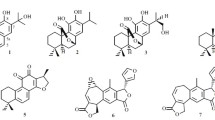
A new amide, beilschamide (1), has been isolated from the stem of Beilschmiedia erythrophloia, together with seven known compounds, N-trans-feruloyltyramine (2), N-trans-feruloyloctopamine (3), vanillin (4), α-tocopheryl quinone (5), β-sitostenone (6), β-sitosterol (7), and 6α-hydroxystigmast-4-en-3-one (8). The structure of the new compound 1 was determined through spectroscopic and MS analyses. N-trans-Feruloyloctopamine (3) and beilschamide (1) exhibited cytotoxic effects with IC50 values of 10.3 and 21.2 μg/mL, respectively, against CCRF-CEM cell line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Beilschmiedia erythrophloia Hay. (Lauraceae) is an evergreen tree, distributed throughout South Mainland China, Hainan Island, the Ryukyus, and Taiwan [1]. Alkaloids [2, 3], flavonoids [4], endiandric acids [5–7], lignans [8, 9], benzenoids [7, 8], steroids [6–9], triterpenoids [7, 9], fatty acid esters [7, 9], and their derivatives are widely distributed in plants of the genus Beilschmiedia. Many of these compounds exhibit diverse biological activities, including cytotoxic [8] and antitubercular [7, 9] activities. In a preliminary screening, the methanolic extract of the root of this species showed cytotoxic activities in vitro. The current phytochemical investigation of the stem of this plant has led to the isolation of a new amide, beilschamide (1), along with seven known compounds. The structural elucidation of 1 and its cytotoxic property are described herein.
Extensive fractionation of the EtOAc-soluble portion of a MeOH extract of leaves of Beilschmiedia erythrophloia using silica gel column chromatography (CC), MPLC, and preparative TLC afforded compounds 1–8.
Compound 1 was isolated as a yellowish amorphous powder. The HR-ESI-MS gave an [M + Na]+ ion at m/z 380.1470 (calcd for C20H23NO5Na, 380.1474), consistent with a molecular formula of C20H23NO5. IR absorptions for NH, OH (3355, 3265 cm–1), and carbonyl (1652 cm–1) functions were observed. The signals of the 1H and 13C NMR spectra of 1 were assigned to a 3,4-dimethoxyphenyl, a 4-hydroxyphenyl, a conjugated double bond, a methoxy, an oxymethine, and a methylene. Comparison of the 1H and 13C NMR data of 1 with those of 3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)-2-methoxyethyl]acrylamide [10] suggested that their structures were closely related, except for the 3,4-dimethoxyphenyl group [δH 3.87 (6H, s, 3, 4-OMe), 6.96 (1H, d, J = 8.5 Hz, H-5), 7.13 (1H, dd, J = 8.5, 2.0 Hz, H-6), 7.16 (1H, d, J = 2.0 Hz, H-2); δC 56.4 (3-OMe), 56.4 (4-OMe), 111.6 (C-2), 116.5 (C-5), 123.1 (C-6), 128.2 (C-1), 149.3 (C-4), 150.7 (C-3)] at C-7 of 1 replacing the 4-hydroxy-3-methoxyphenyl group of 3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)-2-methoxyethyl]acrylamide [10]. This was supported by HMBC correlations between 4-OMe (δH 3.87) and C-4 (δ 149.3) and NOESY correlations between 4-OMe (δH 3.87) and H-5 (δ 6.96). Compound 1 showed levorotary optical activity with [α] 25D –2.3° as in the case of (S)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)-2-methoxyethyl]acrylamide ([α] 23D –2.0°) [10], and the absolute configuration of C-11 in 1 has to be S [11, 12]. The full assignment of 1H and 13C NMR resonances was supported by 1H–1H COSY, DEPT, HSQC, NOESY, and HMBC (Fig. 1) spectral analyses. On the basis of the above data, the structure of 1 was elucidated as (S,E)-3-(3,4-dimethoxyphenyl)-N-(2-(4-hydroxyphenyl)-2-methoxyethyl)acrylamide, named beilschamide.
