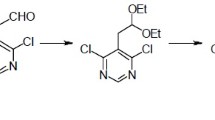
A practical, robust and scalable synthesis of 6-bromo-4-chlorothieno[2,3-d]pyrimidine starting from cheap bulk chemicals has been developed. The method involves four synthetic steps: Gewald reaction, pyrimidone formation, bromination, and chlorination. The process relies on standard laboratory equipment, allowing to obtain the product in an overall yield of 49% without using chromatography for purification of the product and intermediates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The thienopyrimidine scaffold is frequently used in medicinal chemistry, e.g., in antimicrobial [1-3] or antifungal agents [3, 4], for treatment of viral infections [5], treatment of bone diseases including osteoporosis [6, 7], as adenosine A2A receptor antagonists for Parkinson's disease [8, 9], as antiHIV agents [10], as immunosuppressive agents [11], and as anticancer agents [12-19]. One reason for the extensive use of the thienopyrimidine core is its favorable biopharmaceutical profile and its structural similarity to purines [7]. Examples of medicinal use of some thieno[2,3-d]pyrimidines are shown in Figure 1.
Thienopyrimidines can be prepared from both pyrimidine [21, 22] or thiophene derivatives [23, 24]. But 2-aminothiophenes, available by the Gewald reaction, are especially useful precursors for thienopyrimidines [25]. The classical Gewald synthesis is a three-component reaction, where an aldehyde or ketone is condensed with an activated nitrile in the presence of a sulfur source. This leads to 2-aminothiophenes with electron-withdrawing groups, such as cyano or ethoxycarbonyl group in position 3 and alkyl and/or aryl groups in positions 4 and 5 [26].
6-Bromo-4-chlorothieno[2,3-d]pyrimidine (1) has previously been prepared in 27% yield employing Gewald chemistry as the first step [27]. A process starting from methyl 2-aminothiophene-3-carboxylate (2) giving a yield of 16% is also reported [28]. Structurally related compounds have been prepared in 8 and 18% yields [7].
For the synthesis of a library of thienopyrimidine-based kinase inhibitors [19], hundreds of grams of 6-bromo-4-chlorothieno[2,3-d]pyrimidine (1) were required. As we found the price of compound 1 and its precursors unacceptably high and the established methodology unsuited for scaling, we herein present the development of an improved and scalable synthesis of this versatile building block.
We have previously described the synthesis of compound 1, giving 30% yield of a semipure product [29]. Even though the synthesis was applicable, the method had some shortcomings and potential for improvement in yield and ease of work-up. Thus, each of the process steps were tuned and developed.

Gewald reaction. The Gewald reaction was seen as the preferred method to form methyl 2-amino-thiophene-3-carboxylate (2). However, acetaldehyde is not a suitable coupling partner for this transformation due to its low boiling point. Instead, the commercially available dimer of 2-mercaptoacetaldehyde, 1,4-di-thiane-2,5-diol, was used [30]. This also circumvents the need of elementary sulfur as required in the traditional Gewald reaction.
The Gewald reaction of 1,4-dithiane-2,5-diol with methyl cyanoacetate was first tested using both microwave (MW) and conventional heating. Besides a shortening of the reaction time, no major benefit was seen from the use of microwaves. Thus, focus was put on the thermal variant, as equipment size limited the scalability of the MW reactions.

The thermal reactions were run in methanol using 0.4 equivalents of triethylamine as base in scales ranging from 20 to 207 g (Table 1, Entries 1-3). The transformation required 3-3.5 h stirring at 40-60°C to reach full conversion. However, at a 408-gram scaleFootnote 1 (Entry 4) after addition of only a small part (0.03 equiv.) of the total triethylamine amount, the volume-to-surface ratio reached a critical limit, where the reaction became self-sustained with heat. This exothermic release / thermal runaway increases the rate of reaction, consequentially producing more heat. Ice-water cooling was needed, but the increased internal temperature had no influence on the yield or the purity of the isolated product. On further scaling controlling this exothermal reaction could be more challenging.
