The N-arylation of a series of nitroazoles has been studied with the aid of diaryliodonium salts in the presence of CuI under the action of microwave radiation. It was found that alkylation proceeds regioselectively in each actual case with the formation of one of two possible isomers. The correct structure of the N-arylation products was established on the basis of NMR spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nitroazoles modified at the nitrogen atom, including N-arylnitroazoles, possess a broad spectrum of useful properties [1–3]. The difficultly available N-aryl derivatives of nitroazoles containing no electron-withdrawing groups in the aryl fragment are of significant interest. Among the latter effective anti-inflammatory preparations [4], promising antitubercular preparations [5], and synthons for obtaining other heterocyclic compounds have been discovered [6].
The most rational means of obtaining similar N-aryl derivatives of nitroazoles is the direct regioselective introduction of an aryl fragment at the assigned nitrogen atom. In the chemical literature we discovered a single example of the classical phenylation of 3-nitropyrazole under the action of PhBr by the Ullman reaction [7]. However this process is extremely laborious, is brought about under very regirous conditions, and has many limitations. Another approach to obtaining N-aryl derivatives of nitroazoles is electrosynthesis, however this variant of N-arylation requires special apparatus and seldom proceeds chemoselectively, see for example [8].
With the development of methods of catalytic N-arylation according to Buchwald-Hartwig [9] and the modern variant of the Ullman reaction [10, 11] the possibility arose of introducing unactivated aryl groups at the nitrogen atom of the initial azole under mild conditions. However it turned out that the classical arylating agents of the aryl halide type did not react with the anions of low-basicity nitroazoles in the presence of copper and palladium catalysts in the proposed conditions. N-Arylation of similar substrates may be effected only under the action of aryl derivatives of elements existing in the polyvalent state, for example involving diaryliodonium salts.
A single study is known in which arylation was carried out of 2-methyl-5-nitroimidazole (1) by the action of diaryliodonium salts [12]. The authors of this study assigned a structure of 2-methyl-1-(4-methylphenyl)-5-nitro-1H-imidazole (1b) to the reaction product without adequate grounds.

DBU – 1,8-diazobicyclo[5.4.0]undec-7-ene
However our attempts at working on the arylation of ambident azoles possessing a high NH acidity [13–15] led to doubts as to the correctness of this conclusion. With great difficulty we succeeded in repeating this experiment and isolated a product of N-arylation in low yield, which according to our physicochemical characteristics proved to be identical to a sample obtained by us on arylating the initial nitroazole with (p-MeC6H4)2I+BF ¯4 under conditions of microwave irradiation, and to a sample obtained by the interaction of 2-methyl-1,4-dinitroimidazole with p-toluidine by an ANRORC mechanism [6]. Optimization of the conditions for arylation of nitroazoles under conditions of microwave irradiation showed that the best solvents for the reaction with catalysis by CuI were aliphatic nitriles, and the universal base DBU. Preliminary results obtained by us of the investigation of high resolution 1H NMR spectra, two-dimensional NMR COSY, 13C-HSQC, and 13C-HMBC and measurement of NOE factors also showed that the arylation product has a different structure and corresponds to the structure of 2-methyl-1-(4-methylphenyl)-4-nitro-1H-imidazole (1a).
In a similar manner the reaction of 3-methyl-5-nitropyrazole (2), isomeric with compound 1, with (p-MeC6H4)2I+BF ‒4 was investigated under analogous conditions. As in the first case the process proceeded regioselectively with the formation of 5-methyl-1-(4-methylphenyl)-3-nitro-1H-pyrazole (2a) and not compound 2b.

To clarify the general regularities of N-arylation of ambident nitroazoles we investigated a series of nitroazoles, viz. 2-methyl-5-nitroimidazole (1), 3-methyl-5-nitropyrazole (2), 3,5-dimethyl-4-nitropyrazole (3), 4-nitroimidazole (4), 3-nitropyrazole (5), 2,4-dinitroimidazole (6), and 3-nitro-1,2,4-triazole (7), possessing NH acidity in the pKa range 2.85-10.65 units [16], under the action of the readily available Ph2I+OTs-. The data obtained for the N-phenyl derivatives of the indicated nitroazoles 1c-7c are given in Table 1.
