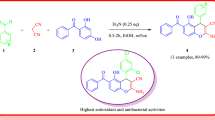
A series of alkyl 2-amino-4-aryl-4H-chromene-3-carboxylates was synthesized by an efficient, solvent-free one-pot three-component cyclocondensation of resorcinol, aromatic aldehydes, and ethyl or methyl cyanoacetate using sodium carbonate as catalyst. The present methodology offers several advantages, such as a simple procedure with ease of handling, short reaction time, high yields, and the absence of any volatile and hazardous organic solvents. The products were characterized on the basis of IR, 1H NMR, and 13C NMR spectral and microanalytical data and evaluated for their antibacterial activity against Gram-positive bacteria (Staphylococcus epidermidis and Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli) using paper disc diffusion technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The chromene moiety has emerged as a privileged scaffold for drug design and discovery and generated great attention because of certain natural and synthetic chromene derivatives possess important biological properties such as antitumor,1 antiviral,2 antivascular,3 antimicrobial,4 antihypertensive,5 anticonvulsant,6 and TNF-α inhibitor activity.7 They have also been widely employed as cosmetics, pigments,8 and potent biodegradable agrochemicals.9 7-Hydroxy-6-methoxy-4H-chromene I (Fig. 1) is an example of naturally occurring 4H-chromene, which was obtained from the flowers of Wisteria sinensis and is one of their fragrance components.10 Among different types of chromene systems, 2-amino-4H-chromenes have been reported to exhibit highly useful proapoptotic properties for the treatment of a wide range of cancer ailments.11 In cancer chemotherapy, 2-amino-4H-chromene II was marked for drug development due to its high inhibition of tumor-associated Bcl-2 proteins.12 A modified 4H-chromene structure III was able to induce apoptosis (programmed cell death) in several cancer cell lines.13 2-Amino-4H-chromenes have also been shown to exhibit antibacterial14 – 16 and antifungal16 activity.
Due to the aforementioned important properties of chromene derivatives, considerable attention has been focused on the development of environmentally friendly methodologies for the synthesis of 2-amino-4H-chromene scaffold. Several methods have been reported for the synthesis of 2-amino-4H-chromene-3-carbonitriles such as potassium carbonate-catalyzed conjugate additioncyclization reaction of malononitrile with Knoevenagel adducts, previously formed from salicylaldehyde, and a range of nucleophiles, such as oxindole, pyrazolone, nitromethane, N,N-dimethylbarbituric acid, or indanedione;17 one-pot reaction of salicylaldehyde, malononitrile, and nitroalkanes catalyzed by DBU;18 reaction of various In conclusion, we have reported a convenient method for the synthesis of some new alkyl 2-amino-4-aryl-4Hresorcinols with benzylidene malononitriles using various bases as catalyst;19 – 21 reaction of malononitrile with in situ generated sensitive ortho-quinone methides from 2-(arylsulfonyl) alkyl phenols;22 reaction of malononitrile with photochemically generated ortho-quinone methides from o-(dimethylaminomethyl)phenols or o-(hydroxymethyl)-phenols;23 or stepwise condensation of salicylaldehydes with 3 equiv of malononitrile.21 An improved method has also been reported for the synthesis of 2-amino-4-aryl-4Hchromene-3-carbonitriles via a one-pot three-component cyclocondensation of resorcinol, an aromatic aldehyde, and malononitrile in the presence of various catalysts.24 – 31 Despite the availability of a wide variety of synthetic routes toward 2-amino-4H-chromene-3-carbonitriles, a closer look at the literature disclosed that the synthesis of alkyl 2-amino-4-aryl-4H-chromene-3-carboxylates has been largely overlooked and only a few examples of the synthesis of ethyl 2-amino-4-aryl-4H-chromene-3-carboxylates have been reported by stepwise reaction of an aromatic aldehyde and ethyl cyanoacetate followed by cyclization with activated phenols or resorcinol in the presence of piperidine as catalyst after refluxing for several hours in ethanol.32 , 33
Inspired by these facts and due to our interest in the synthesis of heterocyclic compounds with potential biological activities,34 – 38 we report here our results from an efficient solvent-free synthesis of alkyl 2-amino-4-aryl-4Hchromene-3-carboxylates by one-pot, three-component cyclocondensation reaction of resorcinol, aromatic aldehydes, and ethyl or methyl cyanoacetate using Na2CO3 as catalyst. The products were also evaluated for their antibacterial activity against Staphylococcus epidermidis (S. epidermidis) ATCC 12228 and Staphylococcus aureus (S. aureus) ATCC 6538 as Gram-positive and Escherichia coli (E. coli) ATCC 8739 as Gram-negative bacteria using paper disc diffusion technique.
