Abstract
The osteogenic potential of autologous cultured osteoblasts mixed with fibrin when transplanted to bone defects was evaluated. Radial shaft defects over 15 mm were made in 30 New Zealand white rabbits. A total of 15 rabbits in the control group underwent an iliac bone graft and 15 rabbits in the experimental group underwent an autologous cultured osteoblast injection mixed with fibrin. Both groups were compared radiologically and 5 rabbits in each group were sacrificed for histological evaluation using H-E and Masson’s trichrome stain at 3, 6, and 9 weeks. Osteogenesis in the control group progressed more rapidly than in the experimental group. However, at 9 weeks, bone formation in both groups were similar and showed no significant difference in terms of the amount of bone formation and the quality of bone union. Autologous cultured osteoblast transplantation mixed with fibrin in bone defects was found to produce bone efficiently.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Partial loss of a bone of the extremities due to trauma, tumor excision, bone diseases, etc., presents as a substantial impediment throughout the life of patients, and numerous efforts have been made to reconstruct the normal function of the skeletal system. However, such efforts still have many limitations and problems. If cultured autologous cells are used instead of bone transplant, problems such as the donor site morbidity occurring generally in autologous bone grafts (Ahlmann et al. 2002), immunological problems, and the spread of diseases occurring in allograft transplantation (Liu et al. 2000) may be overcome, and fast recovery may be achieved (Ahlmann et al.2002). In experiments, it has been reported that bone marrow stromal cells have the ability to differentiate into osteoblasts, chondroblasts, fibroblasts, adipocytes, etc. depending on the environmental condition of adjacent tissues (Bruder et al. 1994; Pittenger et al. 1999). However, as the number of marrow stromal cells in the bone marrow is extremely low (Bruder et al. 1997), cell culture is prerequisite for its utilization in experiments, and it is anticipated that the bone marrow stromal cells with such differentiation potential may be of help in the union of bone fractures (Schneider 1998). Hence, the purpose of this experiment is to investigate whether cells isolated from bone marrow and bone could restore the stability of the fractured area and differentiate into normal bone tissue.
Materials and methods
Experimental animals
A total of 30 New Zealand white rabbits, each weighing approximately 3 Kg, were raised 1 week prior to experiments with the same feed and under the same conditions. Among them, 15 animals were used as the osteoblast transplant group, and the remaining 15 animals were control animals for an autologous iliac bone transplant. The approval for animal experiment of this study was obtained from the Institutional Review Board.
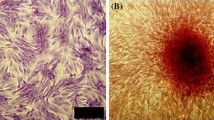
The isolation of bone marrow stromal cells and the culture of osteoblasts
Bone fragments containing bone marrow isolated from the posterior iliac crest of experimental animals were added to a container filled with 30 mL of 10% FBS––α MEM (Sigma Chemical Company, St. Louis, MO, USA) and 350 units of heparin, and were transported to the laboratory. After careful mixing, large bone fragments were removed, and the remaining mixture was centrifuged at 4 °C, 472g, for 10 min. The precipitate was treated with collagenase (Sigma) for 24 h, and centrifuged, after which the supernatant was discarded, and 20 mL of culture medium was added to the remaining pellets. The mixture was filtered with a filter (Falcon, Franklin Lakes, NJ, USA), and 10 mL were added per T-75 culture flask (Corning Science Products, Corning, NY, USA) and culture was initiated (Maniatopoulos et al. 1988). The incubator (Automatic CO2 Incubator, Forma Scientific Inc, Marietta, Ohio, USA) was maintained at 37 °C, and a 5% CO2 condition. The next day, 50 μg L-ascorbic acid (Sigma)/10 mL and dexamethasone 10−7 mol L−1 were added to facilitate cell differentiation into osteoblasts. The cell culture condition was evaluated by a light microscope, and the culture medium was changed on the 5th day of culture. Afterwards, the culture medium was changed every 3 days with a subsequent addition of L-ascorbic acid. On the 14th day of culture, NBT-BCIP (nitro blue tetrazolium chloride––5-bromo-4-chloro-3-indolyl phosphate) staining was performed, confirming the activation of alkaline phosphatase. A period of 24 days after culture, Alizarin red staining which detects newly produced calcium, was performed, and thus it was confirmed that most of the cultured cells were osteoblasts. Around 4 weeks of culture, culture medium was removed, and the cells were washed with 5 mL 0.02% trypsin-ETDA (Gibco BRL, Gettysburg, USA). About 3 mL of 0.02% trypsin-ETDA was again added and incubated in an incubator for 5 min. The trypsin-ETDA activity was stopped by adding 3 mL of culture medium, and all contents were collected in a conical tube, centrifuged at 4 °C, 265g, for 6 min. The supernatant was removed, and the precipitate was collected. After adjusting the cell count to 5 × 106/mL, and the cells were used in the transplant.
