Ring A was transformed and new A-homo-4-aza- and 3-cyano-3,4-seco-olean-4-ene derivatives of 3β-hydroxy18βH-olean-9,12-dien-30-oic acid were synthesized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Synthetic transformations of biologically active natural compounds represent a critical area of modern organic and bioorganic chemistry related to the synthesis of new biologically active compounds and compounds of new structural types [1–3]. Plant triterpenoids are a widely distributed class of natural compounds that have become especially interesting in the last decade due to the observation of several highly active antitumor, antiviral, anti-inflammatory, and anti-ulcer agents among derivatives of oleanolic, betulinic, ursolic, glycyrrhizic, and glycyrrhetic acids, among others [4–12]. The chemistry and pharmacology of glycyrrhetic acid (GLA) (1), the principal oleanane triterpenoid of licorice roots (Glycyrrhiza glabra L. and
G. uralensis Fisher), have been well studied [13]. GLA and its derivatives are effective for treating allergic conditions and are promising for therapy of inflammatory skin diseases, eczema of various etiologies, psoriasis, and allergic dermatitises [14]. GLA amides inhibit reverse transcriptase of HIV-1, a key enzyme in the life cycle of HIV that is necessary in early stages of cell infection [15]. GLA exhibited high antitumor activity in skin tumor models caused by cancerogens [16].
Herein we report the synthesis of new derivatives of 3β-hydroxy-18βH-olean-9,12-dien-30-oic acid (2), a GLA analog modified in ring C.
Reduction of GLA (1) by an excess of NaBH4 in THF:H2O (1:1) in the presence of NaOH produced an epimeric mixture (α/β) of 11-hydroxy derivative 3, which was dehydrated upon refluxing with conc. HCl in THF to 3β-hydroxy9(11),12-diene 2 [17], the yield of which after purification by column chromatography (CC) over Al2O3 was 67%. Methylation by diazomethane formed 30-methyl ester 2a. The structures of the triterpenoids were confirmed by PMR and 13C NMR spectra. Thus, The 13C NMR spectra of dienes 2 and 2a contained additional resonances for olefinic C atoms C-9 at δ 116.36 and 115.71 ppm; C-11, 155.23 and 154.53. Resonances of C-5 shifted to strong field by 3.5-4.0 ppm; of C-14, to weak field by 3 ppm (Table 1).

Oxidation of 2a by pyridinium dichromate (PDC) in CH2Cl2 at room temperature with TLC monitoring produced 3-oxo derivative 4 in 65% yield (Scheme 1). Refluxing 4 with NH2OH·HCl in anhydrous pyridine for 1 h formed 3-hydroxyimine 5. The 13C NMR spectrum of 5 exhibited a resonance for C-3 at δ166.6 ppm (C=N–).
We used the Beckmann rearrangement of ketooximes, which has been well studied for 18α- and 18β-GLA, in order to prepare compounds with a seven-membered ring. Depending on the conditions, the reactions could follow two pathways to form lactams (aza derivatives) and seco-nitriles [18]. Reaction of 5 with SOCl2 in anhydrous dioxane at 10°C formed a single product, A-homo-4-aza derivative 6, in 74% yield according to TLC through a first-order Beckmann rearrangement.
The structure of 6 was elucidated using spectral methods. The IR spectrum of 6 contained absorption bands for CONH at 1664 cm−1. The PMR spectrum had resonances at weak field (δ5.58, 5.62, 5.65 ppm) belonging to NH and olefinic protons (H-11, H-12). The presence of a C=O group in the 3-position of 6 was confirmed by a strong-field shift of the chemical shift (CS) for C-3 (from 166.6 to 175.3 ppm). The resonances of neighboring C atoms C-2 and C-4 shifted to weak field by 2-5 ppm at the same time (Table 1).
Heating 5 with p-TsCl in anhydrous Py for 5 h caused a second-order Beckmann rearrangement to form 3-cyano-3,4-seco-olean-4-ene (7) in 66% yield. The 13C NMR spectrum showed a resonance for the CN group at δ120.3 ppm; of the additional olefinic bond, at 163.7 and 116.5 (Table 1).