In this study, the cytotoxic effects of compounds isolated from the stems of B. erythrophloia were tested in vitro against CCRF-CEM (human lymphoblastic leukemia) cell line. Among the isolated compounds, N-trans-feruloyloctopamine (3) and beilschamide (1) were the most effective with IC50 values of 10.3 and 21.2 μg/mL, respectively, against CCRF-CEM cell line.
The known isolates were readily identified by a comparison of physical and spectroscopic data (UV, IR, 1H NMR, [α]D, and MS) with corresponding authentic samples or literature values, and this included two amides, N-trans-feruloyltyramine (2) [13] and N-trans-feruloyloctopamine (3) [14], a benzenoid, vanillin (4) [15], a benzoquinone, α-tocopheryl quinone (5) [16], and three steroids, β-sitostenone (6) [17], β-sitosterol (7) [18], and 6α-hydroxystigmast-4-en-3-one (8) [8].
Experimental
General Experimental Procedures. Optical rotations were measured using a Jasco DIP-370 polarimeter in CHCl3. Ultraviolet (UV) spectra were obtained on a Jasco UV-240 spectrophotometer. Infrared (IR) spectra (neat or KBr) were recorded on a PerkinElmer 2000 FT-IR spectrometer. Nuclear magnetic resonance (NMR) spectra, including correlation spectroscopy (COSY), nuclear Overhauser effect spectrometry (NOESY), heteronuclear multiple-bond correlation (HMBC), and heteronuclear single-quantum coherence (HSQC) experiments, were recorded on a Varian Inova 500 spectrometer operating at 500 MHz (1H) and 125 MHz (13C), with chemical shifts given in ppm (δ) using tetramethylsilane (TMS) as an internal standard. Electrospray ionization (ESI) and high-resolution electrospray ionization (HR-ESI)-mass spectra were recorded on a Bruker APEX II mass spectrometer. Silica gel (70–230, 230–400 mesh) (Merck) was used for column chromatography (CC). Silica gel 60 F-254 (Merck) was used for thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC).
Plant Material. The stems of B. erythrophloia were collected from Mudan, Pingtung County, Taiwan, in February 2005, and a voucher specimen (Chen 1187) was deposited in the Herbarium of the School of Pharmacy, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan, Republic of China.
Extraction and Separation of Compounds. The dried stems (0.9 kg) of B. erythrophloia were extracted three times with MeOH at room temperature. The methanol extract (82 g) was partitioned between EtOAc and H2O (1:1) to afford EtOAc-soluble (fraction A, 38 g) and H2O-soluble (fraction B, 41 g) fractions. The EtOAc-soluble fraction (38 g) was chromatographed on silica gel (70–230 mesh, 3.1 kg), eluted with CH2Cl2, gradually increasing the polarity with MeOH to give eight fractions (A1–A8). Fraction A2 (4.8 g) was separated by column chromatography on silica gel (230–400 mesh, 220 g), eluted with CH2Cl2–MeOH (12:1–3:1) to yield nine fractions (A2-1–A2-9). Part (132 mg) of fraction A2-3 was purified by preparative TLC (silica gel, CH2Cl2–acetone, 8:1) to afford vanillin (4) (4.5 mg). Part (125 mg) of fraction A2-7 was purified by preparative TLC (silica gel, CH2Cl2–EtOAc, 5:1) to afford α-tocopheryl quinone (5) (4.0 mg). Fraction A4 (4.1 g) was separated by column chromatography on silica gel (230–400 mesh, 185 g), eluted with CHCl3–MeOH (10:1–1:1) to yield eight fractions (A4-1–A4-8). Part (128 mg) of fraction A4-2 was purified by preparative TLC (silica gel, n-hexane–acetone, 3:1) to obtain β-sitostenone (6) (6.5 mg). Fraction A4-6 (320 mg) was separated by MPLC (silica gel column, CH2Cl2–MeOH, 9:1–0:1) to give eight fractions (each 100 mL, A4-6-1–A4-6-8). Fraction A4-6-4 (78 mg) was purified by preparative TLC (silica gel, CHCl3–acetone, 8:1) to provide β-sitosterol (7) (7.2 mg). Part (115 mg) of fraction A4-7 was purified by preparative TLC (silica gel, n-hexane–EtOAc, 6:1) to obtain 6α-hydroxystigmast-4-en-3-one (8) (5.4 mg). Fraction A7 (3.5 g) was separated by column chromatography on silica gel (230–400 mesh, 172 g), eluted with CH2Cl2–MeOH (6:1–0:1) to yield eight fractions (A7-1–A7-8). Fraction A7-3 (280 mg) was separated by MPLC (silica gel column, CHCl3–MeOH, 7:1–0:1) to give nine fractions (each 90 mL, A7-3-1–A7-3-9). A7-3-6 (38 mg) was purified by preparative TLC (silica gel, CHCl3–MeOH, 5:1) to give beilschamide (1) (3.3 mg). Fraction A7-3-9 was purified by preparative TLC (silica gel, CHCl3–MeOH, 4:1) to give N-trans-feruloyltyramine (2) (7.5 mg). Part (108 mg) of fraction A7-7 was purified by preparative TLC (silica gel, CHCl3–MeOH, 2:1) to give N-trans-feruloyloctopamine (3) (4.2 mg).
Beilschamide [( S , E )-3-(3,4-dimethoxyphenyl)- N -(2-(4-hydroxyphenyl)-2-methoxyethyl)acrylamide)] (1). Yellowish amorphous powder. [α] 25D –2.3° (c 0.25, MeOH). UV (MeOH, λmax, nm): 220 (4.42), 294 (4.35), 317 (4.41). IR (KBr, νmax, cm–1): 3355, 3265 (NH, OH), 1652 (C=O). 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 3.21 (3H, s, 11-OMe), 3.41 (1H, dd, J = 14.0, 8.0, H-10), 3.51 (1H, dd, J = 14.0, 4.5, H-10), 3.87 (6H, s, 3, 4-OMe), 4.25 (1H, dd, J = 8.0, 4.5, H-11), 6.47 (1H, d, J = 16.0, H-8), 6.80 (2H, d, J = 8.4, H-14, 16), 6.96 (1H, d, J = 8.5, H-5), 7.13 (1H, dd, J = 8.5, 2.0, H-6), 7.16 (1H, d, J = 2.0, H-2), 7.17 (2H, d, J = 8.4, H-13, 17), 7.46 (1H, d, J = 16.0, H-7). 13C NMR (125 MHz, CD3OD, δ, ppm): 47.0 (C-10), 56.4 (3-OMe), 56.4 (4-OMe), 56.8 (11-OMe), 82.3 (C-11), 111.6 (C-2), 116.2 (C-14), 116.2 (C-16), 116.5 (C-5), 118.7 (C-8), 123.1 (C-6), 128.2 (C-1), 129.2 (C-13), 129.2 (C-17), 131.5 (C-12), 142.2 (C-7), 149.3 (C-4), 150.7 (C-3), 158.4 (C-15), 169.3 (C-9). ESI-MS m/z 380 [M + Na]+. HR-ESI-MS m/z 380.1470 [M + Na]+ (calcd for C20H23NO5Na, 380.1474).
Cytotoxic Assay. Cytotoxic activities of compounds against CCRF-CEM (human lymphoblastic leukemia) were assayed by a modification of the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method [19]. To measure the cytotoxic activities of the purified compounds against the above tumor cells, each cell line was initiated at 5 × 105 cells/well in 96-well microtiter plates (Falcon). Eight concentrations (triplicate) of the test compounds (dissolved in 0.5% DMSO) encompassing a 128-fold range were added to each cell line. Each tumor cell was enumerated using MTT (Sigma) after exposure to the test compounds for 3 days. MTT (15 μL, 1 mg/mL) was added to each well, and plates were incubated at 37°C for a further 4 h. Formazan crystals were redissolved in DMSO (Merck) for 10 min with shaking, and the plate was read immediately on a microtiter plate reader (Dynatech) at a wavelength of 570 nm. The IC50 value was defined as the concentration of the test compound necessary to inhibit the growth to 50% of the control in the MTT assay. The anticancer agent doxorubicin and 0.5% DMSO were used as the positive control and solvent control, respectively. The assays were repeated three times.
