One new furanoid, elevafuranone (1), along with six known compounds constisting of three flavonoids [7-O-methylnaringenin (2), pinocembrin (3), and (–)-epicatechin (4)] and three alphatics [β-sitosterol (5), methyl linoleate (6), and 2,3-dihydroxypropyl palmitate (7)], were isolated from an endophytic fungus Annulohypoxylon elevatidiscus BCRC 34014. Their structures were determined based on their 1D and 2D NMR and HR-ESI-MS spectral data, as well as comparison with previous literature data. All compounds were found for the first time in this species. It is worth mentioning that this is the first study of secondary metabolites isolated from this fungus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
An endophyte refers to a bacterial (including actinomycete) or fungal microorganism that colonizes interior organs of plants but does not have pathogenic effects on its hosts [1, 2]. Recently, endophytes have been recognized as important sources of a variety of structurally novel and biologically active secondary metabolites, including terpenoids, steroids, alkaloids, and isocoumarin derivatives [3–9]. An endophytic fungal strain was isolated from decorticated woods collected from Lan-Yu, Tai-tung County, Taiwan, on August of 2001. This strain was determined to be Annulohypoxylon elevatidiscus (Xylariaceae) based on the cultural and anamorphic data [10, 11]. The Xylariaceae (Xylariales, Ascomycotina) constitutes a large family of fungi, with about 45 genera. Secondary metabolites isolated from the representatives from at least one-third of these genera have now been identified [3]. Members of the Xylariaceae are nearly ubiquitous, and occasionally dominant, endophytic inhabitants of living plants and appear to be the major representatives in most tropical plants that have been studied. The genus Xylaria, in particular, is commonly represented. All sorts of structurally diversified metabolites are widely distributed in Xylaria sp. [3–9]. Thus, contrary to information in the literature, the secondary metabolites of the genus Annulohypoxylon have received less attention than Xylaria species.
In the course of screening for bioactive novel metabolites from Formosan fungal materials, Annulohypoxylon elevatidiscus was chosen for further investigation. This is also being studied and published for the first time. The 95% EtOH extract prepared from rice fermented with the titled fungus A. elevatidiscus was partitioned between n-BuOH and H2O. One new furanoid, named elevafuranone (1), together with six known constituents (2–7), were isolated from the n-BuOH-soluble fraction of the A. elevatidiscus BCRC 34014. Compounds 2–7 were found for the first time from this species. The structures of the isolated metabolites were determined by extensive analysis of their spectroscopic data as well as by comparison with literature reports.
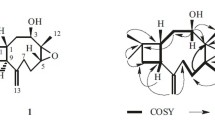
Compound 1, obtained as a colorless powder, has the molecular formula C23H42O3, as determined by HR-ESI-MS data (m/z 389.3035 [M + Na]+; calcd 389.3032) in combination with its 1H NMR, 13C NMR, and DEPT (Table 1), requiring three degrees of unsaturation. The IR spectrum displayed the presence of hydroxyl (3420 cm–1) and one conjugated carbonyl C=O (1715 cm–1) group. The latter was confirmed by the 13C NMR spectrum at δ 207.0. Detailed analysis of the 1D and 2D NMR spectra revealed that 1 was a furanoid derivative [12, 13], very similar to monascuskaoliaone [13], except that one hexadecyl unit [δH 0.89 (3H, t, J = 6.9 Hz, CH3-23), 1.20–1.27 (28H, br.s, CH2-9–22), and 1.72–1.75 (2H, m, CH2-8)] in 1 was replaced by a decyl moiety in the C-2 position in monascuskaoliaone [13]. The C=O group was also located at C-3 according to the HMBC correlations between the C=O group (δC 207.0) and both 2-CH3 (δH 1.33) and H-4 (δH 5.47). The other key correlations of HMBC are illustrated in Fig. 1. The configuration of C-2 remains to be determined due to the small quantity of isolated material and instability of 1. From the above data, compound 1 was characterized as 2-hexadecyl-5-(2-hydroxyethyl)-2-methylfuran-3(2H)-one, named elevafuranone, and its structure was illustrated as 1, which was further confirmed by COSY (Fig. 1 a), HSQC, HMBC (Fig. 1 b), and NOESY (Fig. 1 a) experiments. The fact that the observed optical rotation ([α] 25D value) is (±)–0 suggests that the natural product might have been isolated in racemic form.
The other known isolates, 7-O-methylnaringenin (2) [14], pinocembrin (3) [15], (–)-epicatechin (4) [16], β-sitosterol (5) [17], methyl linoleate (6) [18], and 2,3-dihydroxypropyl palmitate (7) [19], were readily identified by comparison of their spectral data (UV, IR, 1H NMR, MS) with data from the literature.