At first, the synthesis of compound 4a was selected as a model reaction to optimize the reaction conditions. The reaction was carried out by heating equimolar amounts of resorcinol (1), benzaldehyde (2a), and ethyl cyanoacetate (3a) under various conditions. We decided to investigate the efficiency of Na2CO3 in the model reaction under solvent-free conditions, which offers several advantages such as being environmentally friendly, simpler work-ups, cleaner products, enhanced selectivity, reduction of byproducts, and faster reactions. To find the optimum reaction conditions, different parameters were studied for the formation of compound 4a. The results are summarized in Table 1. Low yield of the product was obtained in the absence of the catalyst by heating the reaction mixture at 110°C under solvent-free conditions for 120 min (entry 1). This results indicate that the catalyst is necessary for the reaction. We were pleased to see that the reaction was efficiently catalyzed by Na2CO3 under solvent-free conditions at elevated temperature leading to product 4a in high yield (entry 2). Then the reaction was performed in the presence of various amounts of the catalyst and also at different temperatures under solvent-free conditions. As can be seen, the efficiency of the reaction is affected mainly by the amount of Na2CO3 and reaction temperature. The best result was obtained when the reaction was run at 110°C in the presence of 20 mol % of Na2CO3 (entry 12). For showing the effect of solvent, the same model reaction was also carried out in different solvents including H2O, MeOH, EtOH, MeCO2Et, and CH2Cl2 in the presence of 20 mol % of the catalyst (entries 15–19). As shown, the yield of the product 4a under solvent-free conditions was greater and the reaction time was considerably shorter than with the conventional methods. Therefore, our optimized conditions are 20 mol % of Na2CO3 at 110°C under solventfree conditions. All subsequent reactions were carried out using these conditions.
Encouraged by the remarkable results obtained in the model reaction and to show the generality and scope of this protocol, a range of alkyl 2-amino-4-aryl-4H-chromene-3-carboxylates 4a–j were prepared by the reaction of resorcinol (1), aromatic aldehydes 2a–g, and ethyl or methyl cyanoacetate 3a,b in the presence of Na2CO3 under the optimized reaction conditions. The results are summarized in Table 2. As shown, all reactions proceed very cleanly to give the corresponding products 4a–j in high yields over short reaction times. According to 1H NMR spectral data in aromatic region of chromene ring, resorcinol (1) reacts at position C-4 instead of position C-2 because of the steric hindrance between two hydroxyl groups or crowding of the aryl group with the remaining OH group in the product, and therefore 7-hydroxy-4H-chromenes 4a–j are formed not the corresponding 5-hydroxy isomers 5.25 – 30
The structure of all products 4a–j was deduced from their spectral and microanalytical data. For example, the 1H NMR spectrum of compound 4f in DMSO-d 6 showed two sharp singlets at 3.50 and 4.80 ppm for methoxy and methine groups, respectively, along with a signal at 7.62 ppm for the NH2 group, a signal at 9.62 ppm for the OH proton, as well as the signals in the aromatic region due to seven aromatic protons indicating the formation of compound 4f. The IR spectrum showed absorption bands at 3423, 3308, and 3240 cm−1 for NH2 and OH groups. Further proof came from 13C NMR spectrum which showed the characteristic signals at 50.9, 76.4, and 169.0 ppm for methoxy, methine, and carbonyl groups, respectively, as well as other signals in the aromatic region. This compound also gave satisfactory microanalytical data corresponding to the molecular formula C17H14BrNO4.
The synthesized compounds 4a–j were screened for the antibacterial activity against reference strains of S. epidermidis, S. aureus, and E. coli bacteria. None of the compounds had inhibition zone at a concentration of 2 mg/disc. The results, as shown in Table 3, revealed that compounds 4a–c and 4f are relatively effective against S. epidermidis at concentrations ≥ 3 mg/disc, while compounds 4c, 4g, and 4j show weak antibacterial activity against E. coli only at a concentration of 4 mg/disc. The minimum inhibitory concentration was 3 mg/disc for compounds 4a–c and 4f against S. epidermidis and 4 mg/disc for compounds 4c,g,j against E. coli. None of the compounds showed antibacterial activity against S. aureus at concentration of 3 or 4 mg/disc.