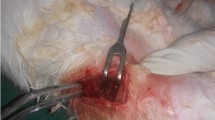
Formation of the bone defect
The rabbits were anesthetized with ketamine and placed in a prone position. Their forearms were incised longitudinally according to a posterior approach, and the shaft of the radius was exposed. Afterwards, using a string saw, a bone defect 15 mm in size was created (Cheng 1994). At that time, the periosteum of the bone defect area was removed completely, and the skin and subcutaneous tissues were sutured carefully after either bone graft or osteoblast injection.
Preparatory experiment
The bone defects of 3 rabbits were not filled with any grafts, and fibrin not containing osteoblasts was injected in the bone defect area in 3 rabbits. They were evaluated radiologically.
Osteoblast and autologous bone graft
In control animals, cancellous bone obtained from the iliac bone was transplanted to the bone defect. In the experimental group, cultured autologous osteoblasts mixed with fibrin were injected to the bone defect area. Animals were injected intramuscularly with gentamicin (40 mg/day), and they were allowed to move freely within their cages.
Examination methods
Radiographs were taken at 3, 6, and 9 weeks after the experiments, and points were allotted according to the degree of bone formation in the bone defect area (Bos et al. 1983). Full formation of bone in the defect was given 3 points, formation over half the area was 2, below half was 1 point, and no formation was 0. At 3, 6, and 9 weeks after the experiments, 5 rabbits from each group were sacrificed, and the area of the defect along with portion of the osteotomy area was extracted, fixed in formalin, decalcified in a mixture of 20% water-soluble formic acid and 10% sodium citrate for 3–4 days, and embedded in paraffin, yielding tissue sections approximately 6 μm in thickness. The sections were stained with hematoxylin-eosin and Masson’s trichrome.
Results
Gross examination
The defects were replaced by firm tissue which afforded stability to the defect area in both groups at 3, 6, and 9 weeks into the experiment. The newly formed tissue showed continuity with the surrounding tissues.
Radiological examination
Preparatory experiment
There is no definite bone formation in the defect of the rabbit that did not receive treatment (Fig. 1a–c). In the fibrin injection group, a small amount of callus formation was observed, with no continuity between the osteotomy site and the defect (Fig. 1d–f).
The radiographs of a rabbit without grafting at 3 weeks (a), 6 weeks (b), and 9 weeks (c) after bone defect operation show no continuity between the osteotomy sites. The radiographs of 3 rabbits in the preparatory experiment at 9 weeks (c) after injection of fibrin without osteoblasts show insufficient bone formation in the defects (d–f)
Three weeks into the experiment
Bone formation of the control group was more prominent than in the experimental group, showing periosteal reaction and continuity between the osteotomy site and the defect (Fig. 2A). The experimental group showed incomplete callus formation in the defect, but continuity between the osteotomy site and the defect is achieved (Fig. 2a).