In conclusions, the chemical characteristics, as well as the biological activities, of many Annulohypoxylon metabolites remain unclear. Thus, the different biological activities of other Annulohypoxylon species and related diverse constituents are worth further research.
Experimental
General Experimental Procedures. Optical rotations were measured on a Jasco P-1020 digital polarimeter, UV spectra were obtained on a Jasco UV-240 spectrophotometer in MeOH, and IR spectra (KBr or neat) were taken on a PerkinElmer System 2000 FT-IR spectrometer. 1D (1H, 13C, DEPT) and 2D (COSY, NOESY, HSQC, HMBC) NMR spectra using CDCl3 as solvent were recorded on a Varian Mercury-400 (400 MHz for 1H NMR, 100 MHz for 13C NMR) spectrometer. Chemical shifts were internally referenced to the solvent signals in CDCl3 (1H, δ 7.26; 13C, δ 77.0) with TMS as the internal standard. Low-resolution ESI-MS spectra were obtained on an API 3000 spectrometer (Applied Biosystems), and high-resolution ESI-MS spectra on a Bruker Daltonics APEX II 30e spectrometer. Low-resolution EI-MS spectra were recorded on a Quattro GC/MS spectrometer having a direct inlet system. Silica gel (70–230, 230–400 mesh) (Merck) was used for column chromatography, and silica gel 60 F-254 (Merck) was used for TLC and preparative TLC.
Fungus Material. Annulohypoxylon elevatidiscus was used throughout this study, and specimens (BCRC 34014) were deposited at the Bioresource Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (FIRDI). A. elevatidiscus was maintained on potato dextrose agar (PDA) and the strain was cultured on potato dextrose agar slants at 25°C for 7 days and the spores harvested by sterile water. The spores (5 × 105) were seeded into 300 mL shake flasks containing 50 mL RGY medium (3% rice starch, 5.6% glycerol, 1.3% polypeptone, 3.2% soybean powder, 0.1% MgSO4, 0.2% NaNO3), and cultivated with shaking (150 rpm) at 25°C for 6 days. After the mycelium enrichment step, an inoculum mixing 100 mL mycelium broth and 100 ml RGY medium was inoculated into plastic boxes (25 cm × 30 cm) containing 2.3 kg sterile rice and cultivated at 25°C to produce the rice, and above-mentioned RGY medium was added to maintain the growth. After 14 days of cultivation, the rice was harvested and used as a sample for further extraction.
Extraction and Separation of Compounds. Dried rice of A. elevatidiscus (2.3 kg) was extracted three times with 95% EtOH at room temperature, and an ethanolic extract was obtained upon concentration under reduced pressure. The EtOH extract, suspended in H2O, was partitioned with n-BuOH to give fractions soluble in n-BuOH (2.4 g) and H2O. The n-BuOH-soluble fraction (2.4 g) was chromatographed over silica gel (60 g, 70–230 mesh) using CH2Cl2–acetone mixtures as eluents to produce five fractions (Fractions 1–5). Fraction 1 (112 mg) was subjected to silica gel chromatography by eluting with n-hexane–EtOAc gradient and enriched with EtOAc to furnish eight fractions (1-1–1-8). Fraction 1-2 (18 mg) was subjected to silica gel chromatography and eluted with CHCl3–EtOAc (8:1), then enriched gradually with EtOAc to obtain methyl linoleate (6) (3.1 mg). Fraction 1-4 (15 mg) was further purified on a silica gel column using n-hexane–acetone (5:1) to obtain 2,3-dihydroxypropyl palmitate (7) (2.8 mg). Fraction 1-7 (45 mg) was further purified on a silica gel column using n-hexane–acetone (5:1) to obtain elevafuranone (1) (2.8 mg). Fraction 3 (425 mg) was subjected to silica gel chromatography by eluting with CH2Cl2–EtOAc (8:1), then enriched with EtOAc to furnish five fractions (3-1–3-5). Fraction 3-2 (19 mg) was further applied on a silica gel column using CH2Cl2–acetone mixtures to obtain 7-O-methylnaringenin (2) (1.8 mg). Fraction 4 (240 mg) was chromatographed over silica gel using CH2Cl2–acetone mixtures as eluents to produce five fractions (4-1–4-5). Fraction 4-1 (86 mg) was subjected to silica gel chromatography by eluting with CH2Cl2–EtOAc (2:1), then enriched gradually with EtOAc to furnish four fractions (4-1-1–4-1-4). Fraction 4-1-3 (36 mg) was further purified on a silica gel column using CH2Cl2–EtOAc (2:1) to obtain pinocembrin (3) (2.7 mg) and β-sitosterol (5) (1.7 mg). Fraction 5 (394 mg) was subjected to silica gel chromatography by eluting with CH2Cl2–MeOH (15:1), then enriched gradually with MeOH to obtain five fractions (5-1–5-5). Fraction 5-1 (74 mg) was further purified on a silica gel column using CH2Cl2–MeOH (5:1→1:1) to obtain (–)-epicatechin (4) (3.2 mg).