In conclusion, we have reported a convenient method for the synthesis of some new alkyl 2-amino-4-aryl-4Hchromene-3-carboxylates by one-pot three-component cyclocondensation of resorcinol, aromatic aldehydes, and ethyl or methyl cyanoacetate using sodium carbonate as catalyst under solvent-free conditions. The method offers several significant advantages, including short reaction times, high yields of products, easy work-up, and the absence of any hazardous organic solvents. Some of the synthesized compounds have weak growth-inhibiting effects on S. epidermidis and E. coli.
Experimental
IR spectra were obtained with KBr pellets using a Tensor 27 Bruker spectrophotometer. 1H and 13C NMR spectra were recorded on a Bruker 400 FT spectrometer (400 and 100 MHz, respectively) in DMSO-d 6 using TMS as internal standard. Elemental analysis was performed on a Thermo Finnigan Flash EA microanalyzer. Melting points were recorded on a Stuart SMP3 melting point apparatus. All chemicals were purchased from Merck and Aldrich and used without additional purification.
Synthesis of alkyl 2-amino-4-aryl-4 H -chromene-3-carboxylates 4a–j (General method). A mixture of resorcinol (1) (0.11 g, 1.0 mmol), an aromatic aldehyde 2a–g (1.0 mmol), ethyl or methyl cyanoacetate 3a,b (1.0 mmol) and anhydrous Na2CO3 (0.2 mmol, 20 mol %) was heated in an oil bath at 110°C for 10–20 min. After completion of the transformation (TLC control, eluent ethyl acetate–n-hexane), the reaction mixture was cooled to room temperature and warm water was added. This resulted in the precipitation of the product, which was collected by filtration. The crude product was washed with warm water repeatedly and recrystallized from EtOH to give compounds 4a–j in high yields.
Ethyl 2-amino-7-hydroxy-4-phenyl-4 H -chromene-3-carboxylate (4a). Yield 95%. IR spectrum, ν, cm−1: 3438, 3372, and 3323 (NH2 and OH), 1666 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 1.01 (3H, t, J = 6.4, CH3); 3.90 (2H, q, J = 6.4, OCH2); 4.75 (1H, s, CH); 6.40 (1H, s, H-8); 6.43 (1H, d, J = 8.0, H-6); 6.94 (1H, d, J = 8.0, H-5); 7.02–7.25 (5H, m, H Ar); 7.57 (2H, br. s, NH2); 9.60 (1H, s, OH).13C NMR spectrum, δ, ppm: 15.8; 60.1; 78.2; 103.7; 113.7; 113.8; 118.3; 127.3; 128.5; 129.7; 131.4; 150.2; 150.6; 158.3; 162.6; 170.0. Found, %: C 69.47; H 5.48; N 4.53. C18H17NO4. Calculated, %: C 69.44; H 5.50; N 4.50.
Methyl 2-amino-7-hydroxy-4-phenyl-4 H -chromene-3-carboxylate (4b). Yield 98%. IR spectrum, ν, cm−1: 3418, 3306, and 3227 (NH2 and OH), 1664 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 3.49 (3H, s, OCH3); 4.79 (1H, s, CH); 6.43 (1H, d, J = 2.0, H-8); 6.47 (1H, dd, J = 8.3, J = 2.0, H-6); 6.99 (1H, d, J = 8.3, H-5); 7.08 (1H, t, J = 7.2, H Ar); 7.14 (2H, d, J = 7.3, H Ar); 7.20 (2H, t, J = 7.6, H Ar); 7.60 (2H, br. s, NH2); 9.60 (1H, s, OH). 13C NMR spectrum, δ, ppm: 50.4; 76.3; 102.0; 112.0; 116.9; 125.7; 126.7; 128.2; 129.6; 148.4; 149.1; 156.7; 161.1; 161.2; 168.6. Found, %: C 68.72; H 5.11; N 4.67. C17H15NO4. Calculated, %: C 68.68; H 5.09; N 4.71.
