocarcinoma cells could be induced by aspirin. The aim of the present study was to evaluate the synergistic antitumor effects of reversine and aspirin on cervical cancer. The inhibition rate of reversine and aspirin on cervical cancer cell lines’ (HeLa and U14) was determined by MTT method, cell cycle of HeLa and U14 cells was analyzed by FACS, mitochondrial membrane potential of HeLa and U14 was detected using a JC-1 kit. HeLa and U14 colony formation was analyzed by soft agar colony formation assay. The expression of caspase-3, Bcl-2/Bax, cyclin D1 and p21 was detected by qRT-PCR and Western Blotting. Moreover, tumor weight and tumor volume was assessed using a murine model of cervical cancer with U14 cells subcutaneously (s.c.) administered into the neck, separately or combined with drug administration via the intraperitoneal (i.p.) route. The inhibition rate of cells in the combination group (10 μmol/L reversine, 10 mmol/L aspirin) increased significantly in comparison to that when the drugs were used alone (P < 0.05); moreover, this combination could synergistically inhibit the proliferation of five cervical cancer cell lines (HeLa, U14, Siha, Caski and C33A). In the therapeutic mouse model, tumor weight and tumor volume of cervical cancer bearing mice was more reduced when compared with the control agents (P < 0.05) in tumor-bearing mice. The combination of reversine and aspirin exerts synergistic growth inhibition and apoptosis induction on cervical cancers cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reversine, a 2, 6-disubstituted purine, originally synthesized at Scripps Research Institute, was used to induce dedifferentiation of murine myoblasts (Chen et al. 2004). Later, some reports showed that reversine had a role in regeneration (Anastasia et al. 2006; Kim et al. 2007; Saraiya et al. 2010; Anastasia et al. 2010; Jung and Williams 2011). Moreover, a recent report showed that reversine had anti-tumor capabilities such as for a myeloma cell line (McMillin et al. 2010), and demonstrated that reversine could suppress the expression of cell cycle related proteins Aurora kinase A (Aur-A) and Aurora kinase B (Aur-B), and also could suppress enzymes involved in cell growth signaling, such as JAK2 and SRC (McMillin et al. 2010; Hua et al. 2012; Shan et al. 2007; Lee et al. 2012; Jemaà et al. 2012; Kuo et al. 2012; Hsieh et al. 2007). However, the definite molecular signaling pathway of the anti-tumor effects of reversine has not yet been understood. Moreover, reversine has never been studied with respect to proliferation and apoptotic induction of cervical carcinoma cell lines.
It is well known that aspirin is associated with a reduced risk of cancer development (Ghosh et al. 2010). Aspirin (acetyl salicylic acid) is a non-steroidal anti-inflammatory drug (NSAID), previous reports demonstrated that Aspirin had anti-inflammatory, anti-pyretic, anti-aggregant, and analgesic properties by suppression of the activity of cyclooxygenase enzymes (COX-1 and COX-2). Traditionally, Aspirin was used only through oral administration (Bunimov and Laneuville 2008; Manrique et al. 2008). Recent research demonstrated that aspirin had another role in anti-tumor effects in several cancer cell lines (De Luna-Bertos et al. 2012; Kumar and Singh 2012; Voutsadakis et al. 2010; Hsieh et al. 2011; Tu et al. 2012) through the suppression of ERK1/2 activation (Im and Jang 2012).
Cervical carcinoma is the second leading malignant tumor among women during their reproductive years, with approximately 500,000 of newly diagnosed cervical cancer patients per year and approximately 200,000 having locally advanced disease and/or die every year (Harper 2009). Most of the cervical cancer patients are diagnosed at the time at which they have already entered an advanced metastatic state for which they are treated by chemotherapy prone to lead to drug resistance of the tumors and systemic side effects. Surgery, radiation and chemicals like 5-Fu or Cisplatin based therapeutic strategies inevitably cause negative effects on patients’ body and spirit. Therefore, better and safer anticancer drugs will be helpful to minimize the suffering of patients and delay cancer progress.
Aspirin
Although aspirin could induce apoptosis in some cancer cells, at higher doses, the main undesirable adverse effects of aspirin taken by mouth are gastrointestinal ulcers, stomach bleeding, and tinnitus (Lewis et al. 1983); today this drug is not longer used in children and adolescents for controlling flu-like symptoms, symptoms of chickenpox or other viral illnesses, because of the risk of Reye’s syndrome (Macdonald 2002). Therefore, there is an urgent need to explore some new treatment options, to find efficient drugs with low side effects for the treatment of cervical cancer.