The unequivocal establishment of the structure of the arylation products is the most important stage of the study. Analysis of literature data shows that the very chemical shifts themselves of the 1H and 13C nuclei of azoles may not unequivocally characterize such fine details of the structure of molecules necessary for the reliable identification of isomers 1a and 2a. For derivatives of azoles the presence of substituents and heteroatoms, containing no magnetic nuclei, introduces additional complications due to the fact that in many cases the possibility of using proton-proton coupling constants is excluded. Nonetheless, NMR spectroscopy itself may give significant information on the structure of these compounds (see, for example, discussions in [17]). The most reliable results may be obtained on the basis of a maximally complete combination of experimental parameters of the NMR spectra.
The 13C–H long range coupling constants [18, 19] and the NOE factors (see [20]) afforded especially important information on structure. The effectiveness of this approach was demonstrated by us previously when establishing the structure of a large series of polysubstituted indoles formed in the course of transforming pyridinium salts [21–23]. A good basis for structural assignments was given by the available 13C–H coupling constants in unsaturated five-membered rings, for cyclopentadiene [24], pyrrole [25], furan [26], and thiophene [27].
The parameters of the NMR spectra in the polysubstituted azoles are linked in a complex way with the structure of the molecule. An important factor determining the properties of the polysubstituted nitroazoles studied by us is the conformation of the molecule, and in particular the orientation of the benzene ring at the ring heteroatom and of the nitro group relative to the plane of the azole. Nonempirical calculations carried out by us on the structure of the molecules (RHF approach with basis functions of the 6 G-311(d,p) type) showed that substitutions affect, to the greatest extent, the degree of turning of the plane of the benzene ring linked with the nitrogen atom. This process may be characterized by the value of the dihedral angle ψC-5,N,C-ipso,C-o , where the atoms C-5 and N are in the azole skeleton, and C-ipso and C-o correspond to the ipso and ortho carbons of the benzene ring. In the absence of substituents at atom C-5 the degree of turning of the plane of the benzene ring was of the order of 35°, which is close to the corresponding value for benzene. However in the presence of substituents at the C-5 atom, due to the emergence of steric hindrance, the turning grows practically to 90°. Oxygen atoms inclined to affect the π-conjugation of the nitro group in the majority of aromatic compounds studied strive to be found in the plane of the molecule, when conjugation with the π-electron system of the molecule is maximal. Our calculations showed that in the compounds studied the oxygen atoms of the nitro group may emerge significantly from the plane of the aromatic framework of the heterocycle. The corresponding dihedral angle φX,C,N,O differs significantly from zero in this case (X and C are skeletal atoms of the azole, N and O are nitrogen and oxygen of the nitro group). This effect depends on the presence and character of substituents in the ortho position relative to the nitro group (see Table 1). The effect of the emergence of a nitro group bonded with an aromatic system from the plane of the molecule has been mentioned in the literature previously in the example of 2,6-difluoronitrobenzene, where the corresponding dihedral angle φC,C,N,O was equal to 53.8(14)° [28].
The conformational heterogeneity of the structure appearing in the course of our calculations evidently does not permit calculation of the fulfillment of the principle of linearity in the series of N-arylnitroazoles, and as a result, of the additivity of the effects of substitution for chemical shifts both for the 1H nucleus and for the 13C nucleus (see discussion in [29]). Data of HMBC experiments, transferring information on long range 13C–H coupling constants and NOE factors, form the basis for the structural determinations in the present study. Only in individual cases did a successful combination of structural factors enable the use of information on proton-proton coupling constants (compounds 4c and 5c).
Assignment of signals in the NMR spectra and of the position of substituents are determined by analysis of multiplet structure and cross-peaks in HSQC and HMBC two-dimensional spectra using NOE factors. Establishment of structure was carried out without any prior assumptions and was based exclusively on the obtained experimental data of one- and two-dimensional NMR spectra within the framework of a strategy of sequential accumulation of structural information (see [17, 30]). Establishment of the structure of substituted azoles comprised three stages. In the first stage recording and analysis of the combination of one-dimensional (1H and 13C) and two-dimensional NMR spectra (COSY, HSQC, and HMBC) was carried out. On the basis of the data obtained (COSY, HSQC) a trial assignment was carried out of the signals in the 1H and 13C spectra. The mutual disposition of structural fragments may be established to a substantial degree on the basis of cross-peaks in the HMBC experiment. The HMBC experiment was carried out with a two-quantum filter from 2 to 8 Hz. This enabled localization of the desired region out of all possible values of the long range 13C–1H constants.
