The lipid fraction of Opuntia ficus indica seeds was extracted and analyzed for its chemical and physical properties such as acid value, free fatty acid percentage (% FFA), iodine index, peroxide value, and saponification value as well as refractive index and density. The yield of seed oil was calculated as 11.75%. The acid and free fatty acid values indicated that the oil has a fairly low acidity. The triacylglycerols, fatty acids, sterols, and tocopherols were identified and their concentrations determined. The main TAGs were LLL (25.60%), OLL (21.53%), PLL (15.53%), and POL + SLL (12.73%). Linoleic acid (60.69%) was the dominant fatty acid, followed by oleic (21.42%) and palmitic (12.76%) acids, respectively. A high level of sterols making up 16.06 g/kg seed oil was present. The sterol marker, β-sitosterol, accounted for 71.60% of the total sterol content in the seed oil. Among the tocopherols present in the oil, γ-tocopherol (421.08 mg/kg) was the main constituent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Opuntia ficus indica (L.), a member of the Cactaceae family, is a tropical or subtropical plant originally grown in South America and cultivated in dry regions as an important nutrient and food source [1, 2]. Cactus pear ‘‘Opuntia sp.’’ grows throughout Tunisia and the fruits are consumed exclusively as fresh fruit [3].
The majority of the products use the juice of the cactus pear, while the seeds are usually discarded [4]. The extracted pigments from prickly pear fruits are used as additives in food, cosmetic, and pharmaceutical preparations [5, 6].
The cactus pear seed oil composition and its chemical characteristics were investigated. The seed oil composition of cactus pear fruits was studied during the maturation period [7], whereas the seeds and pulp were compared in terms of oil composition [8]. All the authors have agreed that Opuntia ficus indica seed oil was rich in polyunsaturated fatty acids and vitamins and may potentially be included in animal and human diets. Recently, the fatty acid composition of Tunisian prickly pear seed oil and its influence on rats were studied [3, 9, 10].
In the present study, we have analyzed the seed lipids in terms of fatty acid composition and triacylglycerol profile and determined the sterol and tocopherol composition to obtain an informative profile that will serve as a basis for further detailed chemical investigations and nutritional evaluation of the prickly seed. The results will furthermore be important as an indication of the potential nutraceutical and economical utility of prickly pear seed as a new source of edible lipids and in the cosmetic industry.
Table 1 presents the chemical composition of Opuntia seeds. Opuntia seeds contained 6.9% moisture. This value is close to that reported by [3]. We found that the total lipids recovered from seeds accounted for 117.5 g/kg, this value being the mean of three replicated extractions made on ground and sieved (500 mesh) seeds. This amount is greater than those reported in previous works where the seed oil content of several species of prickly pear fruits varied between 61 and 109 g/kg [3, 7, 8]. The ash percentage was 1.13%. Opuntia seeds also contained significant amounts of important minerals. The concentration of phosphorus was the highest, followed in descending order by potassium, calcium, magnesium, and sodium.
Opuntia seeds provide relatively high amounts of minerals (Mn, Zn, Cu, Fe, and Si). Lamghari et al. [1] found that the potassium content is highly significant, while calcium, magnesium, and phosphorus occupy second place.
Opuntia seed oil shows a high iodine value (107.38) due to its high content of unsaturated fatty acids (Table 4). This value is comparable with that of other seed oils such as corn oil (103–128), cottonseed oil (99–119), and mustard seed oil (92– 125) [11]. The peroxide value of Opuntia seed oil was evaluated as 1.46 meq/O2, showing the relative stability to oxidation of this oil. The high iodine value and oxidative stability show that the seed oil upholds the good qualities of edible oil [11]. The saponification value, determined as 173.3, is lower than that of safflower, sunflower, and corn oil [12] for which the average range of saponification value is 191–250. The density and refractive index are comparable to values reported by [3].