Reversine
Reversine is a low cost and non-chemotherapeutic alternative drug for oral squamous cell carcinoma and breast cancer treatment (McMillin et al. 2010; Hua et al. 2012; Shan et al. 2007; Lee et al. 2012; Jemaà et al. 2012; Kuo et al. 2012; Hsieh et al. 2007). However, it is still unknown, whether reversine and aspirin could induce apoptosis in cervical cancer cell.
In this paper, we explore the effects of reversine and aspirin and their combination in an in vitro and in vivo cervical cancer cell line model; the obtained data showed the possibility that the combination of reversine and aspirin may be a potential candidate for treating cervical cancer, as a first step to lay a theoretical foundation for the clinical application of reversine and aspirin associated with the treatment of cervical cancer.
Materials and methods
Cells and culture conditions
Mouse cervical carcinoma cells line U14, and the human cervical carcinoma cells line HeLa, Siha, Caski, C33A were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI 1640 medium containing 10 % FBS, l-glutamine (4 mmol/L), penicillin (100 units/mL), and streptomycin (100 μg/mL). All cells were incubated at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air, cells were sub-cultured according to the cell density.
Reagents
Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). RPMI-1640 medium, penicillin and streptomycin were from Gibco (Gibco BRL, Life Technologies, Carlsbad, CA, USA). Bromophenol blue, Coomassie blue R200, sodium chloride, ethanol, and methanol were bought from Yangyuan Chemical (Changshu, China). Phenylmethylsulfonyl fluoride (PMSF), Tween-20, sodium dodecyl-sulphate (SDS), dimethyl sulfoxide (DMSO), trypsin, EDTA, MTT, DAPI, aspirin and reversine were all obtained from Sigma-Aldrich Chemical (St Louis, MO, USA), reversine was prepared as a 1 mM stock in DMSO and kept at −20 °C. Aspirin stock solution (110 mM) was prepared by dissolving aspirin in 0.1 mol/L Tris (pH 8.8). Before use, aliquots of the solution were diluted to desired concentrations. Further kits and reagents were as follows: JC-1 Mitochondrial Membrane Potential Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA), RNeasy kit (QIAGEN, Mississauga, Ontario, Canada), MuLV Reverse Transcriptase (Applied Biosystems, Branchburg, NJ, USA), TaqMan reverse transcription reagents (Applied Biosystems), SYBR Green PCR Master Mix (AB Applied Biosystems). The primary IgG antibodies for cleaved caspase-3, cyclin D1, Bcl-2, Bax and GAPDH and the secondary anti-mouse or anti-rabbit IgG-HRP antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), the primary antibody for p21 was purchased from Abcam (Cambridge, MA, USA), enhanced chemiluminescence (ECL) detection kit was purchased from Amersham (Buckinghamshire, UK). BCA assay was obtained from Pierce (Rockford, IL, USA).
Cell growth analysis using the MTT test
Cell proliferation was evaluated by the MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay (Mosmann 1983). The capacity of reversine and aspirin to interfere with the growth of U14 and HeLa cells was determined using the MTT dye assay. Cells were seeded into the wells of the flat-bottomed 96-well culture plates in RPMI-1640 medium at a density of 5 × 103 of U14 and HeLa cells/100 μL. After an overnight attachment period, the cells were treated with medium only (as control) or medium containing reversine at 2.5, 5, 7.5, 10, 15, and 20 μM, aspirin at 2.5, 5, 7.5, 10, 15, and 20 mM. After incubation for 12, 24, 48, 72 and 96 h, 20 μL of newly prepared MTT (5 g/L) was added into each well, and incubation was continued for 4 h at 37 °C. During this step, the MTT is converted to a blue formazan product by mitochondrial succinate dehydrogenase. Then, supernatant was removed; formazan was solubilized from cells by 150 μL DMSO. The number of metabolically active cells by MTT assay was measured via absorbance (A) at 490 nm by an automatic fluorescence microplate reader. The final results were analyzed by statistical methods in three independent studies. Compusyn software (ComboSyn, Paramus, NJ, USA) was used for determining the combination index (CI) values of the data from the MTT assay. Conventionally, combination of drugs with CI > 1, CI = 1, and CI < 1 is showing antagonism, additive effect, and synergism, respectively.