In the literature there is a widespread opinion that in two-dimensional HMBC experiments it is impossible to determine precisely the value of a coupling constant responsible for the appearance of a cross-peak [31]. We paid attention to the fact that on fulfilling definite conditions the sought-for 13C–1H coupling constant is displayed directly in the form of the splitting of the cross-peak of the two-dimensional spectrum. The structure of the multiplet proved to be particularly simple in this case, when the proton involved in the spin-spin interaction is displayed in the spectrum in the form of a singlet. As our test calculations have shown in this case when, on the coordinate corresponding to the carbon signal magnitude representation and a relatively small numerical resolution is used, the multiplicity of the cross-peak has a particularly simple form. For large long range 13C–1H constants the multiplicity is displayed as a doubling of the cross-peak at the coordinate of the proton spectrum, as shown in Fig. 1 for the cross-peaks of the H-5 proton with the C-2 and C-4 atoms of compound 6c. Displacement of the components of the cross peak enables direct determination of the values of the 3 J C-2,H-5 and 2 J C-4,H-5 constants. In the case of relatively small coupling constants, comparable in value with the width of the line involved in the spin-spin interaction of the proton signal, the value of the desired splitting may be assessed from the extent of deformation of the cross-peak line, which in this case proved to be excessively drawn out at the proton coordinate. Thus, for example, consideration of the shape of the cross-peak lines of the H-5 proton with the C-ipso atom enables assessment with an acceptable precision of the value of 3 J C-ipso,H-5 for compound 6c (see Fig. 1).
In the second stage we carried out a check of the structural assignments using NOE. Even assuming all the possible data on bonding from spin-spin interactions, it is not always successful to assign a compound to an actual molecular structure. In this case measurement of NOE factors may give the necessary information [20, 31], which enables qualitative characterization of the distance in space between the selected pair of nuclei. In the NOEDIFF procedure used by us the NOE factor characterizes the relative reduction in intensity of one signal on irradiation of the other signal. The greater the extent of this reduction the closer they are disposed in space (see [20]). The use of NOE is particularly effective for establishing the relative position of alkyl and aryl groups. In the case of an alkyl group in the α-position relative to aryl an NOE of 3.2-4.5% is observed between the α-proton of the alkyl group and the aromatic ortho proton. The NOE factors for aromatic meta protons are significantly less. para Protons do not show a significant NOE with neighboring alkyl groups. In a series of arylated azoles an NOE of ~7.2% was observed between the α-proton of the alkyl group and the vicinally disposed azole proton. In the case of the N-phenyl group a NOE of the order of 4% was observed between the aromatic ortho proton and the neighboring H-2 or H-5 proton.
The values of the NOE factors in a series of N-phenyl substituted nitroazoles are given in Table 1. The values of the NOE factors for the N-arylation products of nonambident 3,5-dimethyl-4-nitropyrazole, 3a,c, are given for comparison in Fig. 2 and in Table 1.
In the closing stage we carried out calculations of the chemical shifts of protons and of 13C nuclei of the investigated compounds. In recent years new methods have been proposed for nonempirical calculation of chemical shifts, which give a better fit with experiments on model systems in the absence of strong internal strain close to the nuclei being studied (see [32]). The systems studied by us represent a fairly strict test by contemporary methods of calculation since in practically each molecule there are strong steric interactions. Quantum-chemical calculations of chemical shifts were carried out by the GIAO method [33, 34] with basis functions of the 6G311++(d,p) type (for more detail see [35]) using the Gaussian-09W-MP programs [36] in a mode of increased precision for calculating integrals (Grid = UltraFine). Comparison of the experimental and calculated values of the 13C NMR chemical shifts of the series of N-arylnitroazoles investigated (see Fig. 3) is characterized by a fairly high correlation coefficient (R = 0.986), however the absolute deviations were 5.6 ppm on average, in limiting cases reaching 10 ppm and more. The greatest deviations were observed for quaternary carbon atoms linked directly with a nitro group, viz. the signals of the C-4 atom of compound 4c, C-2 and C-4 of compound 6c, and C-4 of compound 3c. Nonetheless it may be concluded that even at this level the calculation approach proved to be useful for structural determinations in the series of polysubstituted azoles.
