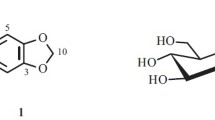
Phytochemical investigation of the methanolic extract of the seeds of Butea monosperma (Lam.) Taub. (Fabaceae) led to the isolation of three higher fatty acids, viz., n-docosanoic, n-octacosanoic, and n-dotriacontanoic acids, together with /-sitosterol xyloside and a zingiberene diglucoside, characterized as 3,4-dihydrozingiberen-14-ol-β-D-glucopyranosyl-(1→4)-/-D-glucopyranoside. All these phytoconstituents were reported from the seeds of B. monosperma for the first time and their structures were established on the basis of spectral data analysis and chemical reactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Butea monosperma (Lam.) Taub. (Fabaceae), commonly known as “calash” in Hindi, is found in the greater part of India and Burma, up to 1000 m, and higher in the outer Himalayas, Khandesh Akrani up to 1200 m, and a hill in south India up to 1300 m [1]. The bark possesses antitumor and antiulcer properties [2] and antifungal activity [3, 4]. The root bark is used as an aphrodisiac, analgesic, and anthelmintic [2]. The flowers are used for the treatment of liver disorders [5] and the seeds are anthelmintic [6].
Flavonoids from the flowers [7] and stem bark [4] and nitrogenous compounds from the seeds [8, 9] have been reported earlier. This paper describes the isolation and structural elucidation of the phytoconstituents of the seeds of B. monosperma.
The air-dried seeds of B. monosperma were extracted with methanol, and the viscous dark green mass was adsorbed on silica gel (60–120 mesh) for preparation of slurry. The slurry was chromatographed over a silica gel column packed in petroleum ether and eluted successively with a mixture of chloroform and methanol (99:1, 98:2, 96:4, 95:5, 97:3, 9:1), yielding pure compounds 1–5.
Compound 1, 2, and 5 are known phytoconstituents, and their structures are characterized as n-docosanoic, n-octacosanoic, and n-dotriacontanoic acids, respectively, on the basis of spectral data analysis and chemical reactions.

Compound 3, named / -sitosterol xyloside, gave a positive Liebermann-Burchard test for sterols and a negative Ehrlich reaction. Its IR spectrum exhibited strong absorption bands at 3450 and 1080 cm–1 characteristic of a glycoside and at 1610 cm–1 for unsaturation. The mass spectrum of 3 showed a molecular ion peak at m/z 546 corresponding to the molecular formula of a steroidal glycoside, C34H58O5. It gave fragment ions at m/z 413 [M–xylose]+, 398 [413–Me]+, 396 [576–C6H12O6]+, 381 [396–Me]+, 273 [413–C10H21, side chain], 255 [273–H2O], 240 [255–Me]+, and 213 [255–ring D fission]+ which were characteristic of / -sitosterol-3-/ -D-glycoside. The 1H NMR spectrum of 3 exhibited a one-proton doublet at 8 5.33 (J = 5.21 Hz) assigned to C-6 vinylic protons. A one-proton doublet at 4.90 (J = 9.61 Hz) was ascribed to anomeric proton H-1' . A one-proton doublet at 8 4.24 with coupling constant 7.2 Hz was ascribed to H-2' . Two doublet signals at 8 3.05 (J = 10.8 Hz) and 8 3.01 (J = 10.8 Hz), both integrated for one proton each, were attributed to oxygenated methylene protons H-5' a and H-5' b. The remaining carbinol protons of the sugar units appeared as multiplets at 8 4.05 (1H) and 8 3.45 (1H). A one-proton broad multiplet at 8 3.34 with W1/2 = 16.5 Hz showed the presence of a 3a -methine proton (axial) interacting with C-2 equatorial, C-2 axial, C-4 axial, and C-4 equatorial protons. Four doublet at 8 0.91 (J = 6.5 Hz), 0.82 (J = 5.69 Hz), 0.80 (J = 6.39 Hz), and 0.89 (J = 7.0 Hz); integrating for three protons each, were ascribed correspondingly to C-21, C-26, and C-27 secondary methyl and C-29 primary methyl protons. The C-18 and C-19 tertiary methyl protons appeared as broad signals at 8 0.65 and 0.95, respectively. The 13C NMR spectrum of 3 displayed 34 carbon signals for a steroidal glycoside, including two vinylic carbons at 8 139.95 (C-5) and 121.35 (C-6) and one carbinol carbon at 8 76.57, as well as anomeric carbons at 8 101.23 (C-1' ) and a sugar carbon between 8 73.54–61.17. The 1H NMR and 13C NMR spectral data of the steroidal skeleton of 3 were compared with the related steroids [10–12]. Acid hydrolysis of 3 yielded xylose and an aglycone that was identified as / -sitosterol by spectral data and by direct comparison with the authentic samples (co-TLC, mp). On the basis of these findings the structure of 3 was established as stigmast-5-en-3-O-/ -D-xylopyranoside. It is a rare steroidal xyloside isolated from B. monosperma for the first time.