Cell cycle analysis
U14 and HeLa cells were seeded on a 6-well plate the day before drug treatment so that they were 60–80 % confluent at the time of treatment. The treatment was divided into 5 groups (group I, untreated; group II, 10 μmol/L reversine; group III, 10 mmol/L aspirin; group IV, 10 μmol/L reversine combined with 10 mmol/L aspirin; group V, 10 μg/mL Cisplatin) for a 48 h treatment. Then 1 × 106 to 5 × 106 U14 and HeLa cells were collected and centrifuged at 1,000 rpm for 5 min, and the culture supernatant was removed; the cells were washed twice by 1 mL PBS, centrifuged, fixed with pre-cooled 70 % alcohol and left overnight at 4 °C; after centrifugation, the supernatant was removed, 1 mL DAPI staining buffer was added to stain cells for 30 min at room temperature in the dark place, and then the solution were sieved by a 400-mesh nylon net. Then, the pellets were resuspended in 500 μL of PBS. The analysis was performed on a Cytopeia InFlux cytometer (BD Biosciences, San Jose, CA, USA) using UV excitation. 50,000 cells were analyzed, if available, and in all cases acceptable histograms contained at least 10,000 cells. The cells with DNA content indicating they were below the G1 phase (peak of hypodiploid DNA below the G1 phase) were regarded as apoptotic cells. The cells in the G0/G1 phase, S phase and G2/M phase were analyzed using the software program Multicycle (Phoenix Flow Systems, San Diego, CA, USA).
Detection of mitochondrial membrane potential (MMP)
U14 and HeLa cells (4 × 105 cells per well in 96-well plate) were treated by reversine (10 μmol/L), aspirin (10 mmol/L) and its combinations for 0, 24 and 48 h, then the membrane potential-dependent stain JC-1 (excitation/emission = 488/530 nm) was used to assess the MPP. A total of 5 × 106 cells per well treated by reversine, aspirin or their combinations incubated in complete DMEM containing a final concentration of 0.1 μmol/L of JC-1 dissolved in DMSO for 20 min in a 37 °C, 5 % CO2 incubator. The cells were sedimented and washed in PBS (pH 7.4) and then incubated in DMEM without dye for 30 min. Cells were sedimented, washed with PBS, and fixed with 4 % paraformaldehyde in PBS for 10 min at 4 °C. After another wash in PBS, the fluorescence intensity of the microplates was read by a fluorescence spectrophotometer. Mean and standard deviation is plotted for 3 replicates from each condition.
Soft agar colony formation assay
HeLa and U14 cell lines were used to define the colony-inducing activity. Briefly, the cells dissociated by trypsin–EDTA treatment were resuspended in culture medium at a concentration of 1.6 × 104 cells per ml, mixed with an equal volume of 0.36 % agar and applied at 0.5 mL per well onto 24-well tissue plates that had been pre-coated with 0.75 % agar. The HeLa and U14 cells were incubated in a CO2 incubator and then reversine, aspirin and its combinations were added, drugs were washed out 24 h posttreatment and the cells were allowed to form colonies for 14 days before being fixed with methanol and stained with 1.25 % Giemsa and 0.125 % crystal violet. The stained cells were observed under a microscope and photographs were taken under low magnification. Colonies containing more than 50 cells were counted under the light microscope, and the colony formation rate (CFR) was calculated as (the number of colonies)/(number of seeded cells (8,000)) × 100 %.
qRT PCR analysis of cell cycle and apoptosis-regulatory genes
Quantitative real time reverse transcription-polymerase chain reaction (qRT-PCR) technique was used to analyse expression levels of relative mRNA. Cell cultures were divided into 5 groups (group I, untreated; group II, 10 μmol/L reversine; group III, 10 mmol/L aspirin; group IV, 10 μmol/L reversine combined with 10 mmol/L aspirin; group V, 10 μg/mL Cisplatin) treated and harvested after 0, 24 and 48 h. The cells were washed twice with ice-cold PBS. Total RNA was isolated using the RNeasy kit. The integrity and purity of RNA was electrophoretically verified by formaldehyde agarose gel stained with ethidium bromide (EB) and optical density (OD) absorption ratio OD260 nm/OD280 nm, respectively. First strand cDNA (1 μg of total RNA was used) was synthesized using MuLV Reverse Transcriptase by priming with oligo-d(T)18. Two microliters (2 μL) of the resultant cDNA products was used for PCR amplification.
