A new flavonoid, 5,7-dihydroxy-2-(1-hydroxy-2,6-dimethoxy-4-oxo-cyclohex)-chromen-4-one (1), was isolated from the roots of Macrothelypteris torresiana (Gaud.) Ching. (Thelypteridaceae). The structure of the product was identified on the basis of detailed spectral analysis, including X-ray structure analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Macrothelypteris torresiana (Gaud.) Ching (Thelypteridaceae), which is widely distributed in the south of China [1], is used in folk medicine for clearing away heat and toxic material. In previous studies, several cytotoxic flavonoids and flavonoid glycosides were found from this species, and their multiple bioactivities (leading to the term bioflavonoid) and medicinal significance have been summarized. For instance, the unique flavonoid protoapigenone showed potent cytotoxic activity against HepG2 and Hep3B (liver), MDA-MB-231 and MCF-7 (breast), and A549 (lung) human cancer cell [2–4]. Recently, we have embarked on a study of the root of M. torresiana and have isolated a new compound, 5,7-dihydroxy-2-(1hydroxy-2,6-dimethoxy-4-oxo-cyclohex)-chromen-4-one (1), which is a flavonoid with a special B-ring. Its structure was elucidated by analysis of spectroscopic data and X-ray diffraction analysis.
The air-dried roots of M. torresiana (4 kg) were extracted with methanol (40 L) at room temperature. The methanolic extract was suspended in H2O and partitioned with CHCl3, EtOAc, and n-BuOH, successively. The EtOAc extract (25 g) was subjected to silica gel column (1.0 kg) chromatography (CHCl3–MeOH, 10:1, 8:1, 6:1, 4:1) to give five fractions I–V. Fraction I was subjected to silica gel column chromatography (CHCl3–MeOH) repeatedly to yield compound 1 (40 mg).
Compound 1 is a colorless solitary crystal. The HR-ESI-MS yielded a pseudomolecular ion peak at m/z 351.1117 [M + 1]+ (calcd for [C17H18O8 + H]+, 351.1080), which was consistent with the molecular formula of C17H18O8. UV (MeOH, A, nm): 232, 250, 299 nm; IR bands (KBr, v, cm–1): 3401, 1710, 1660, 1619, 1581, 1507, 1454. The UV and IR spectra of max1 indicated a typical chromen-4-one core [5]. In the 1H NMR spectrum, meta-coupled aromatic protons at I 6.21 (1H, d, J = 2.0 Hz, H-8) and 6.04 (1H, d, J = 2.0 Hz, H-6) and olefinic protons at I 6.39 (1H, s, H-3) indicated a 5,7-dihydroxychromone moiety. The 13C NMR data of 1 was similar to the reported compound, 5,7-dihydroxy-2-(1-hydroxy-6-methoxy-4-oxo-cyclohex-2-enyl)-chromen-4-one [2], but did not show the presence of a double band in the B-ring. Meanwhile, the 1H NMR and 13CNMR clearly showed the presence of two additional OCH3 (C-7′ and C-8′). For 1H and 13C NMR, see Table 1.
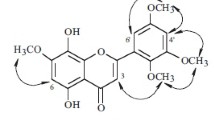
HSQC and HMBC experiments allowed us to define the molecular connectivity. The connections of protons and carbons were unambiguously assigned by the HSQC experiment (see Table 1). The location of the B-ring at C-2 was confirmed by the HMBC correlation of H-3 and C-1′. Cross signals between H-7′ (I 2.99, 3H, s) and C-2′ (I 81.8), between H-8′ (I 3.18, 3H, s) and C-6′ (I 75.4) in the HMBC spectrum were observed, indicating that the two OCH3 could be located at C-2′ and C-6′, respectively. The C-C interconnectivity of all the fragments was established through the response in the HMBC spectrum and the correlations of C-2′ with H-7′ and H-3′, of C-6′ with H-8′, H-5′, and H-2′, and of C-2 with H-2′ (see Fig. 1).
This assumption was subsequently confirmed by X-ray diffraction experiments (see Fig. 2). Thus, the structure of compound 1 was concluded to be 5,7-dihydroxy-2-(1-hydroxy-2,6-dimethoxy-4-oxo-cyclohex)-chromen-4-one.
Experimental
General Methods. UV and IR spectra were recorded on a Shimadzu UV-260 and a Bruker Vertex 70 spectrophotometer. NMR spectra were obtained on a Bruker AM-400 spectrometer using TMS as the internal standard. HR-ESI-MS was measured on an Acquity UPLC-Q-Tof Micro MS instrument. Silica gel plates (GF254) were used for TLC, and silica gel (200–300 mesh and 300–400 mesh) was used for column chromatography. The X-ray data were generated on a Smart Apex CCD system diffractometer.
Plant Material
The roots of M. torresiana were collected in July 2007 from a county of Hubei Province, People's Republic of China, and identified by Prof. Changgong Zhang, College of Pharmacy, Huangzhong University of Science and Technology. A specimen (No. 20070721) was deposited in the College of Pharmacy, Tongji Medical Center, Huangzhong University of Science and Technology.
X-ray Structure Analysis. A colorless single crystal obtained from methanolic solution was measured on a Smart Apex CCD system diffractometer. Crystal data for 1: C17H18O8, monoclinic, C2/c, a = 17.5274 (4) Å, b = 13.3591 (4) Å, c = 14.9103 (4) Å, α = 90°, β = 94.3420 (10)°, γ = 90°, V = 3481.23 (16) A, Z = 2, Dcalcd = 1.406 mg/m3, μ = 0.115 mm–1, F (000) = 1552.
References
The Institute of Botany, Chinese Academy of Sciences, Chinese Flora, Chapter 1, Vol. 4, Science Publisher, Beijing, 1999, pp. 79–80.
A. S. Lin, F. R. Chang, C. C. Wu, C. C. Liaw, and Y. C. Wu, Planta Med., 71, 867 (2005).
A. S. Lin, F. R. Chang, H. F. Yen, H. Bjorkeborn, P. Norlen, and Y. C. Wu, Chem. Pharm. Bull., 55, 635 (2007).
A. S. Lin, N. G. Kyoko, F. R. Chang, D. L. Yu, S. L. M. Natschke, C. C. Wu, S. L. Chen, Y. C. Wu, and K. H. Lee, J. Med. Chem., 50, 3921 (2007).
H. Wada, H. Fujita, T. Murakami, Y. Saiki, and C. M. Chen, Chem. Pharm. Bull., 35, 4757 (1987).
Acknowledgment
The author is grateful to the Analytical and Testing Center of Huazhong University of Science and Technology for measuring the NMR spectrum, and to the Department of Chemistry, Central China Normal University for X-ray structure analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, pp. 175–176, March–April, 2010. Original article submitted October 29, 2008.
Rights and permissions
About this article
Cite this article
Tang, Y., Xiong, C., Zhou, D. et al. A new flavonoid from Macrothelypteris torresiana . Chem Nat Compd 46, 209–211 (2010). https://doi.org/10.1007/s10600-010-9570-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-010-9570-0