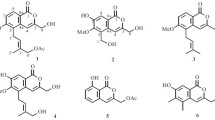
Two new prenylated isocoumarins, versicolols C and D (1 and 2), together with three known isocoumarins (3–5), were isolated from the fermentation products of the endophytic fungus Aspergillus versicolor. Their structures were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. The anti-tobacco mosaic virus (anti-TMV) activities of compounds 1 and 2 were evaluated. The results revealed that compounds 1 and 2 showed moderate anti-TMV activity with inhibition rates of 22.4 and 24.6%, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bioactive secondary metabolites from endophytic fungi, isolated from higher plants, are a major focus of natural product research [1]. Aspergillus, a genus of filamentous fungi, is famous for its medical and commercial importance [2]. Among them, Aspergillus versicolor, a well-studied species, has attracted particular attention as a prolific source of secondary metabolites with diverse structures and biological properties [3,4,5,6,7]. Isocoumarins are an important class of natural products and are known to exhibit a wide range of pharmacological activities, such as antibacterial and antifungal [8], cytotoxicity [9, 10], antiviral [11, 12], antioxidative [13], and anti-inflammatory [14] properties. Here, our further efforts on the fermentation products of the endophytic fungus A. versicolor led to the isolation and identification of two new (1 and 2) and three known (3–5) prenylated isocoumarins. The new compounds were elucidated by means of spectroscopic methods, while the known compounds were identified by comparison with data in the literature. Compounds 1 and 2 were also evaluated for their anti-tobacco mosaic virus (anti-TMV) activity.
A 70% aq. acetone extract prepared from the fermentation products of the endophytic fungus A. versicolor was subjected repeatedly to column chromatography on silica gel, Sephadex LH-20, RP-18, and preparative HPLC to afford compounds 1–5, including two new isocoumarins, versicolols C and D (1 and 2), together with three known isocoumarins (3–5). The 1H and 13C NMR data of compounds 1 and 2 are listed in Table 1. The known compounds, compared with the literature, were identied as versicolols A (3) [9], angelicoin A (4) [15], and tabaisocoumarin A (5) [12].

Compound 1 was obtained as a pale yellow gum. Its molecular formula C16H20O3 was determined by positive HR-ESI-MS at m/z 283.1303 [M + Na]+ (calcd for C16H20NaO3, 283.1310), indicating seven degrees of unsaturation. The IR spectrum showed the absorption bands of hydroxy (3417 cm–1) and aromatic groups (1615, 1546, 1487 cm–1). The 1H NMR spectrum (Table 1) showed signals assignable to a methoxy group at δ 3.82 (1H, s, 6-OMe); a prenyl group at δ 3.37 (2H, d, J = 7.1 Hz, H-1′), 5.33 (1H, t, J = 7.2 Hz, H-2′), 1.54 (3H, s, H3-4′), and 1.75 (3H, s, H3-5′); two oxygenated methylene groups at δ 5.19 (2H, s, H-1) and 4.29 (2H, s, H-9); and three additional olefinic protons at δ 6.22 (1H, s, H-4), 6.59 (1H, d, J = 8.2, H-7), and 6.82 (1H, d, J = 8.2, H-8). In the 13C NMR and DEPT NMR spectra (Table 1), there were signals for three methyls (one oxygenated), three methylenes (two oxygenated), four methines, and six quaternary carbons. These data were closely related to versicolol B [9], an unusual prenylated isocoumarin, except that the methyl group at C-3 in versicolol B was converted into an oxygenated methylene group in 1. This structural assignment was further supported by the further analysis of its HMBC correlations. Thus, compound 1 was named as versicolol C.
Versicolol D (2) was also obtained as a pale yellow gum with the molecular formula C15H18O3 determined by its HR-ESI-MS at m/z 269.1162 [M + Na]+ (calcd 269.1154). Analysis of the 1H and 13C NMR spectra (Table 1) revealed that the structure of 2 was very similar to that of 1, except for the replacement of a methoxy group by a phenolic hydroxyl group in 2. The phenolic hydroxy group located at C-6 was also supported by the HMBC correlations of the phenolic hydroxy proton at δ (9.22, s) with C-5 (δ 133.3), C-6 (δ 155.0), and C-7 (δ 115.4). The structure of 2 was therefore determined.
Since some isocoumarins are known to exhibit potential antivirus activities [11, 12], compounds 1 and 2 were tested for their anti-TMV activities. The anti-TMV activities were tested using the half-leaf method [16], using ningnanmycin (a commercial product for plant disease in China) as a positive control. Compounds 1 and 2 showed high anti-TMV activity with inhibition rates of 22.4 and 24.6% at the concentration of 20 μM, respectively.