Refluxing A-homo-4-aza-3-oxo derivative 6 with Lawesson’s reagent in toluene for 5 h produced 3-thio analog 8 in 65% yield. Its IR spectrum contained absorption bands at 1680 (C=S) and 1572 (NHC=S) cm−1. The 13C NMR spectrum showed a shift of the C-3 resonance from 175.3 ppm to 162.9 as a result of the formation of the C=S bond.
Experimental
PMR and 13C NMR spectra were recorded on Bruker AM-300 spectrometers (operating frequency 300 MHz for 1H and 75.5 MHz for 13C) with TMS internal standard. Resonances in NMR spectra were assigned in normal mode using the program set ACD LABS and literature data for GLA and its derivatives [19, 20].
IR spectra in mineral oil mulls were recorded on an IR Prestige-21 spectrometer (Shimadzu). UV spectra were recorded on a UF-400 spectrophotometer. Molecular ions were determined by LC/MS on a Shimadzu LCMS-2010 instrument using atmospheric pressure chemical ionization and solutions in MeOH or CH3CN.
Optical activity was measured on a Perkin—Elmer 341 polarimeter in a 1-dm tube at 20-22°C (λNa 546 nm). Melting points were determined on a Boetius microstage.
Column chromatography (CC) was carried out over silica gel (KSK, 50-150 fraction, ZAO Sorbpolimer) (SG) or Al2O3 (Brockmann neutral). TLC used Sorbfil (ZAO Sorbpolimer) plates. Spots of compounds were detected using phosphotungstic acid solution (20%) or H2SO4 in EtOH (5%) with subsequent heating at 110-120°C for 2-3 min.
Solvents were purified as usual [21] and were evaporated in vacuo at <50°C.
We used PDC (Aldrich) and GLA prepared by the literature method [22] that was recrystallized twice from aqueous EtOH, mp 292-294°C, [α] 20D +168° (c 0.03, CHCl3), lit. [22] mp 289°C, [α] 20D +163° (c 1.0, CHCl3).
3 β -Hydroxy-18 β H-olean-9(11),12(13)-dien-30-oic Acid (2). A solution of GLA (2.5 g, 5.3 mmol) in THF (100 mL) and H2O (100 mL) was treated with NaOH (1.24 g, 31 mmol) and NaBH4 (12.5 g, 330 mmol), refluxed for 4 h, treated with aqueous NaH2PO4 (500 mL, 5%), and extracted with EtOAc (300 mL ×3). The organic layer was washed with water, dried over MgSO4, and evaporated. The crude product (2.0 g) was dissolved in THF (100 mL), treated with several drops of conc. HCl, refluxed for 6 h, and diluted with cold H2O (300 mL). The precipitate was filtered off, washed with water, and chromatographed over a column of Al2O3 with elution by CHCl3:CH3OH (300:1, 200:1, 100:1, v/v) to afford 2 (2.2 g), which was recrystallized from EtOH. Yield 1.6 g (66.7%) (transparent needles), mp >300°C, [α] 20D +343° (c 0.06, CHCl3), UV spectrum (λmax, MeOH, nm): 280 (log ε 3.98), lit. [17] [α] 20D +374° (c 1.0, THF).
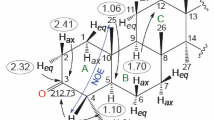
PMR spectrum (CD3OD, δ, ppm, J/Hz): 0.74 (3H, s, CH3-28), 0.80 (3H, s, CH3-27), 0.94 (3H, s, CH3-26), 0.98 (3H, s, CH3-25), 1.08, 1.10 (3H, both s, CH3-23, CH3-24), 1.14 (3H, s, CH3-29), 1.30-2.00 (m, CH, CH2), 3.14 (H α -3, t, J1 = 7.3, J2 = 8.1), 5.52, 5.54 (2H, H-11, H-12).
Table 1 lists the 13C NMR spectrum. C30H46O3. MW 454.7.