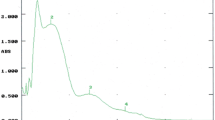
Opuntia seed oil absorbed strongly in the UV-C range (100–290 nm) and showed some absorbance in UV-B (290–320 nm) and UV-A (320–400 nm) ranges. In the UV-B and UV-A ranges, where ultraviolet light is responsible for the most cellular damage. Opuntia seed oil can protect against these radiations and thus may be used in the formulation of UV protectors. Opuntia seed oil exhibited a yellow color, as indicated by absorption ranging between 414 and 478 nm. The carotenoid content of this oil was 8.01 mg/kg (Table 2), indicating that it could be used in applications such as cosmetic formulations with no need to add primary colorants such as carotenes, annattos, and apocarotenals that are commonly used in oil and fat industries [13]. The green pigments, particularly chlorophyll, usually measured at 535–760 nm was low (2.403 mg/kg; Table 2), as indicated by the very low absorption (0.05).
To the best of our knowledge, there are no published studies on the qualitative and quantitative determination of triacylglycerols (TAGs ) from Opuntia ficus indica seed oil.
The attributed TAGs and their relative composition are mentioned in Table 3. The relative peak areas of all identified triglycerides are calculated from RID chromatograms and identified by comparison with olive, corn, sunflower, and soybean oils.
HPLC analysis of Opuntia seed oil (Table 3) showed that the most prominent polyunsaturated TAG was trilinolein (25.60%) followed by OLL (21.53%), PLL (15.53%), and POL + SLL (12.73%). Small amounts of PPL, OOO, and PPO were also detected.
The FAME composition of the seed oil is listed in Table 4. Linoleic acid was the major component (60.69%), followed by oleic (16.41%) and palmitic acids (12.76%). Myristic acid was detected in small amounts. Prickly pear seed oil was found to be highly unsaturated (83.12%).
These results are similar to those of a recent research on a Tunisian cultivar (Sfax, Tunisia) [3]. The observed difference in the percentage of oleic (16.8%) and palmitic (9.32%) acids could be due to the degree of maturity of the fruit. According to the literature, there is an increase in saturated fatty acid content towards the end of fruit maturation [7]. The high amount of linoleic acid could be a particular feature of Tunisian prickly pear seed oil compared to the Turkish cultivar (57.7–53.8%) [7] and the German cultivar (53.5%) [8].
Sterols comprise the bulk of the unsaponifiables in many oils. They are of interest due to their antioxidant activity and impact on health. The phytosterol content in vegetable oils is used as a means of oil characterization, oil derivatives identification, and for quality determination [14]. Recently, sterols have been added to vegetable oils as an example of a successful functional food [8].
Among the different plant sterols, sitosterol has been one of the most intensively investigated with respect to its physiological effects in human health. Many beneficial effects have been shown for sitosterol [15].
The sterol composition determined in this work is presented in Table 5. The sterol amount was estimated and represents 16.06 g/kg of seed oil, which accounts for nearly twice the sterol content of the German cultivar [8]. β-Sitosterol, campesterol, nasterol, and stigmasterol are among the major components. β-Sitosterol and campesterol are the two major sterols, constituting 85.10% of the total content. β-Sitosterol is the sterol marker as it accounts for 71.60% of the total sterol content. Moreover, stigmasterol and Δ5-avenasterol were present at levels of 4.72 and 5.10% of the total sterol content, respectively. Small amounts of Δ7-avenasterol and sitostanol were also detected.
In our study, the tocopherol derivatives identified were α-, γ-, and δ-tocopherols, while β-tocopherol was absent in the seed oil (Table 6). The vitamin E content represents 0.04% of the seed oil, which is comparable to that of the German cultivar [12]. γ-Tocopherol seems to be the major component in seed oil, accounting for 94.12% of the total vitamin E content, whereas δ-tocopherol accounted for 3.42% of the total amount.
These results show that Tunisian prickly pear fruit seeds contain high amounts of unsaturated fatty acids, sterols, and tocopherols compared to Turkish and German cultivars.
Experimental
Materials. Prickly pear fruits (Opuntia ficus indica) were purchased from local markets in Tunis, Tunisia (September 2005). Standards used for phytosterols (ST) characterization, β-sitosterol, stigmasterol, ergosterol, campesterol, cholesterol, and betulin were purchased from Sigma. Standards used for vitamin E (α-, β-, γ-, and δ-tocopherols) characterization were purchased from Roche. All reagents and chemicals used were of the highest purity available.