Isolation of compounds 2 was initially done as described by Hallas et al. [31], by prolonged cooling leading to precipitation directly from the reaction mixture (Entry 1). As indicated by the melting point of the product (72-73°C, lit. mp 79-80°C [31]), this technique caused impurities to co-precipitate with the product. However, organic by-products were not observed by 1H and 13C NMR spectroscopy. This semipure material could be used as such in subsequent steps, however, purification by recrystallization from petroleum ether (bp 60-80°C) was found efficient, yielding large needle-shaped crystals (Fig. 2).
In an alternative purification strategy, the product could be isolated from the crude reaction mixture by multiple extractions using boiling petroleum ether. The procedure gave good yields of the corresponding products (85-87% in three experiments) in high purity of the product, and was successfully performed in up to 408-gram scale (Entries 2-4). Although a continuous extraction process with solvent recovery can be envisioned, this work-up was rather labor intensive. Therefore, as a third alternative, a distillation/sublimation of the crude product was attempted. Methyl 2-aminothiophene-3-carboxylate (2) evaporated readily under reduced pressure at elevated temperature, giving an equally pure product at the expense of approximately 10% in yield, with no use of solvent. Consequently, we believe that the most cost-effective method is sublimation under reduced pressure followed by recrystallization (Entry 5). As the sublimation was done using a homemade apparatus, the yield could be further improved by optimization of equipment.
Compound 2 was found to be somewhat light-sensitive. Upon prolonged storage of the solid in daylight at 22°C, a slight color change was observed. When compound 2 dissolved in either CDCl3 or DMSO-d6 was stored in daylight for 3 months, NMR spectra of both samples showed signs of decomposition. However, when kept as a solid at -18°C in dark bottles, no decomposition was noticed for over a year. Regular handling, however, did not appear to cause major problems.
In conclusion, the Gewald chemistry leading to methyl 2-aminothiophene-3-carboxylate could be performed on a 408-gram scale. Depending on the lab set-up, purification can be done with hot extraction, recrystallization, or sublimation.
Thieno[2,3- d ]pyrimidone formation. Preparation of the bicyclic thieno[2,3-d]pyrimidin-4(3H)-one (3) is a classic example of pyrimidone formation [15, 32] refluxing compound 2 in neat formamide [11, 33]. The first trial reactions at high temperature resulted in extensive tar formation, that complicated isolation and purification of the product. The initial goal was thus to identify conditions giving full conversion at lower temperatures. To achieve this, a more thorough understanding of the reaction was essential.
Reducing the temperature from 215 to 124°C (measured in the reaction mixture) gave a slower process and allowed for a more comprehensive study of the reaction. At this temperature, 28% conversion to compound 3 was observed after 48 h (Fig. 3a ). The condensation proceeds via the formamide intermediate 4 [34], easily observed by 1H NMR spectroscopy.

In accordance with Hesse et al. [34], we also confirmed that addition of ammonium formate accelerates the reaction. This can be seen by comparing the reaction progress of transformations without ammonium formate (Fig. 3a ) with that performed using 1.5 equivalents of ammonium formate (Fig. 3b ).
To optimize the reaction conditions, a 3-level factorial design was used with reaction temperature (124, 140, and 154°C), reaction time (7, 24, and 48 h), and ammonium formate concentration (1.50, 4.75, and 8.00 equiv.) as variables, along with mole fraction of product as the response.
The Pareto chart for the design of experiments (DoE) indicated that the reaction time and temperature, and the product of these, as well as the amount for ammonium formate added to the reaction, were significant variables (Fig. 4).
