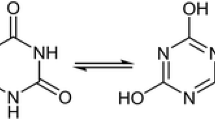
The reaction of 2-methyl-1-nitrоisothiourea with hydrazine in the presence of alkali metal bicarbonates (carbonates) resulted in the formation of 3,5-dinitrimino-1,2,4-triazole salts. The same salts were also formed by a reaction of 2-methyl-1-nitrоisothiourea with alkali metal salts of 4-nitrоsemicarbazide. This represents the first synthesis and characterization of the high-energy 3,5-dinitrimino-1,2,4-triazole, which can be readily isolated by treatment of its disodium salt with HCl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Derivatives of 1,2,4-triazole that contain various explosophoric substituents are clearly of interest as high-energy compounds, with applications in the roles of explosives, components of powders and solid rocket propellants.1 3,5-Dinitrimino-1,2,4-triazole (1) has been considered as one of hypothetical energetic compounds for the development in the future.2
Despite the fact that salts of 3,5-dinitrimino-1,2,4-triazole (previously referred to as salts of 3,5-dinitramino-1,2,4-triazole) have been known for a considerable time,3 compound 1 itself was not previously isolated as a free acid. Unlike with other 3(5)-amino-1,2,4-triazoles, direct nitration of 3,5-diamino-1,2,4-triazole did not result in the formation of the desired dinitrimine, probably due to diazotation of one of the amino groups, followed by complete destruction of the diazonium derivative.4
The previously described attempt to obtain compound 1 from its monoammonium salt by treatment with concd HNO3 was unsuccessful; only the solvate of this salt with HNO3 was isolated, which decomposed upon recrystallization, resulting in recovery of the starting monoammonium salt.3с In order to obtain free acid 1, we developed a method that relied on precipitation of insoluble BaSO4 when solutions of barium salts of dinitrimine 1 (compounds 2b or 3b, Scheme 1) were treated with H2SO4. The method was successful and enabled the first preparation of dinitrimine 1 as a free compound. It is important to note that compound 1 has very high acidity (pK a 1 −2.0, determined by spectrophotometric and potentiometric titration of its salt3c).

Scheme 1
During subsequent studies aimed at the preparation of monosodium salt 3a from disodium salt 2a by the action of HCl, we unexpectedly isolated a precipitate with decomposition temperature of 125°С, which was identified as dinitrimine 1. Thus, compound 1 can be easily obtained by treating a solution of disodium salt 2a with hydrochloric acid (Scheme 1).
The methods used so far to prepare salts of compound 1 3 are not simple as they involve multiple steps. According to these methods, the formation of 1,2,4-triazole ring occurs by cyclization of 2,5-dinitrobiguanidine 4 in alkaline medium. The starting compound 4, in turn, must be synthesized separately or obtained in situ by treatment of 2-nitrоguanidine3c or 1,2-dinitrоguanidine with hydrazine.3b
We have developed a simple one-step procedure for obtaining disodium and dipotassium salts of 3,5-dinitrimino-1,2,4-triazole 2а,c from the readily available 2-methyl-1-nitrоisothiourea (5) (Scheme 2). The formation of 1,2,4-triazole ring according to this method obviously also involved the cyclization of compound 4, which was formed as a result of two sequential nucleophilic substitution reactions. In the first step, compound 5 reacted with hydrazine, forming 1-amino-2-nitrоguanidine (6), which further reacted with compound 5, forming salts of compound 4.

Scheme 2
We identified an alternative route leading to the salts of compound 1 during our study of the reaction of compound 5 with potassium salt of 4-nitrоsemicarbazide (7). This reaction was expected to result in a salt of 2,5-dinitrо-1-ureidoguanidine 8 or products of its further cyclization – 1,2,4-triazole derivatives 9 or 2с (Scheme 3). The isolation of salt 2с from the reaction mixture did not contradict the proposed scheme of transformations. However, kinetic study of the hydrolysis reaction of 4-nitrоsemicarbazide and its salts5 indicated that also in this case the reaction proceeded according to a route similar to Scheme 2. The easy hydrolysis of salt 7 upon heating in aqueous solution merely provided a source of hydrazine.