Experimental
General. UV spectra were obtained using a Shimadzu UV-2401A spectrophotometer (Shimadzu, Kyoto, Japan). IR spectra were obtained in KBr disc on a Bio-Rad Win infrared spectrophotometer (Bio-Rad, Hercules, CA, USA). ESI-MS were measured on a VG Auto Spec-3000 MS spectrometer (VG, Manchester, UK). 1H, 13C, and 2D NMR spectra were recorded on Bruker 400 instrument (Bruker, Karlsruhe, Germany) with TMS as internal standard. Column chromatography was performed on silica gel (200–300 mesh) or on silica gel H (10–40 μm, Qingdao Marine Chemical Inc., Qingdao, China). The second separation was performed on an Agilent 1100 HPLC (Agilent Technologies, Englewood, CO, USA) equipped with a ZORBAX-C18 (21.2 mm × 250 mm, 7.0 μm) column and DAD detector.
Fungal Material. The culture of Aspergillus versicolor 0456 was isolated from the leaves of Nicotiana sanderae, which was collected from Shilin, Yunnan Province, China, in 2014. The strain was identified by one of the authors (Dr. Gang Du) based on the analysis of the ITS sequence. It was cultivated at room temperature for 7 days on potato dextrose agar at 28°C. Agar plugs were inoculated into 250 mL Erlenmeyer flasks each containing 100 mL potato dextrose broth and cultured at 28°C on a rotary shaker at 180 rpm for five days. Large-scale fermentation was carried out in 200 Fernbach flasks (500 mL) each containing 100 g of rice and 120 mL of distilled H2O. Each flask was inoculated with 5.0 mL of cultured broth and incubated at 25°C for 45 days.
Extraction and Isolation. The fermentation products were extracted four times with 70% acetone (4 × 10 L) at room temperature and filtered. The crude extract (226 g) was subject to silica gel (200–300 mesh) column chromatography eluted with a CHCl3–(CH3)2CO gradient system (20:1, 9:1, 8:2, 7:3, 6:4, 5:5) to give six fractions A–F. The further separation of fraction C (8:2, 22.1 g) by silica gel column chromatography eluted with petroleum ether–EtOAc (8:2, 7:3, 6:4, 1:1, 1:2) yielded mixtures C1–C5. Fraction C2 (7:3, 5.08 g) was subjected to preparative HPLC (55% MeOH, flow rate 12 mL/min) to give 1 (13.6 mg), 3 (15.2 mg), and 5 (14.6 mg). Fraction C3 (6:4, 3.67 g) was subjected to preparative HPLC (50% MeOH, flow rate 12 mL/min) to give 2 (13.8 mg) and 4 (12.2 mg).
Anti-TMV Assays. TMV (U1 strain) was obtained from the Key Laboratory of Tobacco Chemistry of Yunnan Province, Yunnan Academy of Tobacco Science, China. The virus was multiplied in Nicotiana tabacum cv. K326 and purified as described. The concentration of TMV was determined as 20 mg/mL with a UV spectrophotometer [Virus concentration = (A 260 × dilution ratio)/E 0.1%, 260 nm/1 cm]. Purified virus was kept at –20°C and was diluted to 32 μg/mL with 0.01 M PBS (phosphate buffer saline) before use.
Nicotiana glutinosa plants were cultivated in an insect-free greenhouse. N. glutinosa was used as a local lesion host. The experiments were conducted when the plants grew to the 5–6 leaf stage. The tested compounds were dissolved in DMSO and diluted with distilled H2O to the required concentrations. A solution of equal concentration of DMSO was used as a negative control. The commercial antiviral agent ningnanmycin was used as a positive control.
For the half-leaf method, the virus was inhibited by mixing with the solution of the compound. After 30 min, the mixture was inoculated on the left side of the leaves of N. glutinosa, whereas the right side of the leaves was inoculated with the mixture of DMSO solution and the virus as control. The local lesion numbers were recorded 3 or 4 days after inoculation. Three repetitions were conducted for each compound. The inhibition rates were calculated according to the formula
where C is the average number of local lesions of the control and T is the average number of local lesions of the treatment.
Versicolol C (1). Obtained as a pale yellow gum, C16H20O3. UV (MeOH, λmax, nm) (log ε): 215 (3.96), 275 (3.69), 290 (3.22), 335 (3.52). IR (KBr, v max, cm–1): 3417, 3084, 2963, 2870, 1615, 1546, 1487, 1359, 1131, 1078. For 1H and 13C NMR (400 and 100 MHz, CDCl3), see Table 1. ESI-MS (positive ion mode) m/z 283 [M + Na]+; HR-ESI-MS (positive ion mode) m/z 283.1303 [M + Na]+ (calcd for C16H20NaO3, 283.1310).