Ethyl 2-amino-4-(4-chlorophenyl)-7-hydroxy-4 H -chromene-3-carboxylate (4c). Yield 90%. IR spectrum, ν, cm−1: 3417, 3303, and 3247 (NH2 and OH), 1661 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 1.03 (3H, t, J = 7.2, CH3); 3.92 (2H, q, J = 7.2, OCH2); 4.78 (1H, s, CH); 6.42 (1H, d, J = 2.0, H-8); 6.45 (1H, dd, J = 8.0, J = 2.0, H-6); 6.94 (1H, d, J = 8.0, H-5); 7.13 (2H, d, J = 8.4, H Ar); 7.25 (2H, d, J = 8.4, H Ar); 7.62 (2H, br. s, NH2); 9.67 (1H, s, OH). 13C NMR spectrum, δ, ppm: 15.9; 60.2; 77.7; 103.7; 113.3; 113.8; 117.7; 129.7; 130.4; 131.4; 131.8; 149.3; 150.4; 158.5; 162.5; 170.0. Found, %: C 62.49; H 4.63; N 4.09. C18H16ClNO4. Calculated, %: C 62.52; H 4.66; N 4.05.
Methyl 2-amino-4-(4-chlorophenyl)-7-hydroxy-4 H chromene-3-carboxylate (4d). Yield 92%. IR spectrum, ν, cm−1: 3417, 3376, and 3320 (NH2 and OH), 1666 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 3.48 (3H, s, OCH3); 4.80 (1H, s, CH); 6.42 (1H, d, J = 1.6, H-8); 6.46 (1H, d, J = 8.4, H-6); 6.98 (1H, d, J = 8.4, H-5); 7.14 (2H, d, J = 8.4, H Ar); 7.25 (2H, d, J = 8.4, H Ar); 7.63 (2H, br. s, NH2); 9.63 (1H, s, OH). 13C NMR spectrum, δ, ppm: 50.9; 76.8; 102.6; 112.6; 117.3; 126.2; 127.1; 127.2; 128.7; 130.1; 149.0; 149.6; 157.3; 161.7; 169.2. Found, %: C 61.56; H 4.23; N 4.19. C17H14ClNO4. Calculated, %: C 61.55; H 4.25; N 4.22.
Ethyl 2-amino-4-(4-bromophenyl)-7-hydroxy-4 H chromene-3-carboxylate (4e). Yield 90%. IR spectrum, ν, cm−1: 3416, 3303, and 3244 (NH2 and OH), 1661 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 1.03 (3H, t, J = 6.8, CH3); 3.92 (2H, q, J = 6.8, OCH2); 4.77 (1H, s, CH); 6.42 (1H, s, H-8); 6.46 (1H, d, J = 8.4, H-6); 6.94 (1H, d, J = 8.4, H-5); 7.08 (2H, d, J = 8.0, H Ar); 7.38 (2H, d, J = 8.0, H Ar); 7.61 (2H, br. s, NH2); 9.64 (1H, s, OH). 13C NMR spectrum, δ, ppm: 15.9; 60.2; 77.6; 103.7; 112.5; 113.8; 117.6; 120.3; 130.8; 131.5; 132.6; 149.7; 150.6; 158.5; 162.6; 169.8. Found, %: C 55.44; H 4.18; N 3.60. C18H16BrNO4. Calculated, %: C 55.40; H 4.13; N 3.59.
Methyl 2-amino-4-(4-bromophenyl)-7-hydroxy-4 H chromene-3-carboxylate (4f). Yield 90%. IR spectrum, ν, cm−1: 3423, 3308, and 3240 (NH2 and OH), 1662 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 3.50 (3H, s, OCH3); 4.80 (1H, s, CH); 6.44 (1H, d, J = 2.4, H-8); 6.48 (1H, dd, J = 8.4, J = 2.4, H-6); 6.99 (1H, d, J = 8.4, H-5); 7.10 (2H, d, J = 8.4, H Ar); 7.40 (2H, d, J = 8.4, H Ar); 7.62 (2H, br. s, NH2); 9.62 (1H, s, OH). 13C NMR spectrum, δ, ppm: 50.9; 76.4; 102.6; 112.7; 116.7; 119.2; 129.5; 130.2; 131.6 (2C); 148.4; 149.5; 157.4; 161.6; 169.0. Found, %: C 54.32; H 3.74; N 3.69. C17H14BrNO4. Calculated, %: C 54.28; H 3.75; N 3.72.