Scheme 3
The erroneous interpretation that salts of compound 1 have primary nitramine structure originated in 1950s.3а The nitrimine structure of nitroguanidine was not yet clearly proven at that time (it was believed to exist in two possible forms – primary nitramine and nitrimine).6 The X-ray structural analysis results for ammonium and potassium salts of compound 1, which were obtained in 1985, were also interpreted according to erroneous assumptions, leading to the assignment of a nitramine structure.7 However, that study did not experimentally determine the positions of hydrogen atoms! The authors of that publication7 deliberately assumed hydrogen atom positions according to their view of compound 1 as a primary nitramine.
When considering the experimental data from the earlier study,7 we noticed that placing the hydrogen atoms at positions corresponding to primary nitramine structure (as proposed in that publication7), would result in very short intermolecular H···H (1.25 Å) and K···Н (2.19 Å) distances in the crystal structure (Fig. 1).8 Such distances are obviously impossible (the typical length of H···H contacts is 2.01–2.31 Å, while the sum of ionic radius of K+ and the van der Waals radius of hydrogen atom is 2.76 Å).9 On the other hand, no such problems would be encountered when placing the hydrogen atoms at positions corresponding to nitrimine, leading to a network of intra- and intermolecular hydrogen bonds, typical for nitrimines (Fig. 2).8 , 10 The nitrimine structure of monosalts derived from compound 1 was unequivocally confirmed by the recently published structural data for the hydrazinium salt.11
The crystal structures of а) ammonium salt and b) potassium salt of compound 1, reproduced from the literature.7
The crystal structures for а) ammonium salt and b) potassium salt of compound 1 according to corrected data from the literature.7
The structure of double salt 2a was established by the method of X-ray crystallography (Fig. 3). All hydrogen atoms were located from the difference electron density synthesis and were freely refined (without imposing constraints) in isotropic approximation. The hydrogen atom position at the triazole ring nitrogen atom, as well as the values of valence angles and bond lengths pointed to nitrimine structure of at least one of the NNO2 groups.
The attempts to grow crystals of compound 1 suitable for X-ray structural analysis were not successful. For this reason, there is still no direct proof available for the nitrimine structure of compound 1. In principle, three different structures are possible for compound 1: nitrimine A, primary nitramine B, and nitramino-nitrimine C (Fig. 4).
We have previously shown that compounds known as 3(5)-nitramino-1,2,4-triazoles actually have the structure of nitrimines.12 This fact was later confirmed by multiple X-ray structural analyses of other similar compounds.1b,4 , 13
The results of quantum-chemical calculations at various levels of theory also reliably showed that the nitrimine structure has the lowest energy.14 However, it was found that 5-nitramino-3-nitrо-1H-1,2,4-triazole, according to the data of X-ray structural analysis, was a primary nitramine.15 At the same time, quantum-chemical calculations still showed a preference for the nitrimine form of this compound.14 Taking into account that the quantum-chemical calculations were performed for an isolated molecule (simulation of the structure in the gas phase), the difference from results of X-ray structural analysis apparently can be explained with the influence of intermolecular interaction forces in the crystal structure. Strong effects of intermolecular interactions, in particular hydrogen bonds, on the molecular structure of nitrimines has been described in the literature.16
In our opinion, X-ray structural analysis data for the salts of compound 1 pointed in favor of the nitrimine structure A also for compound 1 itself, since it was unlikely that the removal of a single proton during salt formation reaction would result in migration of the remaining hydrogen atoms among the nitrogen atoms.