The N-arylation of nitroazoles by diaryliodonium salts in the presence of CuI proceeds regioselectively with the formation of a single isomer, which has been demonstrated correctly with the aid of NMR spectroscopy and quantum-chemical calculations.
Experimental
The 1H and 13C NMR spectra were recorded on Bruker AV-600 (600 and 150 MHz respectively) spectrometers in CDCl3 (Aldrich) at 303 K, internal standard was TMS. The structure of compounds was established using analysis of every combination of data of NMR experiments of 1H, 13C, 1H–1H NOEDIFF and two-dimensional NMR experiments using COSY, HSQC, and HMBC [31]. Recording of 1H and 13C NMR spectra was carried out in impulse mode. Two-dimensional COSY-90, HSQC, and HMBC NMR correlation spectra were recorded at optimal band frequencies with sizes of selected data 4 K*1 K (COSY-90) and 8 K*1 K (HSQC, HMBC) with relaxation delays from 1.5 to 2 sec using a TBI “inverse” broad band pickup, fitted with a control system for impulse field gradients. The HSQC experiments were carried out with a 140 Hz BIRD filter. The HMBC experiments were carried out with three different values of the J-filter (4, 7, and 8 Hz), the best results were obtained for a J-filter of 7 Hz. In the case of the COSY-90 experiments a two-dimensional Fourier conversion (4 K*4 K points) in magnitude representation of data mode was used after preliminary processing with QSIM digital filters at each coordinate. Processing of the HSQC and HMBC experiments was carried out in phase-sensitive mode for 8 K*1 K data stocks using a Lorentz filter (with parameter width 1 Hz for proton coordinates and 20–30 Hz for carbon coordinates).
Chromato-mass spectrometric analysis was carried out on a Finnigan MAT 113 instrument (at 70 eV, only characteristic signals are given).
Reactions were carried out in an every-day microwave oven in Chemglass vessels (www.chemglass.com) at W max = 70 W and in a “Discover CEM” microwave oven (W max = 300 W, P max = 20 atm, www.interanalyt.ru).
Preparation of N-Arylated Nitroazoles by Reaction of Nitroazole with Iodonium Salts under Microwave Irradiation (General Method).
In a “Discover CEM” Oven. Nitroazole (1 mmol), DBU (1 mmol), and diaryl iodonium salt (1 mmol) were added with stirring to absolute PrCN (10 ml) (pyrex flask). After the components had dissolved CuI (20 mol %) was added to the mixture. The flask fitted with a reflux condenser was placed in a microwave oven and boiled at 120°C with variable radiation power for 15 min with stirring. The end of the reaction was checked by TLC and visually (the color of the reaction mixture stopped changing). After this the solvent was distilled in vacuum, the residue treated with hot CCl4, and passed through a layer of alkaline Al2O3 to remove residues of ArI and PrCN. Compounds 1a-3a were isolated by flash chromatography, eluent was EtOAc. The eluate was evaporated and the residue recrystallized from aqueous methanol.
In an Everyday Oven the reaction was carried out in a 25 ml thick-walled glass vessel fitted with a magnetic stirrer with a hermetic Teflon seal and a rubber gasket for working under pressure. Into the vessel containing absolute MeCN (10 ml) was added with stirring nitroazole (1 mmol), DBU (1 mmol), and diaryl iodonium salt (1 mmol). After solution of the components of the mixture, CuI was added (20 mol %). The vessel was sealed and left in the microwave oven, the reaction mixture was irradiated 3–4 times for 5 min each time with intermediate cooling of the reactor to room temperature with stirring. The processing of the reaction mixture and isolation of compounds 1c-7c was carried out analogously to the previous procedure.