Elevafuranone (1). Colorless powder; [α] 25D ±0° (c 0.035, CHCl3). UV (MeOH, nm): 226 (3.89). IR (neat, cm–1): 3420, 1715. ESI-MS m/z 389 [M + Na]+; HR-ESI-MS m/z 389.3035 [M + Na]+ (calcd for C23H42O3Na, 389.3032). 1H and 13C NMR spectra are shown in Table 1.
References
C. Lu and Y. Shen, J. Antibiot., 56, 415 (2003).
R. X. Tan and W. X. Zou, Nat. Prod. Rep., 18, 448 (2001).
A. J. S. Whalley and R. L. Edwards, Can. J. Bot., 73, 802 (1995).
P. C. Healy, A. Hocking, N. Tran-Dinh, J. I. Pitt, R. G. Shivas, J. K. Mitchell, M. Kotiw, and R. A. Davis, Phytochemistry, 65, 2373 (2004).
S. J. Coval, M. S. Puar, D.W. Phife, J. S. Terracciano, and M. Patel, J. Antibiot., 48, 1171 (1995).
H. Huang, Z. She, Y. Lin, L. L. P. Vrijmoed, and W. Lin, J. Nat. Prod., 70, 1696 (2007).
H. Jayasuriya, K. B. Herath, J. G. Ondeyka, J. D. Polishook, G. F. Bills, A. W. Dombrowski, M. S. Springer, S. Siciliano, L. Malkowitz, M. Sanchez, Z. Guan, S. Tiwari, D. W. Stevenson, R. P. Borris, and S. B. Singh, J. Nat. Prod., 67, 1036 (2004).
X. Y. Wu, X. H. Liu, G. C. Jiang, Y. C. Lin, W. Chan, and L. L. P. Vrijmoed, Chem. Nat. Compd., 41, 27 (2005).
R. A. Davis and G. K. Pierens, Magn. Reson. Chem., 44, 966 (2006).
H. M. Hsieh and Y. M. Ju, Mycology, 97, 844 (2005).
Y. M. Ju and J. D. Rogers, Mycotaxon, 73, 371 (1999).
J. He, E. M. K. Wijeratne, B. P. Bashyal, J. Zhan, C. J. Seliga, M. X. Liu, E. E. Pierson, L. S. Pierson, H. D. VanEtten, and A. A. L. Gunatilaka, J. Nat. Prod., 67, 1985 (2004).
M. J. Cheng, M. D. Wu, P. S. Yang, J. J. Chen, I. S. Chen, Y. L. Chen, and G. F. Yuan, J. Chil. Chem. Soc., 55, 107 (2010).
K. Kojima, P. Gombosurengyin, P. Ondognyi, D. Begzsurengyin, O. Zevgeegyin, K. Hatano, and Y. Ogihara, Phytochemistry, 44, 711 (1997).
H. Fukui, K. Goto, and M. Tabata, Chem. Pharm. Bull., 36, 4174 (1988).
L. J. Porter, Z. Ma, and B. G. Chan, Phytochemistry, 30, 1657 (1991).
M. C. Lin and T. D. Lin, J. Chin. Chem. Soc., 22, 167 (1975).
K. R. Markham, K. A. Mitchell, A. L. Wilkins, J. A. Daldy, and Y. Lu, Phytochemistry, 42, 205 (1996).
M. Rejzek, M. Vacek, and Z. Wimmer, Helv. Chim. Acta, 83, 2756 (2000).
Acknowledgment
This investigation was supported by a grant from the Ministry of Economic Affairs of the Republic of China. The authors also thank Senior Technician Mrs. Chyi-Jia Wang of the Center for Resources, Research, and Development (CRRD) of Kaohsiung Medical University for measuring the 2D NMR data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2015, pp. 60–62.
Rights and permissions
About this article
Cite this article
Cheng, MJ., Wu, MD., Cheng, YC. et al. Metabolites Isolated from an Endophytic Fungus of Annulohypoxylon elevatidiscus . Chem Nat Compd 51, 67–70 (2015). https://doi.org/10.1007/s10600-015-1205-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1205-z