Compound 4, designated as buteagibrenol glycoside, was obtained as a colorless crystalline mass from chloroform– methanol (9:1) eluants. It gave a positive test for glycosides. Its IR spectrum showed characteristic absorption bands for hydroxyl groups (3460, 3350, 3210 cm–1). The mass spectrum of 4 displayed a molecular peak at m/z 548 corresponding to the molecular formula of sesquiterpene diglycoside, C27H48O11. It indicated four double equivalents; two of them were adjusted in the diglycoside moiety and one each in the monocyclic carbon skeleton and vinylic linkage. Expulsion of the diglycoside moiety yielded the ion fragments at m/z 223 [C15H27O]+ and 207 [C15H27]+. The ion fragments arising at m/z 168 [223–C4H9]+, 154 [168–CH2]+, 140 [154–CH2]+, 192 [207–CH3]+, 96 [207–C8H15]+, 152 [207–C4H7]+, 138 [152–CH2]+, and 124 [138– CH2]+ suggested a zingiberene type carbon framework of the molecule containing one vinylic linkage at C-10 and an oxygenated methylene group at C-14. The 1H NMR spectrum of 4 showed a one-proton multiplet at 8 5.32 assigned to vinylic H-10. Two one-proton doublets at 8 5.18 (J = 7.1 Hz) and 5.07 (J = 7.3 Hz) were attributed to anomeric H-1' and H-1'' protons, respectively. The two multiplets at 8 4.79 and 4.50, both integrated for two protons each, were ascribed to carbinol protons H-5' , H-5'' , and H-2' , H-2'' , respectively. Four one-proton multiplets at 8 4.42, 4.30, 3.88, and 3.77 were attributed to carbinol protons H-4' , H-4'' , H-3' , and H-3'' , respectively, and deshielding of the H-4' proton to 8 4.42 supported the attachment of the second sugar molecule at this position. A two-proton broad signal at 8 3.56 and two multiplets at 8 3.46 and 3.35, both integrating for two protons each, were associated with the oxygenated methylene protons H2-14, H2-6' , and H2-6'' , respectively. A six-proton broad signal at 8 2.50 was due to C-12 and C-13 methyl protons attached to the (C-11) vinylic carbon. A three- proton doublet at 8 0.85 (J = 6.1 Hz) was attributed to C-15 secondary methyl protons. The remaining methine and methylene protons appeared between 8 2.27–1.23. The 13C NMR spectrum of 4 exhibited signals for vinylic carbons at 8 129.63 (C-11) and 127.72 (C-10), anomeric carbon at 8 104.04 (C-1') and 91.75 (C-1''), an oxygenated methylene carbon at 8 64.62 (C-14), 62.12 (C-6'), and 60.60 (C-6''), and the remaining sugar carbons between 8 82.55–68.56. The shifting of C-4' signal at 8 82.55 supported the attachment of the second sugar moiety at this carbon. The signals at 8 22.09, 24.43, and 13.92 were assigned to C-12, C-13, and C-15 methyl carbons, respectively. The remaining methylene and methine carbons resonated between 8 53.18 and 26.60. The 1H–1H COSY spectrum of 4 showed correlations of H-10 with H3-12 and H3-13; and H2-14 with H-3 and H-1. The 1H–13C HETCOR spectrum exhibited interactions of C-11 with H-10, H3-12, and H3-13; and C-1' with H-2' and H2-14. Acid hydrolysis of 4 yielded D-glucose and buteagibrenol. On the basis of the foregoing discussion, the structure of 4 has been formulated as 3,4-dihydrozingiberen-14-ol-/-D-glucopyranosyl-(1 4)-/-D-glucopyranoside. This is a new phytoconstituents isolated from a plant source for the first time.