2-Methyl-1-(4-methylphenyl)-4-nitro-1H-imidazole (1a). Yield 60%; mp 136-138°C (lit. mp 137–139°C [37]). 1H NMR spectrum, δ, ppm: 2.36 (3H, s, 2-CH3); 2.45 (3H, s, CH3 p-Tol); 7.20 2H, m, H-o); 7.34 (2H, m, H-m); 7.79 (1H, s, H-5). 13 C NMR, δ, ppm (J, Hz); 13.62 (2-CH3); 21.14 (CH3, p-Tol); 120.47 (C-5, 1 JC-5,H--5 = 201.0); 125.27 (C-o); 130.56 (C-m); 133.30 (C-ipso, 3 J C-ipso , H-5 = 1.0); 140.23 (C-p); 144.96 (C-2, 3 J C-2,H-5 = 6.7, 2 J C-2,Me = 7.9); 147.01 (C-4, 2 J C-4,H-5 = 4.3). Mass spectrum, m/z (I rel, %): 217 [M]+ (17), 145 [M-NO2CN]+ (100), 118 [M-NO2CN-HCN]+ (23), 91 [C7H7]+ (47).
2-Methyl-4-nitro-1-phenyl-1H-imidazole (1c). Yield 81%; mp 139°C (lit. mp 139-140°C [38]). 1H NMR spectrum, δ, ppm: 2.36 (3H, s, 2-CH3); 7.33 (2H, m, H-o); 7.51-7.57 (3H, m, H-p and H-m); 7.81 (1H, s, H-5). 13C NMR spectrum, δ, ppm (J, Hz): 14.60 (2-CH3); 121.36 (C-5, 1 J C-5,H-5 = 200.0); 126.45 (C-o); 130.85 (C-p); 131.00 (C-m); 136.75 (C-ipso, 3 J C-ipso,H-5 = 1.0); 145.83 (C-2, 3 J C-2,H-5 = 7.2, 2 J C-5,Me = 8.0); 148.02 (C-4, 2 J C-4,H-5 = 4.2). Mass spectrum, m/z (I rel, %): 203 [M]+ (23), 131 [M-NO2CN]+ (100), 104 [PhNHCN]+ (31 ), 77 [Ph] (85).
5-Methyl-1-(4-methylphenyl)-3-nitro-1H-pyrazole (2a). Yield 59%; mp 69-71°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.32 (3H, d, 4 J = 0.78, 5-CH3); 2.39 (3H, s, CH3 p-Tol); 6.57 (1H, q, 4 J = 0.78, H-4); 7.27 (2H, m, H-m); 7.30 (2H, m, H-o). 13C NMR spectrum, δ, ppm (J, Hz): 12.35 (5-CH3); 20.99 (CH3 p-Tol); 102.45 (C-4, 1 J C-4,H-4 = 185.0); 125.0 (C-o); 129.78 (C-m); 135.54 (C-ipso, 4 J C-ipso,H-4 ~1.0); 139.50 (C-p); 142.31 (C-5, 2 J C-5,H-4 = 7.4, 2 J C-5,Me = 7.9); 155.70 (C-3, 2 J C-3,H-4 ~3.0). Mass spectrum, m/z (I rel, %): 217 [M]+ (100), 170 [M-HNO2]+ (10), 156 [M-NO-HCN]+ (60), 91 [C7H7]+ (28). Found, %: C 60.80; H 5.08; N 19.30. C11H11N3O2. Calculated, %: C 60.82; H 5.10; N 19.34.
5-Methyl-3-nitro-1-phenyl-1H-pyrazole (2c). Yield 85%; mp 93-95°C (lit. mp 92-95°C [39]). 1H NMR spectrum, δ, ppm (J, Hz): 2.38 (3H, d, 4 J = 0.81, 5-CH3); 6.82 (1H, q, 4 J = 0.81, H-4); 7.47 (2H, m, H-o); 7.49 (1H, m, H-p); 7.52 (2H, m, H-m). 13C NMR spectrum, δ, ppm (J, Hz): 13.51 (5-CH3); 103.71 (C-4, 1 J C-4,H-4 = 185.5); 126.32 (C-o); 130.35 (C-m); 130.37 (C-p); 139.10 (C-ipso, 4 J C-ipso,H-4 ~0.5); 143.26 (C-5, 2 J C-5,H-4 = 7.6, 2 J C-5,Me = 7.7); 156.99 (C-3, 2 J C-3,H-4 ~2.0). Mass spectrum, m/z (I rel, %): 203 [M]+ (100), 186 [M-OH]+ (15), 156 [M-HNO2]+ (22), 142 [M-HN2O2]+, 77 [Ph] (37).
