A new phenolic compound, schitenoside C (1), and 23 known compounds (2–24), were isolated from Schizonepeta tenuifolia by repeated column chromatography. Their structures were assigned by spectroscopic data interpretation. Among them, compounds 2–5, 7, and 9–23 were isolated from this genus for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Schizonepeta tenuifolia (Benth.) Briq. belongs to the Labiatae family of an annual herbaceous plant and is widespread in China [1]. The whole plant has been used for cold, cough, and fever [2]. Phytochemical investigations of S. tenuifolia have led to the isolation of several kinds of chemical constituents, such as phenolic compounds and essential oils [3, 4]. In our recent research, 24 compounds were isolated from S. tenuifolia collected in Guizhou Province, P. R. China, in October 2008. Voucher specimens (No. SA080107) are deposited at the College of Bioscience and Biotechnology, Yangzhou University, China.
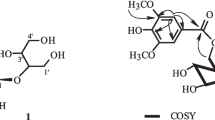
Compound 1 was isolated as a yellow powder (MeOH). It showed positive reaction to Molisch reagent and negative reaction to Turnbull's blue reagent [FeCl3–K3Fe(CN)6]. Compound 1 gave D-glucose after hydrolysis with acid or β-glucosidase. The structure of 1 was determined by 1D (1H, 13C) and 2D (HMQC, HMBC) NMR experiments (Fig. 1, Table 1). The 1H and 13C NMR spectroscopic data of 1 showed a quite similar pattern to those of 7, 1-O-β-D-glucopyranosyloxy-2-hydroxy-4-allylbenzene [5]. The 1H NMR spectrum of 1 indicated the presence of 1,2,4-trisubstituted benzene protons [δ 7.17 (1H, d, J = 8.2 Hz, H-6), 6.57 (1H, br.d, J = 8.2 Hz, H-5), 6.64 (1H, br.s, H-3)], a methylene proton [δ 3.25 (2H, d, J = 6.7 Hz, H-7)], terminal olefinic protons [δ 5.03 (1H, br.d, J = 17.0 Hz, H-9), 5.00 (1H, br.d, J = 10.4 Hz, H-9)], an olefinic proton [δ 5.92 (1H, m, H-8)], and signals characteristic of a glucopyranosyl residue. The coupling constant of the signal at δ 4.82 (1H, d, J = 7.9 Hz, H-1′), assignable to an anomeric proton of the glucose, indicated a β-configuration for the glucosidic linkage. Furthermore, the connectivities of the glucose unit were established based on HMBC cross-peaks indicating long-range 13C–1H couplings: the anomeric proton signal at δ 4.82 (H-1′) gave a strong cross-peak with a carbon signal at δ 145.5 (C-1). A methine proton signal at δ 4.30 (H-2′) showed a weak cross-peak with a carbon signal at δ 148.4 (C-2). Thus, compound 1 was determined to be schitenoside C. However, a paucity and instability of material prevented determination of the MS.
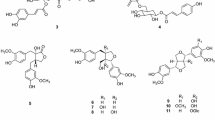
Compounds 2–23, by comparison with the published data, were identified as chrysoeriol 7-O-β-D-glucopyranoside (2) [6], diosmetin 7-O-β-D-glucopyranoside (3) [7], acacetin 7-O-rutinoside (4) [8], luteolin 3′,4′-dimethylether 7-O-rutinoside (5) [9], apigenin 7-O-β-D-glucopyranoside (6) [10], 1-O-β-D-glucopyranosyloxy-2-hydroxy-4-allylbenzene (7) [5], luteolin 7-O-β-D-glucopyranoside (8) [11], apigenin 7-O-rutinoside (9) [12], chrysoeriol 7-O-rutinoside (10) [13], luteolin 3′,4′-dimethylether 7-O-β-D-glucuronic acid methyl ester (11) [14], luteolin 7-O-rutinoside (12) [15], 3-O-feruloylquinic acid methyl ester (13) [16], 4-O-feruloylquinic acid methyl ester (14) [17], 3-methoxyl-4-hydroxycinnamic alcohol 9-O-β-D-glucopyranoside (15) [18], methylconiferin (16) [19], isosyringin (17) [20], vanillactic acid (18) [21], benzeneacetonitrile 7-O-β-D-glucopyranoside (19) [22], 3,9-dihydroxymegastigman-5-ene (20) [23], 12-O-β-D-glucopyranosyl oxyjasmonic acid methyl ester (21) [24], fructose (22) [25], adenosine (23) [26], and daucosterol (24) [27].