Experimental
General Experimental Procedure. The melting points were determined on a Perfit apparatus and are uncorrected. The IR spectra were recorded in KBr pellets on a Win IR FTS 135 instrument (Biorad, USA). 1H (300 MHz), 13C (75 MHz), and 2D NMR spectra were recorded by a Bruker spectrospin NMR instrument in CDCl3, using TMS as internal standard. EIMS were scanned at 70 eV on a Jeol D-300 instrument (Jeol, USA). Column chromatography was performed on silica gel (Merck, 60–120 mesh), and thin-layer chromatography on silica gel G coated TLC plates (Merck).
Plant Material. The seed of B. monosperma was collected from the local market of Khari Baoli, New Delhi and identified by Dr. M. P. Sharma, taxonomist, Department of Botany, Faculty of Science, Jamia Hamdard (Hamdard University). A voucher specimen, No. KB/ND/PRL/BM/18, was deposited in the Herbarium of the Faculty of Pharmacy, Jamia Hamdard, New Delhi.
Extraction and Isolation. The air-dried seeds (2 kg) of B. monosperma were coarsely powdered and extracted exhaustively in a Soxhlet apparatus with methanol for 72 h. The methanolic extract was concentrated under reduced pressure to obtain a dark green viscous mass. A small portion of the extract was analyzed chemically to determine the presence of different chemical constituents. The viscous dark green mass was adsorbed on silica gel (60–120 mesh) for column chromatography after being dissolved in a small quantity of methanol for preparation of the slurry. The slurry (200 g) was air dried and chromatographed over a silica gel column packed in petroleum ether. The column was eluted successively with petroleum ether, a mixture of petroleum ether and chloroform (9:1, 3:1, 1:1 and 1:3), pure chloroform, and finally a mixture of chloroform and methanol (99:1, 98:2, 96:4, 95:5, 97:3, 9:1). Various fractions were collected separately and matched by TLC to check homogeneity. Similar fractions (having the same R f values) were combined and crystallized. The isolated compounds were recrystallized to get the pure compounds.
The physicochemical and spectral data of the isolated compounds are reported below.
n-Docosanoic Acid (1). Elution of the column with chloroform mixture gave colorless crystals of 1, recrystallized from methanol, 110 mg (0.0041% yield), R f 0.29 (CHCl3–MeOH) (99:1); mp 54–56°C; IR (KBr, v, cm–1): 3430, 2922, max2845, 1701, 1450, 1265, 834, 716; 1H NMR (CDCl3, 8, J/Hz): 2.37 (1H, d, J = 7.5, H2-2a), 2.32 (1H, d, J = 7.5, H2-2b), 1.63 (2H, br.s, CH2), 1.61 (2H, br.s, CH2), 1.25 (3H, br.s, 17 x CH2), 0.88 (3H, t, J = 6.1, Me-22); positive FAB-MS m/z (rel. int., %): 340 [M]+ (C22H44O2) (19.5).
n-Octacosanoic Acid (2). Elution of the column with a chloroform–methanol (99:1) mixture gave colorless crystals of 2, recrystallized from acetone, 100 mg (0.0037% yield), R f 0.60 (CHCl3–MeOH, 90:10); mp 49–50°C; IR (KBr, v, cm–1): max3410, 2917, 2850, 1706, 1473, 1204, 886, 717; 1H NMR (DMSO-d6, 8, ppm, J/Hz): 2.50 (2H, br.s, H2-2), 1.49 (2H, br.s, CH2), 1.23 (48H, br.s, 24 x CH2), 0.85 (3H, t, J= 6.3, Me-28); positive FAB-MS m/z (rel. int., %): 424 [M]+ (C28H56O2) (38.6 s).