References
J. C. Liao, Lauraceae in Flora of Taiwan, 2nd Ed., Vol. 2, Editorial Committee of the Flora of Taiwan, Taipei, Taiwan, 1996, p. 433–499.
P. S. Clezy, E. Gellert, D. Y. K. Lau, and A. W. Nichol, Aust. J. Chem., 19, 135 (1966).
E. Tillequin and M. Koch, Heterocycles, 23, 1357 (1985).
J. B. Harborne and J. Mendez, Phytochemistry, 8, 763 (1969).
J. E. Banfield, D. S. C. Black, D. J. Collins, B. M. P. Hyland, and J. J. Lee, Aust. J. Chem., 47, 587 (1994).
P. S. Yang, M. J. Cheng, J. J. Chen, and I. S. Chen, Helv. Chim. Acta, 91, 2130 (2008).
P. S. Yang, M. J. Cheng, C. F. Peng, J. J. Chen, and I. S. Chen, J. Nat. Prod., 72, 53 (2009).
J. J. Chen, E. T. Chou, C. Y. Duh, S. Z. Yang, and I. S. Chen, Planta Med., 72, 351 (2006).
J. J. Chen, E. T. Chou, C. F. Peng, I. S. Chen, S. Z. Yang, and H. Y. Huang, Planta Med., 73, 567 (2007).
C. H. Lee, J. H. Kim, H. J. Lee, S. M. Lee, and Y. H. Kho, J. Nat. Prod., 64, 659 (2001).
A. J. M. Janssen, A. J. H. Klunder, and B. Zwanenburg, Tetrahedron, 47, 7645 (1991).
H. Huang, Q. R. Chao, R. X. Tan, H. D. Sun, D. C. Wang, J. Ma, and S. X. Zhao, Planta Med., 65, 92 (1999).
C. Y. Chen, Y. D. Wang, and H. M. Wang, Chem. Nat. Compd., 46, 448 (2010).
M. DellaGreca, L. Previtera, R. Purcaro, and A. Zarrelli, Tetrahedron, 62, 2877 (2006).
T. H. Chou, I. S. Chen, P. J. Sung, C. F Peng, P. C. Shieh, and J. J. Chen, Chem. Biodivers., 4, 1594 (2007).
C. W. Ting, T. L. Hwang, I. S. Chen, M. H. Yen, and J. J. Chen, Chem. Biodivers., 9, 99 (2012).
J. J. Chen, S. S. Huang, C. H. Liao, D. C. Wei, P. J. Sung, T. C. Wang, and M. J. Cheng, Food Chem., 120, 379 (2010).
J. J. Chen, W. J. Lin, P. C. Shieh, I. S. Chen, C. F. Peng, and P. J. Sung, Chem. Biodivers., 7, 717 (2010).
T. Mosmann, J. Immunol. Methods, 65, 55 (1983).
Acknowledgment
This research was supported by Grants from the National Science Council of the Republic of China (No. NSC 98-2320-B-127-001-MY3 and NSC 101-2320-B-127-001-MY3), awarded to Prof. J.-J. Chen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2015, pp. 266–268.
Rights and permissions
About this article
Cite this article
Chen, JJ., Kuo, WL., Sung, PJ. et al. Beilschamide, a New Amide, and Cytotoxic Constituents of Beilschmiedia erythrophloia . Chem Nat Compd 51, 302–305 (2015). https://doi.org/10.1007/s10600-015-1265-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1265-0