Experimental
The air-dried whole plants of S. tenuifolia (3.2 kg) were extracted with ethanol (95% v/v) three times, 2 h for each. The ethanol extract was concentrated under reduced pressure to yield a crude residue, which was then suspended in 8 L water and partitioned sequentially with petroleum ether, ethyl acetate, and n-butanol to give three portions. The n-butanol portion (83 g) was applied to the macroporous resin HPD 100 column (ethanol–water, 0:100–95:5) to yield two fractions (A, B). Fraction B (61 g) was chromatographed on silica gel CC (CHCl3–MeOH–H2O, 80:20:2–70:30:5–60:40:10) to provide eight fractions (Fr. 1–8).
Fraction 2 was subjected to Sephadex LH-20 (MeOH) to afford five subfractions (Subfr. 2.1–2.5). Subfraction 2.1 was repeatedly purified over Sephadex LH-20 (CHCl3–MeOH, 2:1) to yield compounds 2 (12 mg), 3 (11 mg), 4 (13 mg), 5 (12 mg), 8 (12 mg), and 11 (10 mg). Subfraction 2.2 was chromatographed over preparative thin-layer chromatography (PTLC) on silica gel (CHCl3–MeOH–H2O, 80:20:2) to give compound 6 (8 mg). Subfraction 2.5 was separated by silica gel CC (EtOAc–MeOH, 100:4–100:5–100:10–100:20–100:30) and then by Sephadex LH-20 (MeOH) to obtain compound 20 (7 mg). Fraction 4 was chromatographed over Sephadex LH-20 (MeOH) to yield compound 24 (15 mg). Fraction 5 was subjected to silica gel CC (EtOAc–MeOH–H2O, 18:1:1–10:1:1–7:1:1) to afford three subfractions (Subfr. 5.1–5.3). Subfraction 5.1 was separated by Sephadex LH-20 (MeOH) to yield compounds 9 (3 mg) and 10 (8 mg). Subfraction 5.2 was chromatographed over PTLC on silica gel (CHCl3–MeOH–H2O, 70:30:5) and purified by Sephadex LH-20 (MeOH) to yield compounds 7 (5 mg), 12 (4 mg), and 22 (6 mg). Fraction 6 was purified by Sephadex LH-20 (MeOH) several times to yield compounds 13 (12 mg) and 14 (14 mg). Fraction 7 was subjected to silica gel CC (CHCl3–MeOH, 100:6–100:10–100:20–100:30–100:50) to afford three subfractions (Subfr. 7.1–7.3). Subfraction 7.1 was purified by Sephadex LH-20 (MeOH) to give compound 18 (5 mg). Subfraction 7.2 was subjected to Sephadex LH-20 (MeOH) to yield compounds 1 (1 mg) and 15 (4 mg). Subfraction 7.3 was rechromatographed over Sephadex LH-20 (MeOH) to yield compounds 16 (7 mg), 17 (11 mg), 21 (3 mg), and 23 (8 mg). Fraction 8 was subjected to Sephadex LH-20 (MeOH) to yield compound 19 (9 mg).
Melting points were determined on a Yanaco MP-S3 melting point apparatus and are uncorrected. NMR spectra were measured on a Bruker ARX-600 NMR spectrometer using TMS as an internal standard. Silica gel (200–300 mesh) was from Qingdao Ocean Chemical Group Co. Ltd., P. R. China. TLC was conducted on HSGF254 precoated silica gel plates, 10–40 μm (Yantai Chemical Plant, Yantai, P. R. China). Sephadex LH-20 gel was from Pharmacia.