Real time quantitative RT-PCR was performed using TaqMan reverse transcription reagents and performed on an ABI PRISM 7900HT sequence detection system (Applied Biosystems) to analyse the expression levels of genes related to cell cycle and apoptosis in relation to the housekeeping gene GAPDH. Primers sets for GAPDH, Bax/Bcl-2 and Caspase-3, Cyclin D1 and p21 were designed using the Real-Time Quantitative PCR probe design software (Roche Applied Systems). The primers used for the amplification of the respective genes are listed as Table 1.
PCR reactions for these primers were first optimised using conventional PCR and product sizes were verified by electrophoresis in a 2 % agarose gel. For quantitative RT PCR, SYBR Green PCR Master Mix was used as manufacturer’s instructions, containing cDNA (equivalent to 100 ng reverse-transcribed RNA) and 0.5 μmol/L of each primer shown above. The cycling conditions were: 10 min polymerase activation at 95 °C and 40 cycles at 95 °C for 15 s, 58 °C for 15 s, and 72 °C for 15 s. Molecular concentration of template cDNA for quantification was performed with the standard curve method for relative quantification. The Real-Time PCR efficiencies were calculated for each gene. C t indicates the fractional cycle number when the amount of amplified target genes reaches a fixed threshold within the linear phase of gene amplification, and is inversely related to the abundance of mRNA transcripts in the initial sample. Mean C t of duplicate measurements was used to calculate ΔC t as the difference in C t for target and reference (GADPH) gene. ΔC t for each sample was compared to the corresponding C t of the control experiment and expressed as ΔΔC t . Relative quantitation was expressed as fold-induction or repression of the gene of interest in comparison to the control condition according to the formula (normalized relative ratio = 2−ΔΔCt).
Protein isolation and western blotting
HeLa cells (6-well plate) treated by reversine (10 μmol/L), aspirin (10 mmol/L) and its combinations for 48 h were lysed in lysis buffer (0.5 % sodium deoxycholate, 0.1 % SDS, 1 % NP-40 in PBS containing proteinase inhibitors 100 mg/mL PMSF and 1 mM sodium orthovanadate). Total cell lysates were incubated on ice for 30 min, followed by microcentrifugation at 12,000 rpm for 10 min at 4 °C. Protein concentrations of the supernatants were determined by the BCA assay. Equal amounts of protein were mixed with 2× SDS) sample buffer, boiled for 4 min and separated by 10 % SDS–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). Nonspecific binding was blocked with 5 % non-fat milk in 1× TBST (Tris-buffered saline with 0.1 % Tween-20). Primary antibodies were directed against caspase-3 (1:500), p21 (1:500), cyclin D1 (1:500), Bcl-2 (1:500), Bax (1:200), or GAPDH (1:2,000). After washing three times, each for 10 min in 1× TBST, blots were exposed to the secondary antibody (anti-mouse or anti-rabbit IgG-HRP) at a dilution of 1:2,000 and visualized using ECL chemiluminescence detection system. Band intensities were quantified by scanning densitometry. All studies were performed in triplicate experiments.
Xenograft cervical carcinoma mouse model and in vivo anti-tumor analysis
Female athymic 6–8 weeks old BALB/c nude mice were housed in autoclaved microisolator cages in an air-filtered laminar flow cabinet and were given food and water ad libitum. All procedures were performed under sterile conditions in a laminar flow hood. This animal experiment was approved by the Institutional Animal Care and Use Committee and was in compliance with all regulatory guidelines.
U14 cell suspension (5 × 106 cells in 100 μL of RPMI-1640 medium) was injected subcutaneously. The purpose of developing cervical tumors was to generate histological intact tumors for drug therapy. When the diameter of tumors reached up to about 1 cm, reversine, aspirin or their combinations were administrated by intraperitoneal injection per 3 days, twenty-five of these mice were randomly assigned into one of the following five groups: (a) mice treated with RPMI-1640 medium, (b) mice treated with DMSO, (c) mice treated with reversine (10 mg/kg), (d) mice treated with aspirin (1 μg/kg) and (e) mice treated with a reversine and aspirin combination (Lewis et al. 1983). Body weight and tumor size at the site of inoculation were measured three times a week. Tumor size was measured every 3 days from two diameters, tumor volume was estimated using the formula L × S2/2 (L as the longest diameter, S as the shortest diameter).
Statistical analysis
The results of each series of experiments (performed in triplicates) are expressed as the mean values ± standard deviation of the mean (SD). Levels of the statistical significance were calculated using the paired Student t test when comparing two groups, or by analysis of variance (ANOVA). P values of ≤0.05 were considered significant.