Ethyl 2-amino-4-(4-fluorophenyl)-7-hydroxy-4 H -chromene-3-carboxylate (4g). Yield 95%. IR spectrum, ν, cm−1: 3417, 3304, and 3244 (NH2 and OH), 1662 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 1.03 (3H, t, J = 6.8, CH3); 3.93 (2H, q, J = 6.8, OCH2); 4.79 (1H, s, CH); 6.42 (1H, s, H-8); 6.46 (1H, d, J = 8.4, H-6); 6.94 (1H, d, J = 8.4, H-5); 7.01 (2H, t, J = 8.4, H Ar); 7.14 (2H, t, J = 7.2, H Ar); 7.61 (2H, br. s, NH2); 9.66 (1H, s, OH). 13C NMR spectrum, δ, ppm: 15.8; 60.2; 78.1; 103.7; 113.8; 116.2; 116.4; 118.0; 130.3; 131.4; 146.5; 150.6; 158.4; 160.8; 162.5; 169.9. Found, %: C 65.62; H 4.95; N 4.27. C18H16FNO4. Calculated, %: C 65.65; H 4.90; N 4.25.
Methyl 2-amino-4-(2-chlorophenyl)-7-hydroxy-4 H -chromene-3-carboxylate (4h). Yield 95%. IR spectrum, ν, cm−1: 3427, 3385, and 3315 (NH2 and OH), 1670 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 3.40 (3H, s, OCH3); 5.32 (1H, s, CH); 6.40 (1H, s, H-8); 6.43 (1H, d, J = 8.4, H-6); 6.95 (1H, d, J = 8.4, H-5); 7.00–7.35 (4H, m, H Ar); 7.66 (2H, br. s, NH2); 9.66 (1H, s, OH). 13C NMR spectrum, δ, ppm: 52.0; 76.9; 103.8; 113.9; 117.2; 129.0; 129.1; 130.6; 130.8; 131.3; 131.4; 132.7; 147.4; 150.4; 158.6; 162.8; 170.0. Found, %: C 61.61; H 4.26; N 4.18. C17H14ClNO4. Calculated, %: C 61.55; H 4.25; N 4.22.
Ethyl 2-amino-7-hydroxy-4-(4-methoxyphenyl)-4 H chromene-3-carboxylate (4i). Yield 90%. IR spectrum, ν, cm−1: 3418, 3305, and 3243 (NH2 and OH), 1660 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 1.08 (3H, t, J = 6.8, CH3); 3.68 (3H, s, OCH3); 3.96 (2H, q, J = 6.8, OCH2); 4.74 (1H, s, CH); 6.44 (1H, s, H-8); 6.48 (1H, d, J = 8.4, H-6); 6.78 (2H, d, J = 8.4, H Ar); 6.96 (1H, d, J = 8.4, H-5); 7.05 (2H, d, J = 8.4, H Ar); 7.55 (2H, br. s, NH2); 9.61 (1H, s, OH). 13C NMR spectrum, δ, ppm: 15.9; 56.5; 60.1; 78.4; 103.6; 113.6 (2C); 115.0; 118.7; 129.4; 131.4; 142.4; 150.6; 158.2; 158.7; 162.5; 170.0. Found, %: C 66.84; H 5.57; N 4.12. C19H19NO5. Calculated, %: C 66.85; H 5.61; N 4.10.
Methyl 2-amino-4-(3-bromophenyl)-7-hydroxy-4 H chromene-3-carboxylate (4j). Yield 95%. IR spectrum, ν, cm−1: 3415, 3303, and 3261 (NH2 and OH) 1663 (C=O). 1H NMR spectrum, δ, ppm (J, Hz): 3.49 (3H, s, OCH3); 4.81 (1H, s, CH); 6.43 (1H, s, H-8); 6.48 (1H, d, J = 8.4, H-6); 7.02 (1H, d, J = 8.4, H-5); 7.12–7.35 (4H, m, H Ar); 7.65 (2H, br. s, NH2); 9.65 (1H, s, OH). 13C NMR spectrum, δ, ppm: 52.1; 77.3; 103.8; 113.9; 117.7; 123.1; 127.5; 130.3; 131.0; 131.3; 132.1; 150.6; 152.8; 158.6; 162.8; 162.9; 170.0. Found, %: C 54.30; H 3.77; N 3.69. C17H14BrNO4. Calculated, %: C 54.28; H 3.75; N 3.72.