The goal was to obtain full conversion within 24 h, and the temperature required for achieving this was 154°C, regardless of the amount of ammonium formate added. With 8 equivalents of ammonium formate, at the same temperature, complete conversion was achieved after 7 h. Thus, the most suitable condition for the reaction was the use of 6 equivalents of ammonium formate in formamide at 154°C. A contour plot of the mole fraction of product as a function of the amount of ammonium formate and the reaction temperature using 7 h reaction time is shown in Figure 5.
Conversion of aminothiophene 2 to compound 3 as function of temperature and amount of ammonium formate, after 7 h. Regression analysis of the data points taken at 7 h correlated well with the reaction temperature and equivalents of ammonium formate used (correlation coefficient 0.97, standard deviation 0.08, statistical significance 46.78, nine experiments). The contour plot was generated using the graph function in Minitab 15 from Minitab Inc.
Moreover, the use of more than 6 equivalents of ammonium formate in this process also simplified work-up of the reaction, since the product under these conditions precipitated upon cooling. Subsequently, the optimized conditions were used for a stepwise scale-up ending at 75 g, with a yield of 70-76% in six experiments.
Bromination and chlorination. Various bromination strategies were evaluated for the conversion of compound 3 to 6-bromothieno[2,3-d]pyrimidin-4(3H)-one (5). A similar transformation was performed by Jang et al. [11] using a low-temperature reaction with n-BuLi and CBr4 in THF. However, due to reported low yields and formation of side products, the reaction was not investigated. Instead, a more atom-efficient method employing 2 equivalents bromine was tested [35]. Bromination of compound 3 was performed in acetic acid in a 1-50-gram scale giving the target compound 5 in good yield. Further, the amount of bromine added could be reduced to 1.5 equivalents without noticeable effect on yield or purity.

Work-up of the small-scale reactions was done by quenching the reaction mixture into a blend of saturated aqueous NaHCO3 and ice. However, when the reaction was performed with 50 g of compound 3 in 600 ml of acetic acid, a large total volume was required in the neutralization step. This was solved by allowing the product 5 to precipitate from the reaction mixture and isolation of the product by filtration, followed by neutralization of the solid collected. By using this isolation process, four reactions were performed on a 50-gram scale yielding 91-95% of the product 5.
The chlorination of 6-bromothieno[2,3-d]pyrimidin-4(3H)-one (5) was performed on a 7-50-gram scale in neat POCl3 at 110°C [35]. As with the bromination reaction, full conversion was essential to minimize the need for additional purification. Due to unsuccessful attempts to use a co-solvent in this chlorination, the reaction was run in neat POCl3. To achieve good stirring, a substrate/POCl3 ratio of no more than 0.45 g/ml was found to be essential. The chlorinations were monitored by 1H NMR spectroscopy, and full conversion was achieved in all cases (Table 2). The slightly lower yield experienced in the earlier trials (Entries 1, 2) were judged to be due to a low assay of the starting material.
In contrast to the bromination process, filtration of the reaction mixture after chlorination was unsuccessful due to vigorous generation of gas and heat from the solid material. Thus, a complete work-up of the reaction mixture was initially performed. This led to neutralization of large volumes of unreacted POCl3, which combined with aqueous NaHCO3 to produce a lot of foam. Another problem was the total volume of the solution due to the weak base used. By neutralization of the reaction mixture with a 5 M aqueous NaOH solution with ice, the formation of gas and heat and the total volume were reduced. Another strategy that was investigated was firstly to remove excess POCl3 under high vacuum with a double trap [37]. This led to a simplified neutralization, and the optimized reaction conditions were used for a stepwise scale-up. For the final reactions performed on 30-50-gram scale in five experiments, compound 1 was synthesized in a yield of 90-94%. The obtained 6-bromo-4-chlorothieno[2,3-d]pyrimidine (1) could be used directly in subsequent reactions without problems. However, a slightly lower melting point and a 5-10% loss in mass after silica gel column chromatography indicated an impurity in the product, not detectable by 1H or 13C NMR spectroscopy. By evaluation of various filter aids and solvents, the combination of aluminum oxide and dichloromethane was found to be superior. In this system, a short-path filtration resulted in excellent purity (mp 115-116°C; lit. mp 116°C [36]) with a yield of 89-95% in four experiments.