It would seem that the selection among the various possible structures of compound 1 would be possible by relying on NMR spectra. However, 1Н NMR spectrum of compound 1 featured only one broadened proton signal (11.21 ppm), which is common in systems with rapid exchange of protons. 13C NMR spectrum also contained only one signal with a chemical shift of 148.3 ppm. This provided evidence for symmetrical structure of the molecule in solution phase. 15N NMR spectrum contained only four signals for the seven nitrogen atoms, which can also be explained by the molecular symmetry of compound 1. The signal with chemical shift of −25.1 ppm belonged to the nitrogen atoms of nitrо groups; the signal of NNO2 group appeared at −179.6 ppm, while the signal at −215.4 ppm corresponded to the nitrogen atoms of triazole ring at positions 1, 2, and the signal at −291.2 ppm was due to the nitrogen atom at position 4. Thus, NMR spectra provided evidence for symmetrical molecular structure of compound 1, but these data cannot be considered as unequivocal proof for the nitrimine structure A, since the same result can be observed in the case of rapid proton exchange in solutions (on NMR timescale).
The absorption maximum (due to n→π* transition) in the electronic spectrum of compound 1 had the same wavelength (318 nm) and extinction coefficient (4.21) as those for the monosalts of compound 1, which was not surprising, because compound 1 is a strong acid according to its first dissociation constant and exists mostly as monoanion in aqueous solution. When compared to UV spectra of 1,2,4-triazoles with one nitrimine group,12 UV spectrum of compound 1 showed a bathochromic shift, which can be explained by the presence of conjugation through the molecular structure of the monoanion of compound 1.
The crystal lattice parameters of compound 1 were determined from powder X-ray diffraction data obtained at room temperature. The crystals had monoclinic syngony, space group P21/n, unit cell parameters: a 12.028(2), b 10.521(2), c 10.920(2) Å; β 97.70(1)°; Z 8; V 1369.5(4) Å 3, d calc 1.834 g/cm3.
It is interesting to note that the density of compound 1 is lower than that of its monoammonium (1.84 g/cm3)7 and, in particular, monohydrazinium salts (1.91 g/cm3).11 In the majority of cases, the simplest onium salts of nitrо compounds have lower density than the starting nitrо compounds.1b
The calculated explosive characteristics of compound 1 were sufficiently high and were approximately at the level of such powerful explosive as RDX17 (Table 1). In order to evaluate the practical applicability of compound 1, its sensitivity to thermal and mechanical initiation was studied.17 The sensitivity to thermal initiation was determined from the temperature dependence of time-toexplosion, according to the standard method.18 The flash of compound 1 was accompanied by loud bang and eruption of flame. The impact sensitivity of compound 1 was higher than that of RDX, and was at the level of other similar heterocyclic nitrimines19 (Table 1).
Despite the sufficiently high energetic characteristics, compound 1 has substantial drawbacks: high acidity, low thermal stability, and very pronounced impact sensitivity. At the same time, onium salts of compound 1 are clearly of interest. For example, the velocity of detonation of hydrazinium salt at the density of 1.8 g/cm3 reached 9.0 km/s (experimental data). This salt at the same time had lower impact sensitivity and better thermal stability than compound 1.11 When tested at its maximum density, this compound had detonation parameters that were superior to HMX.
Thus, 3,5-dinitrimino-1,2,4-triazole and its salts can be obtained from the readily available 2-methyl-1-nitrо-isothiourea. Salts of 3,5-dinitrimino-1,2,4-triazole have nitrimine structures, according to X-ray crystallographic analysis. Despite the fact that the spectral data of 3,5-dinitrimino-1,2,4-triazole do not allow to clearly discriminate between the nitramine and nitrimine features, the latter is the most likely structure.