Antibacterial activity. The antibacterial activity of the synthesized compounds was examined against S. epidermidis ATCC 12228 and S. aureus ATCC 6538 as Gram-positive and E. coli ATCC 8793 as Gram-negative bacteria using disc diffusion method according to Kirby–Bauer standards.39 Suspensions of the tested bacteria were prepared with turbidity equivalent to McFarland tube No. 0.5 (107–108 CFU/ml). Standard blank discs, containing 2, 3, and 4 mg of the synthesized compounds, were prepared using DMSO as solvent and placed on a Mueller–Hinton agar pre-inoculated with 0.1 ml of bacterial suspension. Discs of gentamicin 10 μg/disc and DMSO were used as positive and negative controls, respectively. All discs were fully dried before the application on bacterial lawn. Plates were incubated for 18–24 h at 37°C, aerobically, and the diameter of inhibitory zones around the discs was measured in millimeter. All determinations were performed in triplicate, and average value was reported as the inhibition zone (mean ± SEM). Minimum inhibitory concentration was defined as the lowest concentration of compound that inhibited the growth of bacteria.
References
Raj, T.; Bhatia, R. K.; Sharma, M.; Saxena, A.; Ishar, M. Eur. J. Med. Chem. 2010, 45, 790.
Conti, C.; Monaco, L. P.; Desideri, N. Bioorg. Med. Chem. 2014, 22, 1201.
Gourdeau, H.; Leblond, L.; Hamelin, B.; Desputeau, C.; Dong, K.; Kianicka, I.; Custeau, D.; Boudreau, C.; Geerts, L.; Cai, S.-X.; Drewe, J.; Labrecque, D.; Kasibhatla, S.; Tseng, B. Mol. Can. Ther. 2004, 3, 1375.
Ashok, D.; Lakshmi1, B. V.; Ravi, S.; Ganesh, A.; Adam, S. Chem. Heterocycl. Compd. 2015, 51, 462. [Khim Geterotsikl. Soedin. 2015, 462.]
Johannes, C. W.; Visser, M. S.; Weatherhead, G. S.; Hoveyda, A. H. J. Am. Chem. Soc. 1998, 120, 8340.
Bhat, M. A.; Siddiqui, N.; Khan, S. A. Acta Pol. Pharm. 2008, 65, 235.
Cheng, J.-F.; Ishikawa, A.; Ono, Y.; Arrhenius, T.; Nadzan, A. Bioorg. Med. Chem. Lett. 2003, 13, 3647.
Ellis, G. In The Chemistry of Heterocyclic of Compounds. Chromenes, Chromanes and Chromones; Weissberger, A., Taylor, EC, Eds.; John Wiley: New York, 1977., Chapter II, p. 11.
Hafez, E. A. A.; Elnagdi, M. H.; Elagamey, A. G. A.; El-Taweel, F. M. A. A. Heterocycles 1987, 26, 903.
Joulain, D.; Tabacchi, R. Phytochemistry 1994, 37, 1769.
Nolan, K. A.; Zhao, H.; Faulder, P. F.; Frenkel, A. D.; Timson, D. J.; Siegel, D.; Ross, D.; Burke Jr, T. R.; Stratford, I. J.; Bryce, R. A. J. Med. Chem. 2007, 50, 6316.
Doshi, J. M.; Tian, D.; Xing, C. J. Med. Chem. 2006, 49, 7731.
Kemnitzer, W.; Drewe, J.; Jiang, S.; Zhang, H.; Crogan-Grundy, C.; Labreque, D.; Bubenick, M.; Attardo, G.; Denis, R.; Lamothe, S.; Gourdeau, H.; Tseng, B.; Kasibhatla, S.; Cai, S. X. J. Med. Chem. 2008, 51, 417.
Zhang, G.; Zhang, Y.; Yan, J.; Chen, R.; Wang, S.; Ma, Y.; Wang, R. J. Org. Chem. 2012, 77, 878.
Kidwai, M.; Saxena, S.; Rahman Khan, M. K.; Thukral, S. S. Bioorg. Med. Chem. Lett. 2005, 15, 4295.
Shah, N. K.; Shah, N. M.; Patel, M. P.; Patel, R. G. J. Chem. Sci. 2013, 125, 525.