The radiographs of a control group rabbit at 3 weeks (A), 6 weeks (B), 9 weeks (C) after the iliac bone graft. It shows good callus formation and progressive remodeling of the grafted bone. The radiographs of a experimental group rabbit at 3 weeks (a), 6 weeks (b), 9 weeks (c) shows after the osteoblast injection. It shows callus formation in the defect and a steady progression in remodelling
Six weeks into the experiment
In the control group, the bone remodeling is progressed to form near normal mature bone (Fig. 2B). In the experimental group, the hazy bony shadow seen at 3 weeks became more distinct and the defect was completely filled with new bone (Fig. 2b).
Nine weeks into the experiment
In both groups, complete mature bone with marrow space was formed and the maturity was similar. The bone formation score of both groups were all given 3 points, with findings of complete union and bone formation in the defect (Fig. 2C, c).
Tissue and histochemical examination
Three weeks into the experiment
In the experimental group, there was coexisting independent bone (Fig. 3f) and cartilage formation (Fig. 3e) in the defect. The defect area, which was identified as empty space upon X-ray, was filled with these tissues (Fig. 3c, d). Also active collagen formation around the newly formed bone tissue and a connection to the surrounding tissue with some directionality of the collagen fibers was detected (Fig. 3g, h). Immature lamellar bone and enchondral bone formation was noted. In the control group, a more mature form of bone tissue was found as compared to the experimental group (Fig. 3a, b). The graft area was connected to the upper and lower osteotomy area by the formation of periosteum and cortical bone, and the bone marrow cavity was continuous with the surrounding normal bone.
The radiograph of a control group rabbit at 3 weeks shows bone formation connecting both osteotomy sites (a). The defect shows continuity between both osteotomy sites by newly formed bone bridges in H-E stain of Fig. 2a (×6) (b). The radiograph of a experimental group rabbit at 3 weeks shows bone formation connecting both osteotomy sites. The defect is still not completely filled (c). The defect is completely filled with newly formed cartilagenous tissue (black arrow), lamellar bone tissue (dotted arrow) and fibroosseous tissue (white arrow) in H-E stain of Fig. 2c. (×6) (d). Masson’s trichrome stain of the black arrow area in Fig. 2d shows chondrocyte metaplasia and bone formation (×100) (e). Masson’s trichrome stain of the dotted arrow area from Fig. 2d shows immature lamellar bone and vascular formation (×100) (f). H-E stain(g) and Masson’s trichrome stain(h) of the white arrow area from Fig. 2d show active collagen fiber formation and an increase in vascular ingrowth (×100). This formation is partially connected to the surrounding immature lamellar bone (×100) (g, h)
Nine weeks into the experiment
The amount and degree of new bone formation and remodeling was similar in both groups at 9 weeks of experiment, although the control was faster than experimental group in that aspect. Increased blood flow and the formation of mature lamellar bone was found in both groups, and the defect was eventually filled completely with bone (Fig. 4).
The radiograph of a experimental group rabbit at 9 weeks shows bone formation connecting both osteotomy sites. The defect is filled completely (A). The defect is filled completely with newly formed mature bone in H-E stain of Fig. 3A. (×6) (B). The radiograph of a control group rabbit at 9 weeks shows complete bone formation and remodelling in the defect (a). The defect is filled with newly formed mature bone in H-E stain of Fig. 3a (×6)(b)
Discussion
Autologous cultured osteoblast injection is based on bone marrow injection, which is supported by the theory that osteoprogenitor cells in bone marrow induce and facilitate bone formation (Ashton et al. 1980). Bone marrow injection is performed independently or in combination with a bone graft procedure. This procedure is simple and has no donor site morbidity or complication. However, the amount of aspiration volume in one site is limited and the number of bone forming cells is small (Muschler et al. 1997). So it has been considered that the culturing of cells and their subsequent transplant is the most feasible method to overcome such a problem, and by the transplantation between different species using mediators, successful results have been reported (Bruder et al. 1994). The reason why fibrin was used as a carrier in this experiment is that fibrin enables the cultured osteoblasts to attach to the defect area and is safe because it is used in the operation field as a hemostatic agent and is absorbed without a foreign body reaction. Fibrin can also be used as a scaffold loaded with growth factor and can act for the initial settlement of the transplant (Huang et al. 2002). Until now, the information about the origin of cells and the characteristics of cells used for transplant, or the knowledge on factors controlling bone formation and regeneration are limited. In addition, a certain degree of common insight into the effective treatment mode gained by numerous clinical trials and studies on the number of cells required for obtaining effective results, culture methods, and surgical technique, etc. is required.