By optimization and process development of an established route, a practical, robust, and scalable synthesis of 6-bromo-4-chlorothieno[2,3-d]pyrimidine has been developed. All four steps have been demonstrated on a multigram scale, giving the target product in 49% overall yield, which is a considerable improvement from earlier reports. All steps can be performed without the use of traditional chromatography-based purification.
Experimental
1H and 13C NMR spectra were recorded with a Bruker Avance 400 spectrometer (300 or 400 MHz and 100 MHz, respectively) in DMSO-d6, as internal standard – signals of the solvent ( 2.50 ppm for 1H nuclei, 39.5 ppm for 13C nuclei). HPLC (Agilent 110-Series) with a G1379A degasser, G1311A Quatpump, G1313A ALS autosampler and a G1315D Agilent detector (254 nm) was used to determine the purity of the final synthesized compounds. Conditions: Poroshell C18 (100 ×4.6 mm) column, flow rate 0.8 ml/min, elution starting with H2O–MeCN (90:10), 5 min isocratic elution, then linear gradient elution for 35 min ending with pure MeCN. The Agilent ChemStation software was used for the HPLC. All melting points were measured by a Stuart automatic melting point SMP40 apparatus and are uncorrected.
1,4-Dithiane-2,5-diol, methyl cyanoacetate, formamide, ammonium formate, Br2 and POCl3 were purchased from Sigma Aldrich. All the compounds have previously been characterized in other studies from our laboratory [29]. Aluminum oxide 90 active neutral, 63-200 μm (Merck) was used. Petroleum ether of bp 60-80°C was used in the experiments.
Methyl 2-Aminothiophene-3-carboxylate (2) [31]. Methyl cyanoacetate (304.05 g, 3.07 mol), 1,4-dithiane-2,5-diol (312.76 g, 2.05 mol), and MeOH (1000 ml) were mixed and cooled with an ice bath while stirring. Et3N (100 ml, 0.82 mol) was added slowly over 2.5 h. The reaction mixture was heated at 40°C for 1 h, then the mixture was filtered. The liquid phase was evaporated in vacuo, giving a yellow crude product. Yield 583.40 g (121%).
Work-up with extraction
Petroleum ether (750 ml) was added to the crude product, and the mixture was heated to reflux, resulting in the formation of two phases. The clear upper phase was separated and the process repeated ten times. The combined upper phases were evaporated in vacuo to give pure compound 2. Yield 408.20 g (85%), white needle-shaped crystals with a slight hint of yellow, mp 78-80°C (petroleum ether) (mp 77-78°C (petroleum ether) [38], mp 79-80°C (MeOH) [31]).
Work-up with sublimation
The crude product (90.12 g) was heated to 115°C at 0.06 mbar, which gave a liquid phase. The vapor became a solid material upon cooling. Yield 60.37 g (67%), white crystalline solid with a slight hint of yellow, mp 80-81°C (mp 79-80°C (MeOH) [31]).
Work-up with recrystallization
Compound 2 (19.5 g) was dissolved in boiling petroleum ether (19 ml per 1 g of compound 2). The crystals formed upon cooling were isolated by filtration, washed with cold petroleum ether, and dried. Yield 15.8 g (81%), white needle-shaped crystals, mp 80-81°C (petroleum ether) (mp 79-80°C (MeOH) [31]). 1H NMR spectrum (300 MHz), δ, ppm (J, Hz): 3.69 (3H, s, COOCH3); 6.27 (1H, d, J =5.8, H-4); 6.81 (1H, d, J =5.8, H-5); 7.24 (2H, s, NH2). 13C NMR spectrum, δ, ppm: 50.5; 103.7; 106.5; 125.0; 164.0; 164.8. The NMR spectral data for CDCl3 solution correspond to that reported in [39].