Methyl Ester of 3 β -Hydroxy-18 β H-olean-9(10),11(12)-dien-30-oic Acid (2a). A solution of 2 (0.90 g, 2 mmol) in EtOAc (100 mL) was treated with diazomethane in Et2O until a yellow color persisted. The solution was evaporated. The solid was recrystallized from EtOH. Yield 0.83 g (90%), R f 0.70 (CHCl3:CH3OH, 10:1), mp 243-245°C. IR spectrum (ν, cm−1): 3350-3270 (OH), 1722 (COOMe), 1217, 1159, 1103, 1086, 1038, 991, 822, 721. UV spectrum (λmax, MeOH, nm): 282 (log ε5.05).
PMR spectrum (CDCl3, δ, ppm, J/Hz): 0.80 (3H, s, CH3-28), 0.84 (3H, s, CH3-27), 0.98 (3H, s, CH3-26), 1.04 (3H, s, CH3-25), 1.14 (6H, s, CH3-23, CH3-24), 1.20 (3H, s, CH3-29), 1.25-2.10 (CH, m, CH2), 3.24 (H α -3, dd, J1 = 4.2, J2 = 11.0), 3.69 (3H, s, OCH3), 5.60, 5.62 (2H, H-11, H-12).
Table 1 lists the 13C NMR spectrum. [M + H]+ 470. C31H48O3. MW 468.7.
Methyl Ester of 3-Oxo-18 β H-olean-9(10),11(12)-dien-30-oic Acid (4). A solution of 2a (1.5 g, 3.2 mmol) in CHCl3 (10 mL) was treated with PDC (1.0 g, 4.8 mmol); stirred at room temperature for 3 h; diluted with CHCl3 (20 mL); and washed with H2O, Na2CO3 solution (5%), saturated NaCl solution, and H2O again. The organic phase was passed through a small column of Al2O3 and evaporated. The dry solid (1.36 g) was chromatographed over a column of SG with elution by toluene and toluene:EtOAc (200:1, 100:1, v/v). Yield 0.80 g (65.0%), R f 0.38 (benzene), 0.58 (benzene + 1 drop MeOH), mp 233-235°C, [α] 20D +305° (c 0.06, MeOH). IR spectrum (ν, cm−1): 1726 (COOMe), 1217, 1180, 1155, 1030. UV spectrum (λmax, MeOH, nm): 282 (log ε4.66). PMR spectrum (CDCl3, δ, ppm, J/Hz): 0.70 (3H, s, CH3-28), 0.90 (3H, s, CH3-27), 0.96 (3H, s, CH3-26), 1.00 (6H, s, CH3-23, CH3-25), 1.04 (3H, s, CH3-24), 1.15 (3H, s, CH3-29), 1.16-2.50 (CH, m, CH2), 3.57 (3H, s, OCH3), 5.52, 5.54 (2H, H-11, H-12).
Table 1 lists the 13C NMR spectrum. [M + H]+ 469. C31H46O3. MW 466.7.
Methyl Ester of 3-Hydroxyimino-18 β H-olean-9(11),12(13)-dien-30-oic Acid (5). A solution of 4 (0.7 g, 1.5 mmol) in anhydrous Py (28 mL) was treated with NH2OH·HCl (1.4 g), refluxed for 1 h, and treated with cold H2O. The precipitate was filtered off, washed with water, dried, and recrystallized from EtOH. Yield 0.59 g (82.4%), R f 0.74 (toluene:EtOAc, 3:1), 0.57 (benzene:MeOH, 20:1), mp 234-236°C. IR spectrum (ν, cm−1): 3300-3100 (N–OH), 1726 (COOMe), 1217, 1155, 928, 734. UV spectrum (λmax, MeOH, nm, log ε): 282 (4.0), 260 (4.05), 250 (4.1).