Experimental
IR spectra were recorded on a Nicolet Impact 400D FT-IR spectrometer with the resolution of 4 cm−1. UV spectra were recorded on a Shimadzu UV-1601 spectrophotometer for aqueous solutions at 10−4 М concentration, with cuvette thickness of 1 cm. 1H, 13С, and 15N NMR spectra were acquired on a Bruker Avance III instrument (600, 150, and 60 MHz, respectively) at room temperature in acetone-d 6 solution, while using TMS as internal standard (or MeNO2 standard for 15N nuclei). 15N NMR spectra were acquired by using different pulse sequences (zgig and ineptrd). Elemental analysis was performed on a vario EL III instrument. Melting and decomposition temperatures were determined on a PTP apparatus. The impact sensitivity was studied on a K-44-II fall hammer apparatus18с with a 5 kg hammer and 30 mg samples. The methods for calculation of energetic parameters and experimental determination of flash temperature and impact sensitivity for compound 1 have been described in detail previously.17
3,5-Dinitrimino-1,2,4-triazole (1). Method I. Barium salt 2b (1.75 g, 4.2 mmol) was dissolved with heating in H2O (50 ml) and treated with concd HCl (9 ml). Then concd H2SO4 (0.2 ml, 3.9 mmol) was added to the solution and the BaSO4 precipitate was removed by filtration. The filtrate was evaporated under air flow, the residue was treated with acetone (25 ml), filtered, and the filtrate was evaporated. Yield 0.3 g (38%).
Method II. Salt 3b (5.0 g, 8.5 mmol) was dissolved with heating in H2O (75 ml) and treated with concd HCl (9 ml). The solution was then treated with concd H2SO4 (0.4 ml, 7.9 mmol) and the BaSO4 precipitate was removed by filtration. The filtrate was evaporated under air flow, the residue was treated with acetone (60 ml), then filtered and the filtrate was evaporated. Yield 1.4 g (43%).
Method III. A solution of salt 2a (4.9 g, 17 mmol) in H2O (15 ml) at room temperature was treated with concd HCl (19 ml) and cooled to −18°С. The precipitate that formed was filtered off, treated with acetone (60 ml), filtered, and the filtrate was evaporated. Yield 1.8 g (56%), colorless crystals, decomp. temp. 125°С (acetone). IR spectrum (KBr), ν, cm−1: 3337, 3082, 2973, 2916, 2849, 2775, 1640, 1619, 1581, 1532, 1477, 1420, 1401, 1329, 1279, 1246, 1128, 1102, 1045, 993, 893, 832, 774, 751, 715, 515, 471, 437. UV spectrum, λmax, nm (log ε): 213 (3.99), 318 (4.21). 1H NMR spectrum, δ, ppm: 11.21 (br. s). 13C NMR spectrum, δ, ppm: 148.3. 15N NMR spectrum, δ, ppm: −25.1 (NO2); −179.6 (NNO2); −215.4 (N-1,2); −291.2 (N-4). Found, %: C 12.89; H 1.60; N 51.41. C2H3N7O4. Calculated, %: C 12.70; H 1.60; N 51.85.
Trihydrate of 3,5-dinitrimino-1,2,4-triazole disodium salt (2a). A solution of compound 521 (14.9 g, 110 mmol) in H2O (80 ml) was stirred and treated by the addition of hydrazine hydrate (50 mmol), which was analyzed for hydrazine content prior to use. The reaction mixture was maintained at room temperature for 1 h. Then NaHCO3 (8.4 g, 100 mmol) or Na2CO3 (5.3 g, 50 mmol) was added, the mixture was heated to 70°С, and maintained at that temperature for 1.5 h. The reaction mixture was poured into EtOH (200 ml) and cooled to −18°С. The precipitate that formed was filtered off and washed on filter with EtOH. Yield 10.2 g (65%), colorless crystals, decomp. temp. 280°С (H2O). IR spectrum (KBr), ν, cm−1: 3389, 3115, 3109, 1665, 1624, 1549, 1452, 1422, 1402, 1387, 1365, 1330, 1313, 1267, 1168, 1101, 1063, 1032, 996, 880, 834, 812, 753, 706, 695, 556, 488, 452. UV spectrum, λmax, nm (log ε): 206 (4.05), 302 (4.19). Found, %: C 8.20; H 2.42; N 34.14. C2HN7Na2O4·3H2O. Calculated, %: C 8.37; H 2.46; N 34.15.