β-Sitosterol Xyloside (3). Elution of the column with a chloroform–methanol (19:1) mixture gave a colorless amorphous powder of 3, recrystallized from methanol, 286 mg (0.018% yield), R f 0.32 (chloroform–methanol, 4:1); mp 270–272°C; UV (MeOH, λmax, nm): 268 (log ε 4.5); IR (KBr, vmax, cm–1): 3450, 2955, 2363, 1610, 1460, 1375, 1255, 1155, 1100, 1080, 1020, 796; 1H NMR (DMSO-d6, 8, ppm, J/Hz): 5.33 (1H, d, J = 5.21, H-6), 4.90 (1H, d, J = 9.61, H-1'), 4.24 (1H, d, J = 7.2, H-2'), 4.05 (1H, m, H-3'), 3.45 (1H, m, H-4'), 3.34 (1H, br.m, w1/2 = 16.5, H-3a), 3.05 (1H, d, J = 10.8, H2-5'a), 3.01 (1H, d, J = 10.8, H2-5'b), 0.95 (3H, br.s, Me-19), 0.91 (3H, d, J = 6.5, Me-21), 0.89 (3H, d, J = 7.0, Me-29), 0.82 (3H, d, J = 5.69, Me-26), 0.80 (3H, d, J = 6.39, Me-27), 0.65 (3H, br.s, Me-18); 13C NMR (DMSO-d6, 8): 36.74 (C-1), 31.34 (C-2), 76.57 (C-3), 41.18 (C-4), 139.95 (C-5), 121.35 (C-6), 33.37 (C-7), 29.13 (C-8), 49.58 (C-9), 36.74 (C-10), 22.50 (C-11), 35.55 (C-12), 45.24 (C13), 56.16 (C-14), 25.48 (C-15), 28.58 (C-16), 56.16 (C-17), 11.35 (C-18), 20.49 (C-19), 33.37 (C-20), 18.84 (C-21), 31.34 (C22), 29.13 (C-23), 49.58 (C-24), 27.67 (C-25), 19.32 (C-26), 18.84 (C-27), 22.50 (C-28), 11.35 (C-29), 101.23 (C-1'), 72.61 (C2'), 73.54 (C-3'), 70.16 (C-4'), 61.17 (C-5'); positive FAB MS m/z (rel. int., %): 546 [M]+ (C34H58O5) (1.5), 413 [M-sugar]+ (C29H50O) (4.3), 398 (15.5), 396 (20.5), 381 (15.3), 272 (4.3), 255 (11.7), 240 (7.6), 213 (8.2), 186 (10.3), 159 (14.8), 145 (15.9), 138 (13.5), 133 (14.7), 131 (16.1), 121 (18.5), 105 (31.6), 91 (32.7), 81 (37.8), 77 (48.1), 69 (24.3), 57 (63.8), 43 (100).
Hydrolysis of 3. Compound 3 (20 mg) was dissolved in MeOH and 2 N HCl (1:1) and heated to half volume. The solution was dried under reduced pressure and the residue was dissolved in EtOAc (3 x 10 mL). It was washed with H2O (2 x 10 mL), dried over anhydrous Na2SO4, and evaporated to give β-sitosterol, mp 136–138°C, co-TLC comparable. The aqueous phase was concentrated and analyzed by paper chromatography along with standard samples of monosacchrides. n-Butanol–ethanol–water (4:1:2.2) was used as the developing solvent system. The paper was sprayed with aniline hydrogen phthalate. The sugar was identified as D-xylose.