3,5-Dimethyl-1-(4-methylphenyl)-4-nitro-1H-pyrazole (3a). Yield 52%; mp 119-121°C. 1H NMR spectrum, δ, ppm: 2.44 (3H, s, CH3 p-Tol); 2.59 (3H, s, 3-CH3); 2.61 (3H, s, 5-CH3); 7.27 (2H, m, H-o); 7.32 (2H, m, H-m). 13C NMR spectrum, δ, ppm (J, Hz): 12.78 (5-CH3); 13.98 (3-CH3); 21.18 (CH3 p-Tol); 125.48 (C-o); 130.03 (C-m); 132.08 (C-4); 135.45 (C-ipso); 139.60 (C-p); 140.80 (C-5, 2 J C-5,Me = 7.6); 146.77 (C-3, 2 J C-3,Me = 7.6). Found, %: C 62.43; H 5.55; N 18.03. C12H13N3O2. Calculated, %: C 62.33; H 5.67; N 18.17.
3,5-Dimethyl-4-nitro-1-phenyl-1H-pyrazole (3c). Yield 68%; mp 103-105°C (lit. mp 103-105°C [40]). 1H NMR spectrum, δ, ppm: 2.56 (3H, s, 3-CH3); 2.60 (3H, s, 5-CH3); 7.38 (2H, m, H-o); 7.46 (1H, m, H-p); 7.50 (2H, m, H-m). 13C NMR spectrum, δ, ppm (J, Hz): 12.78 (5-CH3); 13.98 (3-CH3); 125.54 (C-o); 129.23 (C-p); 129.36 (C-m); 132.06 (C-4); 137.81 (C-ipso); 140.70 (C-5, 2 J C-5,Me = 7.9); 146.71 (C-3, 2 J C-3,Me = 8.2). Mass spectrum, m/z (I rel, %): 217 [M]+ (100), 200 [M-OH]+ (10), 170 [M-HNO2] + (14), 132 [M-MeC2NO2]+, 118 [M-HNO2-C4H4]+ (61), 104 [PhNCH]+ (31), 77 [Ph] (79).
4-Nitro-1-phenyl-1H-imidazole (4c). Yield 76%; mp 187°C (lit. mp 186-187°C [37]). 1H NMR spectrum, δ, ppm (J, Hz): 7.47 (1H, m, H-p); 7.53 (2H, m, H-m); 7.71 (2H, m, H-o); 8.26 (1H, s, H-2); 8.62 (1H, d, 2 J = 0.61, H-5). 13C NMR spectrum, δ, ppm: 119.84 (C-o); 125.81 (C-5); 128.67 (C-p); 129.85 (C-m); 136.70 (C-2); 137.35 (C-4); 138.70 (C-ipso). Mass spectrum, m/z (I rel, %): 189 [M]+ (100), 104 [PhNHCN]+ (64), 77 [Ph] (60).
3-Nitro-1-phenyl-1H-pyrazole (5c). Yield 89%; mp 127°C (lit. mp 126-128°C [41]). 1H NMR spectrum, δ, ppm (J, Hz): 7.10 (1H, d, 3 J = 2.61, H-4); 7.43 (1H, m, H-p); 7.52 (2H, m, H-m); 7.75 (2H, m, H-o); 7.98 (1H, d, 3 J = 2.61, H-5). 13C NMR spectrum, δ, ppm: 104.33 (C-4, 1 J C-4,H-4 = 188.0, 2 J C-4,H-5 = 8.4); 120.08 (C-o); 128.67 (C-p); 129.49 (C-5, 1 J C-5,H-5 = 192.0, 2 J C-5,H-4 = 8.2); 129.77 (C-m); 138.87 (C-ipso, 4 J C-ipso,H-4 ~1.0, 3 J C-ipso,H-5 ~1.0); 157.07 (C-3, 2 J C-3,H-4 = 2.0, 3 J C-3,H-5 = 10.2). Mass spectrum, m/z (I rel, %): 189 [M]+ (100), 142 [M-HNO2]+ (21), 77 [Ph] (60).