Experimental Design of the Thieno[2,3- d ]pyrimidin-4(3 H )-one (3) Synthesis. Methyl 2-amino-thiophene-3-carboxylate (2) (1.00 g, 6.40 mmol), HCONH2 (12 ml), and HCOONH4 (0, 1.50, 4.75 or 8.00 equiv.) were mixed under N2 atmosphere. The reaction mixtures were heated to 124, 140, or 154°C (measured in the reaction mass). Samples were withdrawn after 7, 24, and 48 h, diluted with DMSO-d6, and analyzed by 1H NMR spectroscopy. Selected experiments were more comprehensively sampled. Pure samples of methyl 2-aminothiophene-3-carboxylate (2) and thieno[2,3-d]pyrimidin-4(3H)-one (3) were used as reference standards, while the identity of methyl 2-formamidothiophene-3-carboxylate (4) was proved by comparing the 1H NMR spectrum of a semipure sample with that reported by Hesse [34]. The product distribution and thus the mole fraction of compounds were determined from 1H NMR spectra by integration of the methyl group signal at 3.69 ppm for compound 2, the methyl group signal at 3.84 ppm for compound 4, and the signal of H-2 proton at 8.12 ppm for compound 3. Analysis of the design of experiments was done using Minitab 15 from Minitab Inc. at a confidence level of 95%. The Pareto Chart was obtained using the experimental data shown in Table S-1 in the supporting information file. Plotting was performed by Minitab 15 from Minitab Inc.
Thieno[2,3- d ]pyrimidin-4(3 H )-one (3). Methyl 2-aminothiophene-3-carboxylate (2) (75.21 g, 0.48 mol), HCOONH4 (202.67 g, 3.21 mol), and HCONH2 (500 ml) were mixed under N2 atmosphere. The reaction mixture was heated to 155°C (measured in the reaction mass) for 19 h. Upon completion, the mixture was cooled to -18°C for 24 h. The formed crystals were filtered off, washed with water (3×100 ml), and dried. Yield 55.07 g (76%), yellow crystalline solid, mp 241-245°C (decomp.) (mp 245°C (decomp., HCONH2) [34]). 1H NMR spectrum (300 MHz), δ, ppm (J, Hz): 7.39 (1H, d, J =5.8, H-5); 7.58 (1H, d, J =5.8, H-6); 8.12 (1H, s, H-2); 12.46 (1H, s, NH). 13C NMR spectrum, δ, ppm: 121.6, 123.8, 124.6, 145.6, 157.5, 164.2. The NMR spectroscopic data correspond to that previously reported [34].
Methyl 2-(Formylamino)thiophene-3-carboxylate (4) [34]. An analytical sample of compound 4 was prepared from methyl 2-aminothiophene-3-carboxylate (2) (1.00 g, 6.40 mmol), HCONH2 (12 ml), and HCOONH4 (0.61 g, 9.6 mmol) by heating the reaction mixture at 124°C for 7 h under N2 atmosphere. 1H NMR (300 MHz), δ, ppm (J, Hz): 3.84 (3H, s, COOCH3); 7.05 (1H, d, J =5.8, H-4); 7.19 (1H, d, J =5.8, H-5); 8.56 (1H, s, NHCHO); 11.32 (1H, br. s, NH). The spectrum correlated well with that reported in [34].
6-Bromothieno[2,3- d ]pyrimidin-4(3 H )-one (5). Compound 3 (50.03 g, 329 mmol) and glacial AcOH (600 ml) were mixed. Elementary bromine (30 ml, 582 mmol) was added dropwise with stirring at room temperature. The mixture was heated at 80°C for 1 h, then cooled to room temperature and filtered. The solid material was washed with water, saturated aq. NaHCO3 solution, and water again until neutral pH of the washing liquid. The product was dried for 24 h in air, then under reduced pressure. Yield 72.24 g (95%), sand-colored solid, mp 299-301°C (decomp., H2O) (mp 301-304°C (decomp., CH2Cl2) [40]). 1H NMR spectrum (400 MHz), δ, ppm: 7.55 (1H, s, H-5); 8.15 (1H, s, H-2); 12.64 (1H, s, NH). 13C NMR spectrum, δ, ppm: 110.3; 124.6; 125.6; 146.5; 156.0; 164.9. The NMR spectroscopic data correspond with that reported in [27, 35].