Oil was extracted from 50 g of seed powder in a Soxhlet extractor for 10 h using hexane as a solvent. The results are expressed as lipid percentage of seed powder (dry matter).
The organic phase was removed using a rotary evaporator under reduced pressure; the oil was flushed with a stream of nitrogen and stored at –20°C prior to analysis.
Analytical Methods. All measurements of the analytical moisture, the ash, the mineral constituents (Ca, K, Mg, Fe, Cu, Zn, P, Si, and Mn), the acid value, the iodine value, the saponification value, the peroxide value, refractive index, the density, and the chlorophyll content (mg/kg) were in accordance with the International standard.
Absorbances of oil solutions in hexane were measured with a UV/VIS spectrophotometer (JASCO V-530, WITEG Labortechnik).
Carotene Content. The AOAC (1998) official method, used to evaluate the oil carotenoid content expressed as micrograms of β-carotene per gram of oil, was applied by a calibration curve constructed by preparing solutions of increasing concentrations, from 0.5 to 2.5 μg of β-carotene/mL of hexane. The absorbance was recorded at 440 nm (JASCO V-530, WITEG Labortechnik) using hexane as a blank. The oil was diluted with hexane before being analyzed.
The triacylglycerol (TAGs) profile was obtained by reverse-phase high-performance liquid chromatography (RP-HPLC) using an Agilent liquid chromatograph equipped with an auto-injector and a refractive index detector. The TAGs of sample were separated using a hypersil ODS column (125 × 4 mm) with a particle size of 5 μm and was eluted from the column with a mixture of acetonitrile–acetone (65/35, v/v) at a flow rate of 0.5 mL/min; 10 μL of sample solution prepared in chloroform– acetone (1:1, v/v) was injected into the HPLC system. The total run time was 45 min. TAG peaks were identified based on their equivalent carbon number (ECN). Their elution occur according to the ECN, being ECN = CN (carbon number) – 2ND (number of double bond) by reference to the AOAC (1998). Common oils (olive, corn, sunflower, and soybean oils) were used as external standard. Peak areas produced by the data integrator were used to quantify the components based on relative percentages.
The fatty acid composition of the seed oil sample was analyzed by GC-HRMS after transesterification. Fatty acid methyl esters (FAME) were prepared in the presence of a solution of 2 N potassium hydroxide in methanol and analyzed on a Hewlett Packard 6890 (Agilent) gas chromatograph equipped with an FID detector and a HP-INNOWAX capillary column (polyethyleneglycol, 30 m × 0.32 mm i.d, film thickness 0.25 μm). The operational conditions were: injector temperature 270°C, oven temperature program 75°C for 1 min, then a gradient of 5°/min to 240°C for 20 min; the carrier gas was helium at a flow rate of 1 mL/min.
Separation of ST was performed according to the ISO 12228 method. Lipids (250 mg) were refluxed for 15 min with 5 mL ethanolic KOH solution (3%, w/v) after addition of betulin (1 mg; Fluka) as an internal standard and a few antibumping granules. The mixture was immediately diluted with 5 mL of ethanol. The unsaponifiable part was eluted over a glass column packed with a slurry of aluminum oxide (Scharlau) in ethanol (1:2, w/v) with 5 mL of ethanol and 30 mL of diethyl ether at a flow rate of 2 mL/min. The extract was evaporated in a rotary evaporator at 40°C under reduced pressure, and the ether was completely evaporated under a steam of nitrogen. For the characterization of sterols, a silica gel F254 plate (Fluka) was developed in the solvent system n-hexane–diethyl ether (1:1, v/v). For the detection of sterols, the thin-layer plate was sprayed with methanol; the sterol bands were scraped from the plate and recovered by extraction with diethyl ether. The extract was then evaporated in a rotary evaporator and in nitrogen. Finally, the sterols were derivatized using a sylilant reagent (1 mL of pure 1-methyl-imidazole and 50 μL of N-methyl-N-(trimethylsilyl-heptafluorobutyramide purchased from Fluka).