2,4-Dinitro-1-phenyl-1H-imidazole (6c). Yield 57%; mp 164°C. 1H NMR spectrum, δ, ppm: 7.41 (2H, m, H-o); 7.60 (2H, m, H-m); 7.64 (1H, m, H-p); 7.96 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 123.72 (C-5); 125.43 (C-o); 130.12 (C-m); 131.13 (C-p); 134.97 (C-ipso); 140.91 (C-4); 143.93 (C-2). Mass spectrum, m/z, (I rel, %): 234 [M]+ (9), 130 [M + H-PhNCN]+ (23), 115 [M + H-PhNCO]+ (20), 77 [Ph] (100). Found, %: C 46.10; H 2.51; N 23.81. C9H6N4O4. Calculated, %: C 46.16; H 2.58; N 23.92.
3-Nitro-1-phenyl-1H-1,2,4-triazole (7c). Yield 46%; mp 133°C (lit. mp 132°C [42]). 1H NMR spectrum, δ, ppm: 7.51 (1H, m, H-p); 7.57 (2H, m, H-m); 7.74 (2H, m, H-o); 8.65 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 120.47 (C-o); 130.00 (C-p); 130.12 (C-m); 135.69 (C-ipso); 142.30 (C-5); 163.25 (C-3). Mass spectrum, m/z (I rel, %): 190 [M]+ (100), 117 [M-HCN-NO2]+ (16), 77 [Ph] (42).
The work was carried out with the support of the Russian Fund for Fundamental Investigations (grant 09-03-00779).
References
J. H. Boyer, Nitroazoles, Wiley-VCH, Hamburg (1987), Chaps. 1–5 (1987).
N. G. Huilgol, C. K. K. Nair, and V. T. Kagiya, in: Radiation Sensitizers: A Contemporary Audit, Narosa, Mumbai (2001), Chaps. 1–3.
J. P. Agrawal and R. Hodgson, in: Organic Chemistry of Explosives, Wiley, Chichester (2007), Chaps 1–6 (2007).
M. J. Graneto, US Patent 5521207; Chem. Abs., 125, 114612 (1996).
K. Walczak, A. Gondela, and J. Suwinski, Eur. J. Med. Chem., 39, 849 (2004).
R. Jedrysiak, M. Sawicki, P. Wagner, and J. Suwinski, ARKIVOC, vi, 103 (2007).
M. R. Grimmett, K. H. R. Lim, and R. T. Weavers, Aust. J. Chem., 32, 2203 (1979).
V. A. Chauzov, V. Z. Parchinskii, E. V. Sinel’shchikova, A. V. Burasov, B. I. Ugrak, N. N. Parfenov, and V. A. Petrosyan, Izv. Akad. Nauk, Ser. Khim., 1402 (2002).
A. R. Muci and S. L. Buchwald, Top. Curr. Chem., 219, 131 (2002).
S. V. Ley and A. W. Thomas, Angew. Chem. Intern. Edit. Eng., 42, 5400 (2003).
P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 248, 2337 (2004).
L. Wang and Z.-C. Chen, J. Chem. Res. (S), 367 (2000).
I. P. Beletskaya, D. V. Davydov, and M. Moreno-Manas, Tetrahedron Lett., 39, 5621 (1998).
D. V. Davydov, I. P. Beletskaya, and M. S. Gorovoy, Tetrahedron Lett., 43, 6221 (2002).
D. V. Davydov, I. P. Beletskaya, B. B. Semenov, and Y. A. Smushkevich, Tetrahedron Lett., 43, 6217 (2002).
J. Catalan, J. L. M. Abboud, and J. Elguero, Adv. Heterocycl. Chem., 41, 187 (1987).
V. A. Chertkov and M. A. Yurovskaya, Khim. Geterotsikl. Soedin., 899 (1993). [Chem. Heterocycl. Comp., 29, 762 (1993)].
V. A. Chertkov and N. M. Sergeyev, J. Magn. Reson., 21, 159 (1976).
L. Ernst, V. Wray, V. A. Chertkov, and N. M. Sergeyev, J. Magn. Reson., 25, 123 (1977).
H. Mo and T. C. Pochapsky, Prog. Nucl. Magn. Reson. Spectrosc., 30, 1 (1997).
M. A. Yurovskaya, V. A. Chertkov, A. Z. Afanas'ev, F. V. Ienkina, and Yu. G. Bundel', Khim. Geterotsikl. Soedin., 509 (1985). [Chem. Heterocycl. Comp., 21, 424 (1985)].