Hydrate of 3,5-dinitrimino-1,2,4-triazole sodium salt (3a). A heated solution of salt 2a (2 g, 7 mmol) in H2O (20 ml) was treated with 68% nitric acid (1 ml, 15 mmol), the solution was cooled to room temperature and then to −18°С. The precipitate was filtered off and washed on filter with EtOH. Yield 0.9 g (56%), colorless crystals, decomp. temp. 185°С (H2O). IR spectrum (KBr), ν, cm−1: 3588, 3539, 3438, 3153, 1647, 1589, 1560, 1495, 1425, 1335, 1328, 1275, 1233, 1117, 1079, 1052, 1011, 988, 881, 855, 809, 773, 753, 722, 659, 610, 539, 479, 446. UV spectrum, λmax, nm (log ε): 210 (4.06), 318 (4.20). Found, %: C 10.04; H 1.78; N 42.28; C2H2N7NaO4·H2O. Calculated, %: C 10.49; H 1.76; N 42.80.
Hydrate of 3,5-dinitrimino-1,2,4-triazole dipotassium salt (2c). Method I. This method was analogous to the procedure for preparation of salt 2a, except that KHCO3 (10 g, 100 mmol) or K2CO3 (6.9 g, 50 mmol) was used instead of NaHCO3 or Na2CO3. Yield 9.7 g (62%).
Method II. Compound 5 (1 g, 7.4 mmol) was dissolved with heating in H2O (10 ml), treated by the addition of salt 7 (1.17 g, 7.4 mmol),22 and maintained for 3 h on a refluxing water bath. The reaction solution was cooled to room temperature and poured into EtOH (30 ml), then cooled to −18°С. The precipitate was filtered off, washed with EtOH, and air-dried. Yield 0.7 g (67%), colorless crystals, decomp. temp. 220°С (H2O) (decomp. temp. 221°С3с). IR spectrum (thin layer), ν, cm−1: 3537, 3168, 3019, 1649, 1525, 1481, 1431, 1386, 1354, 1321, 1257, 1155, 1101, 1058, 1015, 991, 868, 798, 760, 693. UV spectra, λmax, nm (log ε): 206 (3.99), 305 (4.19).
Pentahydrate of 3,5-dinitrimino-1,2,4-triazole barium salt (2b). A hot solution of Ba(NO3)2 (1.8 g, 6.9 mmol) in H2O (5 ml) was added with stirring to a hot solution of salt 2a (1.8 g, 6.3 mmol) in H2O (10 ml). The reaction solution was cooled to room temperature, the obtained precipitate was filtered off, washed with H2O and EtOH. Yield 2.2 g (86%), colorless crystals, decomp. temp. ~350°С. IR spectrum (KBr), ν, cm−1: 3576, 3505, 3415, 1666, 1624, 1541, 1482, 1438, 1396, 1360, 1327, 1300, 1163, 1119, 1089, 1072, 1006, 993, 880, 871, 856, 756, 732, 713, 704, 693, 625, 550, 473, 452. UV spectrum, λmax, nm (log ε): 206 (4.06), 302 (4.19). Found, %: C 5.72; H 1.92; N 24.52. C2HBaN7O4·5H2O. Calculated, %: C 5.80; H 2.68; N 23.66.
Tetrahydrate of barium di(3,5-dinitrimino-1,2,4-triazolate) (3b). Method I. A hot solution of salt 2a (5.00 g, 17.4 mmol) in H2O (50 ml) was stirred and treated by the addition of 68% HNO3 (1.5 ml), followed by adding a hot solution of Ba(NO3)2 (2.28 g, 8.7 mmol) in H2O (12.5 ml). The solution was cooled to room temperature, the precipitate was filtered off, washed with H2O and EtOH. Yield 4.9 g (96%).