PMR spectrum (CDCl3, δ, ppm, J/Hz): 0.84 (3H, s, CH3-28), 1.00, 1.02 (6H, both s, CH3-26, CH3-27), 1.09 (3H, s, CH3-25), 1.12, 1.15 (6H, both s, CH3-23, CH3-24), 1.19 (3H, s, CH3-29), 1.30-2.40 (CH, m, CH2), 3.69 (3H, s, OCH3), 5.62, 5.65 (2H, H-11, H-12), 8.92 (br.s, 1H, N–OH).
Table 1 lists the 13C NMR spectrum. C31H47O3N. MW 481.7.
Methyl Ester of A-Homo-4-aza-3-oxo-18 β H-olean-9(11),12(13)-dien-30-oic Acid (6). A solution of 5 (0.5 g, 1 mmol) in anhydrous dioxane (50 mL) at 10°C was treated with freshly distilled SOCl2 (1 mL), stirred for 10 min, and poured into cold KOH solution (1%). The precipitate was filtered off, washed with water, and dried. The dry solid was recrystallized from MeOH:CHCl3, yield 0.37 g (74%), R f 0.54 (benzene + 1 drop MeOH), mp 264-266°C, [α] 20D +442° (c 0.04, CHCl3). IR spectrum (ν, cm−1): 3300-3100 (NH), 1726 (COOMe), 1664 (CONH), 1271, 1249, 1215, 1186, 1107, 1085, 1016, 997, 923, 894. UV spectrum (λmax, nm, MeOH): 285 (log ε 4.14).
PMR spectrum (CDCl3, δ, ppm): 0.83 (3H, s, CH3-28), 0.97, 1.00 (6H, both s, CH3-26, CH3-27), 1.10, 1.12 (6H, both s, CH3-25, CH3-23), 1.24 (3H, s, CH3-29), 1.34 (3H, s, CH3-24), 1.40-2.10 (CH, m, CH2), 3.68 (3H, s, OCH3), 5.58, 5.62, 5.65 (3H, NH, H-11, H-12).
Table 1 lists the 13C NMR spectrum. C31H49O3N. MW 483.7.
Methyl Ester of 3-Cyano-3,4- seco -18 β H-olean-4,9,12-trien-30-oic Acid (7). A solution of 5 (0.1 g, 0.1 mmol) in anhydrous Py (2 mL) was treated with p-TsCl (0.3 g), refluxed without admitting moisture for 5 h, and poured into HCl solution (5%, 10 mL). The precipitate was filtered off, washed with water, and dried to afford 7 (0.06 g, 66%), homogeneous according to TLC, R f 0.23 (benzene:EtOH, 20:1), mp 253-256°C. IR spectrum (ν, cm−1): 2200-2100 (CN), 1728 (COOMe), 1217, 1155, 1088, 926, 735.
PMR spectrum (CDCl3 + DMSO-d6, δ, ppm): 0.52, 0.66, 0.76, 0.80, 0.83, 0.95 (18H, all s, 6CH3), 1.00-2.00 (CH, m, CH2), 3.38 (3H, s, OCH3), 5.30, 5.32, 5.34 (4H, =CH2, H-11, H-12).
Table 1 lists the 13C NMR spectrum. C31H46O2N. MW 464.7.
Methyl Ester of A-Homo-4-aza-3-thioxo-18 β H-olean-9(11),12(13)-dien-30-oic Acid (8). A solution of 6 (50 mg, 0.1 mmol) in anhydrous toluene (5 mL) was treated with Lawesson’s reagent (160 mg, 0.4 mmol), refluxed without admitting moisture for 5 h, and filtered. The filtrate was washed with Na2CO3 solution (5%) and water and evaporated. The solid was chromatographed over a column of SG with elution by CHCl3. Yield 65.2%, R f 0.77 (toluene:EtOAc, 3:1). IR spectrum (ν, cm−1): 2967 (NH), 1680 (S=C), 1572 (NHC=S).
PMR spectrum (CDCl3, δ, ppm): 0.80, 0.84, 1.00, 1.02, 1.13, 1.26 (21H, all s, 7CH3), 1.40-2.7 (CH, m, CH2), 3.70 (3H, s, OCH3), 5.62, 5.68 (2H, H-11, H-12), 7.00 (1H, br.s, NH).