Buteagibrenol Glycoside (4). Elution of the column with chloroform–methanol (9:1) afforded colorless crystals of 4, recrystallized from methanol, 500 mg (0.018% yield), R f 0.43 (chloroform–methanol) (3:1); mp 170–171°C; IR (KBr, vmax, cm–1): 3460, 3350, 3210, 2922, 2852, 1620, 1375, 1103, 834; 1H NMR (DMSO-d6, 8, ppm, J/Hz): 5.32 (1H, m, H-10), 5.18 (1H, d, J = 7.1, H-1'), 5.07 (1H, d, J = 7.3, H-1''), 4.79 (2H, m, H-5', H-5''), 4.50 (2H, m, H-2', H-2''), 4.42 (1H, m, H-4'), 4.30 (1H, m, H-4''), 3.88 (1H, m, H-3'), 3.77 (1H, m, H-3''), 3.56 (2H, br.s, H2-14), 3.46 (2H, m, H2-6'), 3.35 (2H, m, H2-6''), 2.73 (2H, m, H2-9), 2.50 (6H, br.s, H3-12, H3-13), 2.27 (1H, m, /-H-6), 2.05 (2H, m, H-3, H-7), 1.50 (2H, m, H2-8), 1.23 (8H, br.s, H2-1, H2-2, H2-4, H2-5), 0.85 (3H, d, J = 6.1, H3-15); positive FAB-MS m/z (rel. int., %): 548 [M]+ (C27H48 O11), (5.3), 223 (36.5), 207 (16.1), 192 (21.3), 168 (38.5), 154 (71.3), 152 (36.6), 140 (21.2), 138 (34.8), 124 (31.9), 104 (46.2), 96 (27.3); 13C NMR (DMSO-d6): see Table 1.
Hydrolysis of 4. Compound 4 (15 mg) was dissolved in ethanol (5 mL); dilute HCl (2 mL) was added, and the reaction mixture was heated for one hour on a steam bath. The reaction mixture was dried under reduced pressure, and the residue was dissolved in chloroform to separate buteagibrenol. The remaining residue was mixed with water (2 mL) and chromatographed over TLC using n-butanol–acetic acid– water (4:1:5, top layer) as developing solvent mixture; R f 0.12, co-TLC comparable with a standard sample of D-glucose.
n-Dotriacontanoic Acid (5). Elution of the column with chloroform–methanol (17:3) mixture gave colorless crystals of 5, recrystallized from methanol, 90 mg, (0.0034% yield), R f 0.9 (chloroform–methanol) (3:1); mp 212–214°C; IR (KBr, vmax, cm–1): 3410, 2920, 2855, 1705, 1470, 1210, 946, 718; 1H NMR (DMSO-d6, 8, ppm, J/Hz): 10.21 (1H, br.s, COOH), 2.50 (2H, br.s, H2-2), 1.49 (2H, br.s, CH2) 1.23 (56H, br.s, 28 x CH2), 0.86 (3H, t, J = 6.1, Me-32); positive ion FAB-MS m/z (rel. int., %): 480 [M]+ (C32H64O2) (12.8).
References
K. R. Kritikar and B. D. Basu, Indian Medicinal Plants, Sri Satguru Publications, Shakti Nagar, Delhi, India, 1999, 4, pp. 2312–2313.
Anonymous, The Wealth of India-Raw Material, PID, CSIR, New Delhi, 1988, pp. 341–346.
B. M. R Bandara, N. S. Kumar, and K. M. S. Samaranayake, J. Ethanopharmacol., 25, 73 (1989).
B. M. R. Bandara, N. S. Kumar, and K. M. S. Wimalasiri, J. Natl. Sci. Council (Sri Lanka) 18, 97 (1990).
H. Wagner, B. Geyer, M. Fiebig, Y. Kiso, and H. Hikino, Planta Med., 52, 77 (1986).
J. Lal, S. Chandra, and M. Sabir, Indian J. Pharm. Sci., 40, 97 (1978)
S. R. Gupta, B. Ravindranath, and T. R. Seshadri, Phytochemistry, 9, 2231 (1970).
P. K. Guha, R. Poi, and A. Bhattacharya, Phytochemistry, 29, 2271(1990).
B. K. Mehta and M. M. Bokadia, Chem. Ind., 3, 98 (1981).
10. M. D. Greca, P. Monaco, and L. Previtera, J. Nat. Prod., 53, 960, 1430 (1990).
S. Gupta, M. Ali, M. S. Alam, M. Niwa, and T. Sakai, Phytochemistry, 31, 2558 (1992).
M. Ali, Techniques in Terpenoid Identification, Birla Publications, Shahdara, Delhi, 2001, p. 471–529.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, pp. 41–44, January–February, 2010.
Rights and permissions
About this article
Cite this article
Alam, S., Ali, M., Alam, P. et al. Phytochemical investigation of the seeds of Butea monosperma . Chem Nat Compd 46, 44–48 (2010). https://doi.org/10.1007/s10600-010-9521-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-010-9521-9