Result
Cytotoxic and antiproliferation assay
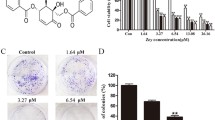
Reversine concentration of 2.5, 5, 7.5, 10, 15 and 20 μmol/L, and aspirin concentration of 2.5, 5, 7.5, 10, 15 and 20 mmol/L were assessed on U14 and HeLa cells, the results showed that reversine and aspirin inhibited HeLa (Fig. 1a) and U14 (Fig. 1b) cell growth with time and in dependence of concentration, the comparison of each group compared with the control group showed a statistically significant difference (P < 0.05). Therefore, in this study, 10 μmol/L reversine, with 10 mmol/L aspirin or their combinations were used to analysis the synergistic effect of inhibition or apoptosis on U14 and HeLa cells using different detection methods. The inhibition of the combined administration was significantly higher than when reversine and aspirin were used separately, the differences between each group and the control group were statistically significant (P < 0.05) (Fig. 1c). The combined administration of reversine and aspirin led to a synergistic inhibition of U14 and HeLa cell growth. At the same time, different cells had different drug sensitivities when treated by reversine combined with aspirin (Fig. 1d); the difference was statistically significant (P < 0.05) for each group compared with the control group. From the data shown above, we found that the combination of reversine and aspirin can inhibit growth of cervical cancer cell lines. Finally, we analyzed the results from the MTT assay using the Compusyn software to determine the combination of both drugs (CI = 0.72), CI values <1.0 indicate a synergistic interaction. Therefore, reversine and aspirin could synergistically inhibited growth of cervical cancer cells.
Inhibition of growth of cervical cancer cells by reversine and aspirin a inhibition rate of different drug concentrations on HeLa cells treated with reversine at 10 μmol/L increased compared with mock group, P < 0.05, however, inhibition rate of aspirin group at 10 mmol/L increased significantly, P < 0.05. b reversine (10 μmol/L), aspirin (10 mmol/L) and their combination on U14 cells with different time treatment, inhibition rate of combination group decreased significantly similar to HeLa cells compared with the drug used separately, P < 0.05. c Inhibition rate of the combination of both agents on HeLa and U14 cells at different processing times. d Effect of reversine, aspirin, or their combination on different cervical cancer cell lines (Siha, Caski and C33A) for 48 h; inhibition rate of the combination group significantly increased compared with that when the drug was used separately in different cell lines, P < 0.01
Cell cycle arrest of HeLa and U14 cells and MMP decrease
The reduction of cell growth may be mediated by cell cycle arrest and cell death, therefore, we examined whether cell cycle was changed after treatment with the different drugs. The human cervical cancer cells HeLa and mouse cervical cancer cells U14 were used. Cell cycle and the percentage of apoptosis of HeLa and U14 cells were evaluated by flow cytometry. 48 h after combined treatment with reversine and aspirin HeLa (Fig. 2a) and U14 (Fig. 2b) cells showed an hypodiploid cell population peak (apoptotic cells). In the combination group, the area of the apoptotic peak (appearing before the G0/G1 peak) was significantly increased compared for the drugs used separately.
Cell cycle and mitochondria potential of HeLa and U14 cells a cell cycle analysis of HeLa cells after treatment with reversine, aspirin or a combination of both (10 μmol/L reversine, 10 mmol/L aspirin). b Cell cycle analysis of U14 cells after the same treatments as in a. c Mitochondrial membrane potential (MMP) assay on HeLa cells treated with reversine (10 μmol/L), aspirin (10 mmol/L) and their combination after different incubation times, for the reversine and aspirin group, MMP decreased to a larger extent compared with the mock group, P < 0.05; whereas for the combination group the difference was significant at P < 0.01 when compared with the mock group. d MMP assay for U14 cells as for HeLa cells
During apoptosis, cell volume loss is a fundamental characteristic, moreover, the electrochemical gradient across the mitochondrial membrane collapses, and then, the MMP decreases. Fluorescent probe JC-1 has been shown for efficiently measuring MMP changes and was used for our analysis of MMP in this study. Compared with reversine and aspirin alone at similar concentrations, MMP of the HeLa (Fig. 2c) and U14 (Fig. 2d) cells was significantly decreased, the difference was statistically significant (P < 0.05).
Therefore, as shown by cell cycle analysis and MMP assay a reversine and aspirin combination could be used to induce apoptosis in the cervical cancer cells HeLa and U14 cells.