Method II. A solution of Ba(NO3)2 (0.18 g, 0.69 mmol) in H2O (2 ml) was added to a hot stirred solution of salt 3a (0.31 g, 1.35 mmol) in H2O (3 ml). The solution was cooled to room temperature, the precipitate was filtered off, washed with H2O and EtOH. Yield 0.24 g (61%), colorless crystals, decomp. temp. >320°С. IR spectrum (KBr), ν, cm−1: 3582, 3448, 3422, 3362, 3294, 3063, 2837, 1596, 1550, 1486, 1407, 1366, 1344, 1214, 1121, 1080, 1043, 1010, 980, 873, 830, 764, 723, 672, 544, 448. UV spectrum, λmax, nm (log ε): 210 (4.04), 315 (4.22). Found, %: C 7.91; H 1.89; N 33.25. C4H4BaN14O8·4H2O. Calculated, %: C 8.20; H 2.07; N 33.49.
Powder X-ray diffraction analysis was performed on a Bruker D8 Advance diffractometer. X-ray structural analysis of salt 2a was performed on a Bruker SMART APEX II instrument with a Bruker AXS CCD detector at 296(2) K temperature. Crystals of salt 2a that were suitable for X-ray structural analysis were obtained by slow evaporation from aqueous solution. The complete X-ray structural dataset for compound 2а was deposited at the Cambridge Crystallographic Data Center (deposit CCDC 1535107).
Dehydration and hydration studies for salts of compound 1. The content of solvated water in salts was not only reflected in the data of elemental analysis and UV spectra, but also determined by mass loss upon heating.23 A 1 g sample of the studied salt, weighed with accuracy to 0.1 mg, was placed in a drying oven heated to 120°С. The sample was weighed at certain time intervals. The content of water was calculated after a constant sample mass was achieved. The dried sample was left open under ambient air at room temperature and weighed to determine the amount of water absorbed from the air.
References
(a) Pevzner, M. S. Ross. Khim. Zh. 1997, 41, 73. (b) Gao, H.; Shreeve, J. M. Chem. Rev. 2011, 111, 7377.
Bottaro, J. In Advanced Series in Physical Chemistry: Overviews of Recent Research on Energetic Materials; Shaw, R. W.; Brill, T. B.; Thompson, D. L., Eds.; World Scientific: Singapore, 2005, Vol. 16, p. 473.
(a) Henry, R. A.; Skolnik, S.; Smith, G. B. L. J. Am. Chem. Soc. 1953, 75, 955. (b) Astrat'yev, A. A.; Dashko, D. V.; Kuznetsov, L. L. Russ. J. Org. Chem. 2003, 39, 501. [Zh. Org. Khim. 2003, 39, 537.] (c) Metelkina, E. L. Russ. J. Org. Chem. 2004, 40, 543. [Zh. Org. Khim. 2004, 40, 572.]
Klapötke, T. M.; Nordheider, A; Stierstorfer, J. New J. Chem. 2012, 36, 1463.
Astachov, A. M.; Antishin, D. V.; Buka, E. S. In 20th International Seminar “New Trends in Research of Energetic Materials”; University of Pardubice: Pardubice, 2017, p. 478.
McKay, A. F. Chem. Rev. 1952, 51, 301. Dyugaev, K. P.; Kuzubov, A. A.; Nasluzov, V. A.; Vasiliev, A. D.; Buka, É. S. J. Struct. Chem. 2009, 50, 201. [Zh. Strukt. Khim. 2009, 216.]
Astachov, A. M.; Revenko, V. A.; Vasiliev, A. D.; Buka, E. S. In 13th International Seminar “New Trends in Research of Energetic Materials”; University of Pardubice: Pardubice, 2010, p. 390.
(a) Andreev, K. K.; Belyaev, A. F. Theory of Explosives [in Russian]; Oborongiz: Moscow, 1960, p. 325. (b) Gol'binder, A. I. Laboratory Work for the Theory of Explosives Course [in Russian]; Rosvuzizdat: Moscow, 1963, p. 100. (с) Andreev, S. G.; Babkin, A. V.; Baum, F. A.; Imkhovik, N. A.; Kobilkin, I. F.; Kolpakov, V. I.; Ladov, S. V.; Odintsov, V. A.; Orlenko, L. P.; Okhitin, V. N.; Selivanov, V. V.; Solov'ev, V. S.; Stanyukovich, K. P.; Chelyshev, V. P.; Shekhter, B. I. Physics of Explosion [in Russian]; Orlenko, L. P., Ed.; Fizmatlit: Moscow, 2002, Vol. 1, p. 204.