6-Bromo-4-chlorothieno[2,3- d ]pyrimidine (1). Compound 5 (50.00 g, 216 mmol) and POCl3 (110 ml, 1180 mmol) were mixed and stirred at 110°C for 2.5 h. Then the mixture was cooled to room temperature and treated as described below.
Work-up with neutralization
The total reaction mixture was poured into ice water (1500 ml) and neutralized with 5 M aq. NaOH solution (900 ml). The mixture was filtered and the solid material washed with H2O (500 ml) and dried. Yield 50.57 g (94%), a sand-colored solid, mp 113-114°C (H2O) (mp 116°C (Et2O) [36]).
Work-up with evaporation and neutralization
The excess POCl3 was removed under high vacuum with a double trap. Subsequent neutralization was performed similarly to the work-up without evaporation.
Purification of 6-bromo-4-chlorothieno[2,3-d]pyrimidine (1)
Dry 6-bromo-4-chlorothieno[2,3-d]pyrimidine (1) (29.41 g) was applied to a column packed with Al2O3 (100 ml) and washed through with CH2Cl2 (33 ml per 1 g of compound 1). The first fraction (100 ml) was discarded, while the rest was collected, evaporated, and dried. Yield 27.66 g (94%), off-white solid, mp 115-116°C (mp 116°C (Et2O) [36]). HPLC purity >99%, t R 23.8 min. Compound 1 could also be recrystallized from EtOAc to give X-ray quality crystals. 1H NMR spectrum (400 MHz), δ, ppm: 7.90 (1H, s, H-5), 8.96 (1H, s, H-2). 13C NMR spectrum, δ, ppm: 118.5; 122.9; 130.2; 152.5; 153.2; 169.0. The NMR spectroscopic data correspond with that reported in [35].
The Supplementary file containing 1H and 13C NMR spectra, alongside experimental data for the Design of Experiments for compound 3 is available at the webpage http://hgs.osi.lv.
Anders Jahres Foundation and NTNU Technology Transfer AS (TTO-NTNU Trondheim) are acknowledged for financial support. Roger Aarvik and Svein Jacob Kaspersen are acknowledged for their contributions.
Notes
* As an exception, scale in this case is based on amount of product produced in the reaction. If nothing else is indicated, scale is based on the amount of substrate for the reaction.
References
Z. A. Hozien, F. M. Atta, K. M. Hassan, A. A. Abdel-Wahab, and S. A. Ahmed, Synth. Commun., 26, 3733 (1996).
P. J. Hill, A. Abibi, R. Albert, B. Andrews, M. M. Gagnon, N. Gao, T. Grebe, L. I. Hajec, J. Huang, S. Livchak, S. D. Lahiri, D. C. McKinney, J. Thresher, H. Wang, N. Olivier, and E. T. Buurman, J. Med. Chem., 56, 7278 (2013).
S. B. Kanawade, R. B. Toche, and D. P. Rajani, Eur. J. Med. Chem., 64, 314 (2013).
N. Tani, M. Rahnasto-Rilla, C. Wittekindt, K. A. Salminen, A. Ritvanen, R. Ollakka, J. Koskiranta, H. Raunio, and R. O. Juvonen, Eur. J. Med. Chem., 47, 270 (2012).
J. S. De, L.-J. Gao, P. Herdewijn, J. Herman, M. Jang, P. Leyssen, T. Louat, J. Neyts, C. Pannecouque, and B. Vanderhoydonck, WO Pat. Appl. 2011147753.