References
J. S. Yoo, D. K. Kim, S. H. Kim, and T. Y. Shin, Nat. Prod. Sci., 17, 239 (2011).
D. Fung and C. Lau, J. Clin. Pharmacol., 42, 30 (2002).
J. H. Shao, C. C. Zhao, R. Zhao, X. C. Wang, and B. B. Zhang, Biochem. Syst. Ecol., 51, 83 (2013).
J. Y. Yang and H. S. Lee, J. Agric. Food Chem., 61, 11511 (2013).
T. N. Ly, R. Yamauchi, M. Shimoyamada, and K. Kato, J. Agric. Food Chem., 50, 4919 (2002).
M. Olszewska and J. M. Roj, Phytochem. Lett., 4, 151 (2011).
A. F. Li, A. L. Sun, R. M. Liu, and Y. Q. Zhang, J. Chin. Med. Mat., 37, 428 (2014).
J. Zhang, Z. Q. Yin, P. Cao, Y. B. Li, and J. A. Duan, Chem. Nat. Compd., 44, 701 (2008).
M. Tripathi, L. Jain, and V. B. Pandey, Fitoterapia, 67, 477 (1996).
X. L. Ouyang, L. X. Wei, X. M. Fang, H. S. Wang, and Y. M. Pan, Chem. Nat. Compd., 49, 428 (2013).
Q. L. Liang and L. S. Ding, J. Chin. Pharm. Univ., 27, 205 (1996).
Y. Tian, X. Q. Liu, and J. X. Dong, Acta Pharm. Sin., 44, 496 (2009).
Y. Chen, H. Z. Deng, and L. Liang, J. Southern Med. Univ., 28, 858 (2008).
C. Souleles, J. Nat. Prod., 52, 1311 (1989).
J. Zhao, F. Xu, J. H. He, W. Tan, Z. Y. Gu, and L. Ma, J. Chin. Med. Mat., 36, 54 (2013).
Y. Z. Li, C. Ma, and J. Huang, Chin. Pharm. J., 44, 1294 (2009).
E. H. Dong, A. G. Wang, J. B. Yang, T. F. Ji, and Y. L. Su, J. Chin. Med. Mat., 35, 1441 (2012).
R. Guo, Y. H. Wang, Y. N. Shi, X. Y. Li, W. C. Li, and C. L. Long, Nat. Prod. Res. Dev., 24, 1007 (2012).
P. Huang, K. Gloria, and G. W. Peter, Nat. Prod. Res. Dev., 16, 403 (2004).
C. Yan, Y. Wang, and X. J. Hao, Chin. J. Chin. Mater. Med., 34, 2895 (2009).
L. Tang, X. F. Li, S. X. Yang, Y. Qiu, and K. Yuan, Chin. J. Chin. Mater. Med., 39, 2284 (2014).
H. J. Ye, H. Dai, L. X. Wu, Y. H. Guo, and W. X. Gu, J. Trop. Subtrop. Bot., 22, 307 (2014).
H. Achenbach, M. Lottes, R. Waibel, G. Karikas, A. M. D. Correa, and M. Gupta, Phytochemistry, 38, 1537 (1995).
T. Fujita, K. Terato, and M. Nakayama, Biosci. Biotechnol. Biochem., 60, 732 (1996).
L. J. Wu, Shiyong Youji Huahewu Guangpu Jiexi [M], People's Medical Publishing House, Bei Jing, 2009, p. 116.
H. X. Kuang, X. Yang, P. Xin, Z. B. Wang, Y. Su, B. Li, and Q. H. Wang, J. Chin. Med. Mat., 37, 621 (2014).
W. Li, Y. F. Chen, N. Wu, M. Y. Chi, and J. Fei, Zhong Cao Yao, 43, 1712 (2012).
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 31201563) and partially supported by the program for the Analytical Detective Center, Yangzhou University. We are also grateful to Prof. QiShi Sun for identification of the plant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2016, pp. 863–865.
Rights and permissions
About this article
Cite this article
Huang, XH., Chen, J., Xu, XQ. et al. A New Phenolic Compound from Schizonepeta tenuifolia . Chem Nat Compd 52, 1005–1007 (2016). https://doi.org/10.1007/s10600-016-1847-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-016-1847-5