Table 1 lists the 13C NMR spectrum. C32H51NO2S.
References
K.-H. Lee, J. Nat. Prod., 67, 273 (2004).
M. S. Butler, Nat. Prod. Rep., 22, 162 (2005).
J. M. Rollinger, T. Langer, and H. Stuppner, Curr. Med. Chem., 13, 1491 (2006).
W. N. Setzer and M. C. Setzer, Mini Rev. Med. Chem., 3, 540 (2003).
R. H. Cichewicz and S. A. Kouzi, Med. Res. Rev., 29, 90 (2004).
J. Liu, J. Ethnopharmacol., 100, 92 (2005).
Z. Ovesna, A. Vachalkova, K. Horvathova, and D. Tothova, Mini Rev. Neoplasma, 51, 327 (2004).
L. A. Baltina, Curr. Med. Chem., 10, 155 (2003).
M. M. Yore, K. T. Liby, T. Honda, G. W. Gribble, and M. B. Sporn, Mol. Cancer Ther., 5, 3232 (2006).
S. Chintharlapalli, S. Papineni, S. Liu, I. Jutooru, G. Chadalapaka, S. Cho, R. S. Murthy, Y. You, and S. Safe, Carcinogenesis, 28, 2337 (2007).
T. Honda, G. W. Gribble, N. Suh, H. J. Finlay, B. Rounds, L. Bore, F. G. Favaloro, Y. Wang, and M. B. Sporn, J. Med. Chem., 43, 1866 (2000).
M. Urban, J. Sarek, J. Klinot, G. Korinkova, and M. Hajduch, J. Nat. Prod., 67, 1100 (2004).
G. A. Tolstikov, L. A. Baltina, V. P. Grankina, R. M. Kondratenko, and T. G. Tolstikova, Licorice: Biological Variability, Chemistry, and Use in Medicine [in Russian], Akad. Izd. Geo, Novosibirsk, 2007.
G. A. Tolstikov, L. A. Baltina, and N. G. Serdyuk, Khim.-farm. Zh., 32, 5 (1998).
T. V.Il′ina, E. A. Semenova, O. A. Plyasunova, N. V. Fedyuk, E. I. Petrenko, N. V. Elantseva, E. E. Shul′ts, G. A. Tolstikov, and A. G. Pokrovskii, Byull. Sib. Otd. Ross. Akad. Med. Nauk, No. 2, 20 (2002).
K. Kitagawa, H. Nishino, and A. Iwashima, Oncology, 43, 127 (1986).
R. Pellegata, M. Pinza, G. Pifferi, and C. Farina, Org. Prep. Proced. Int., 31, 181 (1999).
G. A. Tolstikov, Kh. A. Alibaeva, and M. I. Goryaev, Zh. Org. Khim., 5, 1625 (1969).
G. A. Tolstikov, L. M. Khalilov, L. A. Baltina, R. M. Kondratenko, A. A. Panasenko, and E. B. Vasil′eva, Khim. Prir. Soedin., 645 (1985).
N. I. Petrenko, V. Z. Petukhova, M. M. Shakirov, E. E. Shul′ts, and G. A. Tolstikov, Zh. Org. Khim., 36, 1013 (2000).
A. J. Gordon and R. A. Ford, A Chemist’s Companion, Wiley-Interscience, New York, 1972.
L. A. Baltina, O. B. Flekhter, Zh. M. Putieva, R. M. Kondratenko, L. V. Krasnova, and G. A. Tolstikov, Khim.farm. Zh., 30, 47 (1996).
Acknowledgment
The work was supported financially by the RFBR (Grant 08-03-00366a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 3, pp. 335–338, May–June, 2009.
Rights and permissions
About this article
Cite this article
Mikhailova, L.R., Khudobko, M.V., Baltina, L.A. et al. Synthesis of new derivatives of 3β-hydroxy18βH-olean-9,12-dien-30-oic acid. Chem Nat Compd 45, 393–397 (2009). https://doi.org/10.1007/s10600-009-9336-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-009-9336-8