C.-Y. Leung, A. M. Langille, J. Mancuso, and Y. S. Tsantrizos, Bioorg. Med. Chem., 21, 2229 (2013).
C. Y. Leung, J. Park, J. W. De Schutter, M. Sebag, A. M. Berghuis, and Y. S. Tsantrizos, J. Med. Chem., 56, 7939 (2013).
B. C. Shook, D. Chakravarty, J. K. Barbay, A. Wang, K. Leonard, V. Alford, M. T. Powell, S. Rassnick, R. H. Scannevin, K. Carroll, N. Wallace, J. Crooke, M. Ault, L. Lampron, L. Westover, K. Rhodes, and P. F. Jackson, Bioorg. Med. Chem. Lett., 23, 2688 (2013).
J. K. Barbay, D. Chakravarty, B. C. Shook, and A. Wang, WO Pat. Appl. 2010045006.
C. Tintori, I. Laurenzana, F. La Rocca, F. Falchi, F. Carraro, A. Ruiz, J. A. Este, M. Kissova, E. Crespan, G. Maga, M. Biava, C. Brullo, S. Schenone, and M. Botta, ChemMedChem, 8, 1353 (2013).
M.-Y. Jang, S. De Jonghe, K. Van Belle, T. Louat, M. Waer, and P. Herdewijn, Bioorg. Med. Chem. Lett., 20, 844 (2010).
E. R. Wood, L. M. Shewchuk, B. Ellis, P. Brignola, R. L. Brashear, T. R. Caferro, S. H. Dickerson, H. D. Dickson, K. H. Donaldson, M. Gaul, R. J. Griffin, A. M. Hassell, B. Keith, R. Mullin, K. G. Petrov, M. J. Reno, D. W. Rusnak, S. M. Tadepalli, J. C. Ulrich, C. D. Wagner, D. E. Vanderwall, A. G. Waterson, J. D. Williams, W. L. White, and D. E. Uehling, Proc. Natl. Acad. Sci. USA, 105, 2773 (2008).
Y. Dai, Y. Guo, R. R. Frey, Z. Ji, M. L. Curtin, A. A. Ahmed, D. H. Albert, L. Arnold, S. S. Arries, T. Barlozzari, J. L. Bauch, J. J. Bouska, P. F. Bousquet, G. A. Cunha, K. B. Glaser, J. Guo, J. Li, P. A. Marcotte, K. C. Marsh, M. D. Moskey, L. J. Pease, K. D. Stewart, V. S. Stoll, P. Tapang, N. Wishart, S. K. Davidsen, and M. R. Michaelides, J. Med. Chem., 48, 6066 (2005).
H.-P. Hsieh, S. M. Coumar, T.-A. Hsu, W.-H. Lin, Y.-R. Chen, and Y.-S. Chao, US Pat. Appl. 20100120805.
C.-H. Wu, M. S. Coumar, C.-Y. Chu, W.-H. Lin, Y.-R. Chen, C.-T. Chen, H.-Y. Shiao, S. Rafi, S.-Y. Wang, H. Hsu, C.-H. Chen, C.-Y. Chang, T.-Y. Chang, T.-W. Lien, M.-Y. Fang, K.-C. Yeh, C.-P. Chen, T.-K. Yeh, S.-H. Hsieh, J. T.-A. Hsu, C.-C. Liao, Y.-S. Chao, and H.-P. Hsieh, J. Med. Chem., 53, 7316 (2010).
A. Zhao, X. Gao, Y. Wang, J. Ai, Y. Wang, Y. Chen, M. Geng, and A. Zhang, Bioorg. Med. Chem., 19, 3906 (2011).
J. Zhang, CN Pat. Appl. 102872018.
T. P. Heffron, B. Q. Wei, A. Olivero, S. T. Staben, V. Tsui, S. Do, J. Dotson, A. J. Folkes, P. Goldsmith, R. Goldsmith, J. Gunzner, J. Lesnick, C. Lewis, S. Mathieu, J. Nonomiya, S. Shuttleworth, D. P. Sutherlin, N. C. Wan, S. Wang, C. Wiesmann, and B.-Y. Zhu, J. Med. Chem., 54, 7815 (2011).