The numbers of HeLa and U14 cell clones decreased more in the reversine: aspirin combination group
The colony formation assay (CFA) is the gold standard for measuring the effects of cytotoxic agents on cancer cells in vitro. The cell survival assay is based on the ability of a single cell to grow into a colony. The colony formation assay showed that the number of colonies was about 3-fold higher in the mock group than in the reversine, aspirin and the combination groups, while in the combination group, colony number further decreased than when reversine and aspirin were used separately. Moreover, suppression of colony formation was similar for HeLa and U14 cultures (Fig. 3a, b).
Soft agar colony formation analysis and expression of cell cycle and apoptosis related genes in HeLa or U14 cells after treatment with reversine and aspirin for 48 h a HeLa and U14 cells colony formation decreased significantly in the combination group. b Quantitative analysis of soft agar clone formation after different treatments, P < 0.05. c qRT PCR analysis of the expression level of cell cycle and apoptosis related genes, Cyclin D1 and Bcl-2 decreased while p21, Caspase-3 and Bax expression increased in the combination group compared with the drugs used separately, P < 0.05. d Expression of genes related to cell cycle and apoptosis by Western blotting. e Scanning densitometric analysis of Western blotting
Expression of cell cycle and apoptosis related genes
To further investigate the mechanisms involved in reversine and aspirin combination-mediated apoptosis in cervical cancer cells (HeLa and U14), qRT-PCR and western blotting was used to analyze the expression of apoptosis and cell cycle related genes. Thus the expression of proteins caspase-3 and Bcl-2/Bax and of proteins p21 and cyclin D1 were determined after drug treatment of cervical cancer cells; mRNA (Fig. 3c) and protein levels (Fig. 3d, e) of caspase-3, Bax and p21 increased largely in the combined treatment groups compared with the groups for which drugs were used alone, the difference was statistically significant (P < 0.05); while the expression of Bcl-2 and cyclin D1 was reduced in the combined treatment groups.
Effect of drug combination on tumor volume and weight in a mouse model
In this study, we demonstrated that the combination of reversine and aspirin could more efficiently induce cell cycle arrest and apoptosis.To evaluate the anti-tumor effect of this combination, we established a xenograft nude mouse model by s.c. injection. Mice inoculated with cervical cancer cells had lost about 10 % of their initial body weight by about 16 days after tumor inoculation. However, tumor growth (tumor weight, Fig. 4a; tumor volume, Fig. 4b) was reduced and the mice survived longer in the combination group.
Cervical cancer model and its treatment a tumor weight under different treatment conditions, tumor weight of combination group was significantly decreased compared with the reversine or aspirin group, P < 0.05. b Tumor volume analysis, in the combination group, tumor volume of tumor bearing mouse in drug combination decreased to an higher extent than when reversine and aspirin were used separately
Discussion
Cancer is one of the leading causes of death in humans, the development of cancer involves a complex interplay among cellular processes, and treatment with a single agent is rarely effective. So, chemotherapy of cancer needs to assess many chemicals to treat various types of cancer, such as cervical cancer. Moreover, cancer chemotherapeutic strategies commonly require multiple agents. Therefore, novel and effective treatments are urgently needed to deal with the current treatment dilemma in incurable cervical cancer. Combination therapy is now considered to be a standard approach to chemotherapy (Sohma et al. 2011). However, drug combinations must be optimized to increase tumor response and lower their toxicity on normal tissues and cells, and increase patient tolerance (Mellman et al. 2011; Topalian et al. 2011; Ma and Waxman 2008). To improve efficacy and reduce adverse drug reactions, effective synergy to reduce the dose of each drug provide us with a new basis for induction of differentiation therapy for cervical cancer.
In the present study, reversine, a substituted purine, could induce fibroblasts transformation or dedifferentiation through protein kinases (such as JAK2, SRC, and Akt) and modulation of phosphorylation (Hua et al. 2012; Shan et al. 2007; Lee et al. 2012). Later on, research showed that it exhibited a significant antitumor activity against human cancer cells characterized by virtually uncontrolled tumour growth and spread of abnormal cells (Jemaà et al. 2012; Kuo et al. 2012; Hsieh et al. 2007). Aspirin is usually used for long-term treatment to prevent heart attack, stroke, and blood clot formation in people at high risk of developing blood clots at low doses (Lewis et al. 1983; Macdonald 2002). However, at higher doses, the main undesirable adverse effects of aspirin when taken by mouth are gastrointestinal ulcers, stomach bleeding, and tinnitus, thus it is no longer in use in children and adolescents. Previous research showed that aspirin had a prophylactic and therapeutic action against a variety of malignancies particularly those of the gastrointestinal tract (Khan et al. 2011; Wang et al. 2011) based not only on its direct tumoricidal action but also on the inhibiting of the pro-inflammatory status of tumor microenvironment. Whether aspirin-dependent decline of tumor activity can augment the anti-tumor activity of the anticancer drug reversine needs to be investigated.