Preparation of standard solutions: a mixture of standard solutions of sterols was prepared by derivatization (cholesterol, sitosterol, stigmasterol, betulin, ergosterol, and campesterol).
Gas chromatographic analysis of sterols was carried out using a Hewlett Packard 6890 (Agilent) gas chromatograph equipped with an FID detector and a capillary column HP5 (5% phenylmethylsiloxane, 30 m × 0.32 mm id, film thickness 0.25 μm). The operational conditions were: injector temperature 320°C, column temperature: a gradient of 4°/min from 240°C to 255°C (65 min); carrier gas helium at a flow rate of 1 mL/min. Sterol peak identification was carried out according to the ISO 12228 reference method and confirmed by mass spectrometry using the NIST 2002 database.
Normal Phase HPLC Separation, Identification, and Quantification of Tocopherols. Tocopherols were analyzed by NP-HPLC with fluorescent detection. A silica column (150 × 3.2 mm, Pinnacle II Silica 3 μm) was used. The mobile phase was the solvent system hexane–isopropanol (99.5:0.5, v/v). The flow rate was 0.5 mL/min, and the column was held at 30°C. The fluorescent detector was set at 290 and 330 nm, respectively, for excitation and emission. Peak areas are used for quantification. Tocopherol isomers were identified by comparing their retention times with those of pure standards.
Preparation of standard solutions: standard solutions were prepared by serial dilution to concentrations of approximately 5 mg/mL of each tocopherols. Standard solutions were prepared from a stock solution stored in the dark at –20°C.
References
R. Lamghari El Kossori, C. Villaume, E. El Boustani, Y. Sauvaire, and L. Mejean, Plant Foods Hum. Nutr., 52, 263 (1998).
D. Trombetta, C. Puglia, D. Perri, A. Licata, S. Pergolizzi, E. R. Lauriano, A. De Pasquale, A. Saija, and F. P. Bonina, Phytomedicine, 13, 352 (2006).
M. Ennouri, B. Evelyne, M. Laurence, and A. Hamadi, Food Chem., 93, 431 (2005).
C. Saenz, J. Arid Environ., 46, 209 (2000).
A. Piga, J. Prof. Assoc. Cactus, 6, 9 (2004).
J. A. Fernandez-Lopez, R. Castellar, J. M. Obon, and L. Almela, Chromatographia, 56, 591 (2002).
Y. Coskuner and A. Tekin, J. Sci. Food Agric., 83, 846 (2003).
M. F. Ramadan and J. T. Morsel, Food Chem., 82, 339 (2003).
M. Ennouri, H. Fetoui, E. Bourret, N. Zeghal, F. Guermazi, and H. Attia, Bioresour. Technol., 97, 1382 (2006).
M. Ennouri, H. Fetoui, E. Bourret, E. N. Zeghal, F. Guermazi, and H. Attia, Bioresour. Technol., 97, 136 (2006).
O. Yong and S. Salimon, Ind. Crops Prod., 24, 146 (2006).
R. D. O′Brien, Fats and Oils, Second Ed., CRC Press, Washington, DC, 2004.
D. B. Oomah, S. Ladet, V. D. Godfrey. J. Liang, and B. Giarard, Food Chem., 69, 187 (2000).
M. F. Ramadan, G. Sharanabasappa, Y. N. Seetharam, M. Seshagiri, and J-T. Moersel, Food Chem., 98, 359 (2006).
B. Yang, R. M. Karlsson, P. H. Oksman, and H. P. Kallio, J. Agric. Food. Chem., 49, 5620 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, pp. 521–524, September–October, 2009.
Rights and permissions
About this article
Cite this article
El Mannoubi, I., Barrek, S., Skanji, T. et al. Characterization of Opuntia ficus indica seed oil from Tunisia. Chem Nat Compd 45, 616–620 (2009). https://doi.org/10.1007/s10600-009-9448-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-009-9448-1