Versicolol D (2). Obtained as a pale yellow gum, C15H18O3. UV (MeOH, λmax, nm) (log ε): 215 (3.98), 272 (3.62), 288 (3.28), 332 (3.46). IR (KBr, v max, cm–1): 3452, 3069, 2970, 2865, 1612, 1550, 1479, 1363, 1145, 1070. For 1H and 13C NMR (400 and 100 MHz, CDCl3), see Table 1. ESI-MS (positive ion mode) m/z 271 [M + Na]+; HR-ESI-MS (positive ion mode) m/z 269.1162 [M + Na]+ (calcd for C15H18NaO3, 269.1154).
References
A. H. Aly, A. Debbab, and P. Proksch, Pharmazie, 68, 499 (2013).
J. F. Sanchez, A. D. Somoza, N. P. Keller, and C. C. C. Wang, Nat. Prod. Rep., 29, 351 (2012).
M. P. Sobolevskaya, E. V. Leshchenko, M. V. Pivkin, V. A. Denisenko, N. N. Slinkina, and S. S. Afiyatullov, Chem. Nat. Compd., 47, 796 (2011).
M. Zhou, G. Du, H. Y. Yang, C. F. Xia, J. X. Yang, Y. Q. Ye, X. M. Gao, X. N. Li, and Q. F. Hu, Planta Med., 81, 235 (2015).
Y. Q. Ye, C. F. Xia, J. X. Yang, Y. C. Yang, Y. Qin, X. M. Gao, G. Du, X. M. Li, and Q. F. Hu, B. Korean Chem. Soc., 35, 3059 (2014).
U. W. Hawas, A. A. El-Beih, and A. M. El-Halawany, Arch. Pharm. Res., 35, 1749 (2012).
N. Y. Ji, X. H. Liu, F. P. Miao, and M. F. Qiao, Org. Lett., 15, 2327 (2013).
R. X. Li, S. X. Chen, S. B. Niu, L. D. Guo, J. Yin, and Y. S. Che, Fitoterapia, 96, 88 (2014).
M. Zhou, J. Lou, Y. K. Li, Y. D. Wang, K. Zhou, B. K. Ji, W. Dong, X. M. Gao, G. Du, and Q. F. Hu, Arch. Pharm. Res., 38, 786 (2015).
S. L. Luo, X. J. Huang, Y. Wang, R. W. Jiang, L. Wang, L. L. Bai, Q, L. Peng, C. L. Song, D. M. Zhang, and W. C. Ye, Fitoterapia, 95, 115 (2014).
Y. Q. Ye, C. F. Xia, J. X. Yang, Y. C. Yang, X. M. Gao, G. Du, H. Y. Yang, Y. K. Li, and Q. F. Hu, Heterocycles, 89, 2369 (2014).
S. Z. Shang, W. X. Xu, L. Li, J. G. Tang, W. Zhao, P. Lei, M. M. Miao, H. D. Sun, J. X. Pu, Y. K. Chen, and G. Y. Yang, Phytochem. Lett., 11, 53 (2015).
K. Tianpanich, S. Prachya, S. Wiyakrutta, C. Mahidol, R. Somsak, and P. Kittakoop, J. Nat. Prod., 74, 79 (2011).
L. C. Di Stasi, D. Camuesco, A. Nieto, W. Vilegas, A. Zarzuelo, and J. Galvez, Planta Med., 70, 315 (2004).
M. Shibano, H. Naito, M. Taniguchi, N. H. Wang, and K. Baba, Chem. Pharm. Bull., 54, 717 (2006).
M. Zhou, K. Zhou, X. M. Gao, Z. Y. Jiang, J. J. Lv, Z. H. Liu, G. Y. Yang, M. M. Miao, C. T. Che, and Q. F. Hu, Org. Lett., 17, 2638 (2015).
Acknowledgment
This research was financially supported by the National Natural Science Foundation of China (Nos. 21462051, 31560099) and the Foundation of Yunnan Province (No. 2012BA015 and Category of Industry Guide No. 2014-01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2017, pp. 372–374.
Rights and permissions
About this article
Cite this article
Hu, QF., Xing, HH., Wang, YD. et al. Prenylated Isocoumarins from the Fermentation Products of the Endophytic Fungus Aspergillus versicolor and Their Anti-Tobacco Mosaic Virus Activities. Chem Nat Compd 53, 436–439 (2017). https://doi.org/10.1007/s10600-017-2017-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2017-0