At present, reports on reversine for the treatment of cervical cancer are still limited. Using different methods of detection, this study established that the combination of reversine and aspirin led to growth inhibition of cervical cancer cells to a certain degree. Meanwhile, flow cytometry assay revealed that reversine and aspirin synergistically arrested the cell cycle of cervical cancer cells; the MPP assay showed that these two drugs in combination could induce apoptosis of cervical cancer cell. Moreover, based on the data of soft agar colony formation assay, the colony formation ability of cervical cancer cells decreased. Lastly, we found that in the combination group the weight and volume of the tumors decreased in a tumor bearing mouse model.
The aim of the present study was to evaluate the effects of reversine and aspirin with respect to anti-proliferation and induction of apoptosis activity; when reversine and aspirin were used in combination, there was a certain synergistic effect to inhibit tumor cell growth. Based on this finding, we suggest that the combination of reversine and aspirin may emerge as an attractive strategy for the treatment cervical cancer.
References
Anastasia L, Sampaolesi M, Papini N, Oleari D, Lamorte G, Tringali C, Monti E, Galli D, Tettamanti G, Cossu G, Venerando B (2006) Reversine-treated fibroblasts acquire myogenic competence in vitro and in regenerating skeletal muscle. Cell Death Differ 13:2042–2051
Anastasia L, Pelissero G, Venerando B, Tettamanti G (2010) Cell reprogramming: expectations and challenges for chemistry in stem cell biology and regenerative medicine. Cell Death Differ 17:1230–1237
Bunimov N, Laneuville O (2008) Cyclooxygenase inhibitors: instrumental drugs to understand cardiovascular homeostasis and arterial thrombosis. Cardiovasc Hematol Disord Drug Targets 8:268–277
Chen S, Zhang Q, Wu X, Schultz PG, Ding S (2004) Dedifferentiation of lineage-committed cells by a small molecule. J Am Chem Soc 126:410–411
De Luna-Bertos E, Ramos-Torrecillas J, García-Martínez O, Díaz-Rodríguez L, Ruiz C (2012) Effect of aspirin on cell growth of human MG-63 osteosarcoma line. ScientificWorldJournal 2012:834246
Ghosh N, Chaki R, Mandal V, Mandal SC (2010) COX-2 as a target for cancer chemotherapy. Pharmacol Rep 62:233–244
Harper DM (2009) Current prophylactic HPV vaccines and gynecologic premalignancies. Curr Opin Obstet Gynecol 21:457–464
Hsieh TC, Traganos F, Darzynkiewicz Z, Wu JM (2007) The 2, 6-disubstituted purine reversine induces growth arrest and polyploidy in human cancer cells. Int J Oncol 31:1293–1300
Hsieh CC, Hernández-Ledesma B, de Lumen BO (2011) Lunasin-aspirin combination against NIH/3T3 cells transformation induced by chemical carcinogens. Plant Foods Hum Nut 66:107–113
Hua SC, Chang TC, Chen HR, Lu CH, Liu YW, Chen SH, Yu HI, Chang YP, Lee YR (2012) Reversine, a 2,6-disubstituted purine, as an anti-cancer agent in differentiated and undifferentiated thyroid cancer cells. Pharm Res 29:1990–2005
Im SR, Jang YJ (2012) Aspirin enhances TRAIL-induced apoptosis via regulation of ERK1/2 activation in human cervical cancer cells. Biochem Biophys Res Commun 424:65–70
Jemaà M, Galluzzi L, Kepp O, Boilève A, Lissa D, Senovilla L, Harper F, Pierron G, Berardinelli F, Antoccia A, Castedo M, Vitale I, Kroemer G (2012) Preferential killing of p53-deficient cancer cells by reversine. Cell Cycle 11:2149–2158
Jung DW, Williams DR (2011) Novel chemically defined approach to produce multipotent cells from terminally differentiated tissue syncytia. ACS Chem Biol 6:553–562
Khan Z, Khan N, Tiwari RP, Sah NK, Prasad GB, Bisen PS (2011) Biology of Cox-2: an application in cancer therapeutics. Curr Drug Targets 12:1082–1093