Astachov, A. M.; Revenko, V. A.; Buka, E. S. In 12th International Seminar “New Trends in Research of Energetic Materials”; University of Pardubice: Pardubice, 2009, p. 396.
Gogulya, M. F.; Brazhnikov, M. A. Khim. Fizika 1994, 13, 52.
Fishbein, L.; Gallaghan, J. A. J. Am. Chem. Soc. 1954, 76, 1877.
Il'yasov, S. G.; Lobanova, A. A.; Popov, N. I.; Sataev, R. R. Russ. J. Org. Chem. 2002, 38, 1731. [Zh. Org. Khim. 2002, 1793.]
Kreshkov, A. P. Introduction to Analytical Chemistry. Theoretical Foundations. Quantitative Analysis; Khimiya: Moscow, 1971, Vol. 2, p. 369.
Astakhov, A. M.; Dyugaev, K. P.; Kuzubov, A. А.; Buka, E. S. In Energetic Condensed Systems. Proceedings of 3rd All- Russia Conference [in Russian]; Yanus-K: Chernogolovka, 2006, p. 13.
Dippold, A. A.; Klapötke, T. M.; Martin, F. A.; Wiedbrauk, S. Eur. J. Inorg. Chem. 2012, 2429.
Astakhov, A. M.; Dyugaev, K. P.; Kuzubov, A. A.; Nasluzov, V. A.; Vasiliev, A. D.; Buka, É. S. J. Struct. Chem. 2009, 50, 201. [Zh. Strukt. Khim. 2009, 216.]
Astachov, A. M.; Revenko, V. A.; Vasiliev, A. D.; Buka, E. S. In 13th International Seminar ''New Trends in Research of Energetic Materials''; University of Pardubice: Pardubice, 2010, p. 390.
(a) Andreev, K. K.; Belyaev, A. F. Theory of Explosives [in Russian]; Oborongiz: Moscow, 1960, p. 325. (b) Gol'binder, A. I. Laboratory Work for the Theory of Explosives Course [in Russian]; Rosvuzizdat: Moscow, 1963, p. 100. (с) Andreev, S. G.; Babkin, A. V.; Baum, F. A.; Imkhovik, N. A.; Kobilkin, I. F.; Kolpakov, V. I.; Ladov, S. V.; Odintsov, V. A.; Orlenko, L. P.; Okhitin, V. N.; Selivanov, V. V.; Solov'ev, V. S.; Stanyukovich, K. P.; Chelyshev, V. P.; Shekhter, B. I. Physics of Explosion [in Russian]; Orlenko, L. P., Ed.; Fizmatlit: Moscow, 2002, Vol. 1, p. 204.
Astachov, A. M.; Revenko, V. A.; Buka, E. S. In 12th International Seminar ''New Trends in Research of Energetic Materials''; University of Pardubice: Pardubice, 2009, p. 396.
Gogulya, M. F.; Brazhnikov, M. A. Khim. Fizika 1994, 13, 52.
Fishbein, L.; Gallaghan, J. A. J. Am. Chem. Soc. 1954, 76, 1877.
Il'yasov, S. G.; Lobanova, A. A.; Popov, N. I.; Sataev, R. R. Russ. J. Org. Chem. 2002, 38, 1731. [Zh. Org. Khim. 2002, 1793.]
Kreshkov, A. P. Introduction to Analytical Chemistry. Theoretical Foundations. Quantitative Analysis; Khimiya: Moscow, 1971, Vol. 2, p. 369.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(6/7), 722–727
Rights and permissions
About this article
Cite this article
Astakhov, A.M., Antishin, D.V., Revenko, V.A. et al. A simple method for the preparation of 3,5-dinitrimino-1,2,4-triazole and its salts. Chem Heterocycl Comp 53, 722–727 (2017). https://doi.org/10.1007/s10593-017-2116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2116-7