S. Bugge, S. J. Kaspersen, S. Larsen, U. Nonstad, G. Bjørkøy, E. Sundby, and B. H. Hoff, Eur. J. Med. Chem., 75, 354 (2014).
J. K. Barbay, K. Leonard, D. Chakravarty, B. C. Shook, and A. Wang, WO Pat. Appl. 2010045013.
T. Sakamoto, Y. Kondo, R. Watanabe, and H. Yamanaka, Chem. Pharm. Bull., 34, 2719 (1986).
T. Sakamoto, Y. Kondo, and H. Yamanaka, Chem. Pharm. Bull., 30, 2417 (1982).
M. Robba, J. M. Lecomte, and M. Cugnon de Sevricourt, Bull. Soc. Chim. Fr., 587 (1975).
V. P. Arya, Indian J. Chem., 10, 1141 (1972).
R. W. Sabnis, Sulfur Rep., 16, 1 (1994).
R. W. Sabnis, D. W. Rangnekar, and N. D. Sonawane, J. Heterocycl. Chem., 36, 333 (1999).
J. Grembecka, S. He, A. Shi, T. Purohit, A. G. Muntean, R. J. Sorenson, H. D. Showalter, M. J. Murai, A. M. Belcher, T. Hartley, J. L. Hess, and T. Cierpicki, Nat. Chem. Biol., 8, 277 (2012).
P. Herdewijn, J. S. De, L.-J. Gao, M.-Y. Jang, B. Vanderhoydonck, M. J. A. Waer, Y. Lin, J. F. Herman, and T. A. M. Louat, WO Pat. Appl. 2010103130.
S. Bugge, S. J. Kaspersen, E. Sundby, and B. H. Hoff, Tetrahedron, 68, 9226 (2012).
K. Wang, D. Kim, and A. Domling, J. Comb. Chem., 12, 111 (2010).
G. Hallas and A. D. Towns, Dyes Pigm., 33, 319 (1997).
F. D. Therkelsen, M. Rottländer, N. Thorup, and E. B. Pedersen, Org. Lett., 6, 1991 (2004).
J. Herman and T. Louat, WO Pat. Appl. 2012035423.
S. Hesse, E. Perspicace, and G. Kirsch, Tetrahedron Lett., 48, 5261 (2007).
T. R. Caferro, S. D. Chamberlain, K. H. Donaldson, P. A. Harris, M. D. Gaul, D. E. Uehling, and D. E. Vanderwall, WO Pat. Appl. 2003053446.
M. Robba, J. M. Lecomte, and M. Cugnon de Sevricourt, Bull. Soc. Chim. Fr., 592 (1975).
T. Park, M. F. Mayer, S. Nakashima, and S. C. Zimmerman, Synlett, 1435 (2005).
K. Gewald, Chem. Ber., 98, 3571 (1965).
Y. Huang, S. Wolf, M. Bista, L. Meireles, C. Camacho, T. A. Holak, and A. Domling, Chem. Biol. Drug Des., 76, 116 (2010).
J. F. Atherall, T. L. Hough, S. D. Lindell, M. J. O'Mahony, E. A. Saville-Stones, and J. H. Parsons, WO Pat. Appl. 9849899.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1275-1285, August, 2014.
Rights and permissions
About this article
Cite this article
Bugge, S., Skjønsfjell, E.M., Willumsen, F.B. et al. Improved and Scalable Preparation of 6-Bromo-4-Chlorothieno[2,3-d]Pyrimidine. Chem Heterocycl Comp 50, 1177–1187 (2014). https://doi.org/10.1007/s10593-014-1579-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1579-z