In our experiment, we created several defects of varying size in the rabbit radius, the largest being 15 mm, which, according to a study by Herold et al. (1971) is the size where spontaneous bone formation does not occur. This is a critical size defect and the result of follow ups show that the fracture area on both sides become slender and thin, and bone formation does not occur any further (Fig. 1). Therefore, because bone formation was assessed in a bone defect area larger than 15 mm after osteoblast transplantation, it can be concluded that bone formation by autologous cultured osteoblast injection mixed with fibrin has occurred.
Transcutaneous application of autologous cultured osteoblasts can produce satisfactory bone formation in that it cause less tissue damage, and consequently there is no impairment of the blood supply. On the other hand, in autologous grafts or allografts, surgery is needed for the transplant, causing impaired blood circulation, in addition to resorption and reformation of the transplanted graft, which is needed for bone union. In osteoblast transplantation, it is considered that without undergoing such processes, immature bone tissues formed by osteoblasts are connected to the adjacent tissues more readily (Fig. 3g, h).
For the union of fractures, autologous bone transplant is a very fast and effective method, however, considering the pain in the donor area due to surgery, the limitation of the volume of bone graft, and the fact that additional surgery is required for transplant, osteoblast transplantation may be an adequate substitution for autologous bone graft.
Conclusion
It was confirmed in our study that autologous osteoblast transplantation in bone defects induce ossification. It is anticipated that the procedure may be applicable to various situations requiring bone transplant.
References
Ahlmann E, Patzakis M, Roidis N (2002) Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg 84-A:716–720
Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M (1980) Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop 151:294–307
Bos GD, Goldberg VM, Powell AE, Heiple KG, Zika JM (1983) The effect of histocompatibility matching on canine frozen bone allografts. J Bone Joint Surg 65-A:89–96
Bruder SP, Fink DJ, Caplan AI (1994) Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem 56:283–294
Bruder SP, Jaiswal N, Haynesworth SE (1997) Growth kinetics, self-renewal and the osteogenic potentials of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Bioch 64:278–294
Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV (1994) Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology 134:277–286
Herold HZ, Hurvitz A, Tadmor A (1971) The effect of growth hormone on the healing of experimental bone defects. Acta Orthop Scand 42:377–384
Huang Q, Goh JC, Hutmacher DW, Lee EH (2002) In vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor beta1 and the potential for in situ chondrogenesis. Tissue Eng 8:469–482
Liu J, Wang Z, Hu Y (2000) Complications of massive allografts after segmental resection of malignant bone tumors. Zhongh ua Wai Ke Za Zhi 38:332–335
Maniatopoulos C, Sodek J, Melcher AH (1988) Bone formation in vitro by stromal cells obtained from bone marrow of young adults rat. Cell Tissue Res 254:317–330
Muschler GF, Boehm C, Easley K (1997) Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg 79-A:1699–1709
Pittenger MF, Mackay AM, Beck SC (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Schneider U (1998) Autogenous bone cell transplantation. Orthopade 27:143–146
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SJ., Jang, JD. & Lee, SK. Treatment of long tubular bone defect of rabbit using autologous cultured osteoblasts mixed with fibrin. Cytotechnology 54, 115–120 (2007). https://doi.org/10.1007/s10616-007-9084-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-007-9084-1