M. A. Yurovskaya, A. Z. Afanas’'ev, V. A. Chertkov, A. M. Gizatullina, and Yu. G. Bundel’, Khim. Geterotsikl. Soedin., 1625 (1987). [Chem. Heterocycl. Comp., 23, 1305 (1987)].
M. A. Yurovskaya, A. Z. Afanas'ev, V. A. Chertkov, and Yu. G. Bundel', Khim. Geterotsikl. Soedin., 1213 (1988). [Chem. Heterocycl. Comp., 24, 1000 (1988)].
V. A. Chertkov, Yu. K. Grishin, and N. M. Sergeyev, J. Magn. Reson., 24, 275 (1976).
T. Bundgaard, H. J. Jakobsen, and E. J. Rahkamaa, J. Magn. Reson., 19, 345 (1975).
M. Hansen, R. S. Hansen, and H. J. Jakobsen, J. Magn. Reson., 13, 386 (1974).
V. A. Chertkov and Yu. K. Grishin, Zh. Struk. Khim., 18, 616 (1977).
O. V. Dorofeeva, A. V. Ferenets, N. M. Karasev, L. V. Vilkov, and H. Oberhammer, J. Phys. Chem., A, 112, 5002 (2008).
N. M. Sergeev and V. A. Chertkov, Dokl. Akad. Nauk, 286, 1186 (1986).
W. R. Croasmun and R. M. K. Carlson, Two-dimensional NMR Spectroscopy. Applications for Chemists and Biochemists, Methods in Stereochemical Analysis, Vol. 9, Verlag Chemie, Weinheim (1987).
T. D. W. Claridge, High-Resolution NMR Techniques in Organic Chemistry, 2nd ed., Elsevier, Oxford (2008).
J. A. Jones, Prog. Nucl. Magn. Reson. Spectrosc., 38, 325 (2001).
T. A. Keith and R. F. W. Bader, Chem. Phys. Lett., 210, 223 (1993).
J. R. Cheeseman, M. J. Frisch, G. W. Trucks, and T. A. Keith, J. Chem. Phys., 104, 5497 (1996).
A. K. Shestakova, A. V. Makarkina, O. V. Smirnova, M. M. Shtern, and V. A. Chertkov, Izv. Akad. Nauk, Ser. Khim., 1309 (2006).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09W, Revision A.1, Gaussian Inc., Wallingford (2009).
E. Salwinska and J. Suwinski, Pol. J. Chem., 64, 813 (1990).
J. Suwiski, W. Pawlus, E. Salwiska, and K. Swierczek, Heterocycles, 37, 1511 (1994).
D. Dal Monte Casoni, Gazz. Chim. Ital., 89, 1539 (1959).
M. R. Grimmett, S. R. Hartshorn, K. Schofield, and J. B. Weston, J. Chem. Soc., Perkin Trans. 2, 1654 (1972).
G. S. Predvoditeleva, T. V. Kartseva, and M. N. Shchukina, Khim.-farm. Zh., 8, 525 (1974).
V. A. Petrosyan, M. E. Niyazymbetov, M. S. Pevzner, and B. I. Ugrak, Izv. Akad. Nauk SSSR. Ser. Khim., 1643 (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 1, pp. 63–74, January, 2011.
Rights and permissions
About this article
Cite this article
Chertkov, V.A., Shestakova, A.K. & Davydov, D.V. Regioselective N-arylation of nitroazoles. Determination of the structure of N-arylnitro-azoles on the basis of NMR spectroscopic data and quantum-chemical calculations. Chem Heterocycl Comp 47, 45–54 (2011). https://doi.org/10.1007/s10593-011-0718-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0718-z



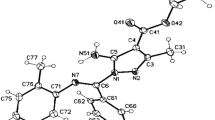


 , data of HMBC experiments) and NOE factors (
, data of HMBC experiments) and NOE factors ( , data of NOEDIFF experiments).
, data of NOEDIFF experiments).