Kim YK, Choi HY, Kim NH, Lee W, Seo DW, Kang DW, Lee HY, Han JW, Park SW, Kim SN (2007) Reversine stimulates adipocyte differentiation and downregulates Akt and p70(s6k) signaling pathways in 3T3-L1 cells. Biochem Biophys Res Commun 358:553–558
Kumar A, Singh SM (2012) Priming effect of aspirin for tumor cells to augment cytotoxic action of cisplatin against tumor cells: implication of altered constitution of tumor microenvironment, expression of cell cycle, apoptosis, and survival regulatory molecules. Mol Cell Biochem 371:43–54
Kuo CH, Lu YC, Tseng YS, Shi CS, Chen SH, Chen PT, Wu FL, Chang YP, Lee YR (2012) Reversine induces cell cycle arrest, polyploidy, and apoptosis in human breast cancer cells. Breast Cancer. doi:10.1007/s12282-012-0400-z
Lee YR, Wu WC, Ji WT, Chen JY, Cheng YP, Chiang MK, Chen HR (2012) Reversine suppresses oral squamous cell carcinoma via cell cycle arrest and concomitantly apoptosis and autophagy. J Biomed Sci 19:9
Lewis HD Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE 3rd, Schnaper HW, LeWinter MM, Linares E, Pouget JM, Sabharwal SC, Chesler E, DeMots H (1983) Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med 309:396–403
Ma J, Waxman DJ (2008) Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther 7:3670–3684
Macdonald S (2002) Aspirin use to be banned in under 16 year olds. BMJ 325:988
Manrique C, Lastra G, Palmer J, Gardner M, Sowers JR (2008) Aspirin and Diabetes Mellitus: revisiting an old player. Ther Adv Cardiovasc Dis 2:37–42
McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, Mitsiades N, Schlossman RL, Munshi NC, Kung AL, Griffin JD, Richardson PG, Anderson KC, Mitsiades CS (2010) Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med 16:483–489
Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480:480–489
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Saraiya M, Nasser R, Zeng Y, Addya S, Ponnappan RK, Fortina P, Anderson DG, Albert TJ, Shapiro IM, Risbud MV (2010) Reversine enhances generation of progenitor-like cells by dedifferentiation of annulus fibrosus cells. Tissue Eng Part A 16:1443–1455
Shan SW, Tang MK, Chow PH, Maroto M, Cai DQ, Lee KK (2007) Induction of growth arrest and polycomb gene expression by reversine allows C2C12 cells to be reprogrammed to various differentiated cell types. Proteomics 7:4303–4316
Sohma I, Fujiwara Y, Sugita Y, Yoshioka A, Shirakawa M, Moon JH, Takiguchi S, Miyata H, Yamasaki M, Mori M, Doki Y (2011) Parthenolide, an NF-κB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics 8:39–47
Topalian SL, Weiner GJ, Pardoll DM (2011) Cancer immunotherapy comes of age. J Clin Oncol 29:4828–4836
Tu Z, Li H, Ma Y, Tang B, Tian J, Akers W, Achilefu S, Gu Y (2012) The enhanced antiproliferative response to combined treatment of trichostatin A with raloxifene in MCF-7 breast cancer cells and its relevance to estrogen receptor β expression. Mol Cell Biochem 366:111–122
Voutsadakis IA, Patrikidou A, Tsapakidis K, Karagiannaki A, Hatzidaki E, Stathakis NE, Papandreou CN (2010) Additive inhibition of colorectal cancer cell lines by aspirin and bortezomib. Int J Colorectal Dis 25:795–804
Wang YH, Wu MW, Yang AK, Zhang WD, Sun J, Liu TR, Chen YF (2011) COX-2 Gene increases tongue cancer cell proliferation and invasion through VEGF-C pathway. Med Oncol 28:S360–S366
Acknowledgments
This work was supported by Grant of a Research Contact with the Education Bureau of Henan Province (No.: 2011A320017).
Conflict of interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, Hx., Yang, J., Cui, Hk. et al. Synergistic antitumor activity of reversine combined with aspirin in cervical carcinoma in vitro and in vivo. Cytotechnology 65, 643–653 (2013). https://doi.org/10.1007/s10616-012-9520-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-012-9520-8