He, Y.; Hu, R.; Tong, R.; Li, F.; Shi, J.; Zhang, M. Molecules 2014, 19, 19253.
Zonouzi, A.; Mirzazadeh, R.; Safavi, M.; Ardestani, S. K.; Emami, S.; Foroumadi, A. Iranian J. Pharm. Res. 2013, 12, 679.
Mohammed, F. K.; Soliman, A. Y.; Ssawy, A.; Badre, M. G. J. Chem. Pharm. Res. 2009, 1, 213.
Kolla, S. R.; Lee, Y. R. Tetrahedron 2011, 67, 8271.
Anderson, D. R.; Hegde, S.; Reinhard, E.; Gomez, L.; Vernier, W. F.; Lee, L.; Liu, S.; Sambandam, A.; Snider, P. A.; Masih, L. Bioorg. Med. Chem. Lett. 2005, 15, 1587.
Caruana, L.; Mondatori, M.; Corti, V.; Morales, S.; Mazzanti, A.; Fochi, M.; Bernardi, L. Chem.–Eur. J. 2015, 21, 6037.
Fujiwara, M.; Sakamoto, M.; Komeyama, K.; Yoshida, H.; Takaki, K. J. Heterocycl. Chem. 2015, 52, 59.
Khaksar, S.; Rouhollahpour, A.; Talesh, S. M. J. Fluorine Chem. 2012, 141, 11.
Habibi-Khorassani, S. M.; Hazeri, N.; Shahraki, M.; Abbasi, M.; Karima, M.; Ali, M. Iran. J. Org. Chem. 2013, 5, 1163.
Dekamin, M. G.; Eslami, M.; Maleki, A. Tetrahedron 2013, 69, 1074.
Qareaghaj, O. H.; Mashkouri, S.; Naimi-Jamal, M. R.; Kaupp, G. RSC Adv. 2014, 4, 48191.
Safari, J.; Zarnegar, Z.; Heydarian, M. Bull. Chem. Soc. Jpn. 2012, 85, 1332.
Makarem, S.; Mohammadi, A. A.; Fakhari, A. R. Tetrahedron Lett. 2008, 49, 7194.
Kundu, S. K.; Mondal, J.; Bhaumik, A. Dalton Trans. 2013, 42, 10515.
Shekhar, A. C.; Kumar, A. R.; Sathaiah, G.; Raju, K.; Rao, P. S.; Sridhar, M.; Narsaiah, B.; Srinivas, P. V. S. S.; Sridhar, B. Helv. Chim. Acta 2012, 95, 502.
Al-Mousawi, S. M.; Elkholy, Y. M.; Mohammad, M. A.; Elnagdi, M. H. Org. Prep. Proced. Int. 1999, 31, 305.
Radwan, S. M.; Bakhite, E. A.; Kamal El-Dean, A. M. Phosphorus, Sulfur Silicon Relat. Elem. 1995, 101, 207.
Davoodnia, A.; Bakavoli, M.; Vahedinia, A.; Rahimizadeh, M.; Roshani, M. Heterocycles 2006, 68, 801.
Davoodnia, A.; Bakavoli, M.; Soleimany, M.; Tavakoli- Hoseini, N. Monatsh. Chem. 2009, 140, 355.
Davoodnia, A.; Bakavoli, M.; Moloudi, R.; Khashi, M.; Tavakoli-Hoseini, N. Chin. Chem. Lett. 2010, 21, 1–4.
Davoodnia, A.; Khashi, M.; Tavakoli-Hociseini, N.; Moloudi, R.; Zamani, H. A. Monatsh. Chem. 2013, 144, 677.
Khashi, M.; Davoodnia, A.; Chamani, J. Phosphorus, Sulfur Silicon Relat. Elem. 2014, 189, 839.
Bauer, A. W.; Kirby, W. M. M.; Sherris, J. C.; Turck, M. Am. J. Clin. Pathol. 1966, 45, 493.
The authors express their gratitude to the Islamic Azad University, Mashhad Branch for its financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(9), 808–813
Rights and permissions
About this article
Cite this article
Gholipour, S., Davoodnia, A. & Nakhaei-Moghaddam, M. Synthesis, characterization, and antibacterial evaluation of new alkyl 2-amino-4-aryl-4H-chromene-3-carboxylates. Chem Heterocycl Comp 51, 808–813 (2015). https://doi.org/10.1007/s10593-015-1779-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1779-1