Abstract
The southeastern United States and Florida support an unusually large number of endemic plant species, many of which are threatened by anthropogenic habitat disturbance. As conservation measures are undertaken and recovery plans designed, a factor that must be taken into consideration is the genetic composition of the species of concern. Here we describe the levels, and partitioning, of genetic diversity in 17 populations of the rare and threatened Florida endemic, Euphorbia telephioides (telephus spurge). Species-wide genetic diversity was high (Ps = 91%, APs = 3.81, As = 3.57 and Hes = 0.352) as was mean population genetic diversity (Pp = 81%, APp = 2.98, Ap = 2.59 and Hep = 0.320) which ranks it among the highest 10% of plant species surveyed. Partitioning of genetic variation (Gst = 0.106) was low compared to other herbaceous outcrossing perennials indicating high historical gene flow across its limited geographic range. Among population Gst values within the three Florida counties in which it occurs, Gulf (0.084), Franklin (0.059) and Bay Counties (0.033), were also quite low. Peripheral populations did not generally have reduced genetic variation although there was significant isolation by distance. Rarefaction analysis showed a non-significant relationship between allelic richness and actual population sizes. Our data suggest that E. telephioides populations were probably more continuously distributed in Bay, Gulf and Franklin Counties and that their relative contemporary isolation is a recent phenomenon. These results are important for developing a recovery plan for this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The southeastern United States harbors an unusually large number of endemic plant taxa (Delcourt and Delcourt 1991) with approximately 385 species endemic to Florida alone (Gentry 1986). Many of these species are now threatened by anthropogenic habitat loss and degradation related to factors such as urbanization, habitat conversion, invasive species, fire suppression and/or alteration of natural fire regimes (Slapcinsky et al. 2010). The value and importance of at risk species, and the need to protect them, are increasingly being recognized. For the conservation and recovery of such species in their native habitat to be effective, a multi-faceted approach is necessary that includes habitat management and protection, studies of the species’ life history characteristics and interactions with other organisms (e.g., pollinators and seed dispersers), and estimates of their genetic diversity. Species with more genetic variation are more likely to have the capacity to adapt to biotic or abiotic fluctuations in their environment (Fisher 1930; Dobzhansky 1937) such as novel pathogens, climate change, etc. Thus, preservation of a species’ genetic diversity is a factor that should improve its chances of surviving over evolutionary time. In this context, knowledge of the levels and distribution of genetic variation within species of conservation concern, and the identification of populations with the greatest evolutionary potential, are essential for the development of effective conservation and recovery practices. As an example of how such knowledge can be applied, populations with rare alleles or with elevated levels of genetic diversity may be given higher priorities for preservation than less genetically diverse and unique populations. Plants from the most genetically diverse populations would also be the most valuable propagule sources for recovery projects. Thus, direct empirical data regarding the levels and partitioning of genetic variation are of fundamental importance before effective conservation decisions can be made. This is particularly true of species with small, isolated populations which is a pattern that currently characterizes many endemic plant taxa.
Euphorbia telephioides Chapman (telephus spurge), which is restricted to the Florida panhandle, is a southeastern endemic with endangered world status in the 1997 IUCN Red List of Threatened Plants (Walter and Gillett 1998). It was also designated as threatened in the Federal Register, May 8, 1992 (designation effective June 8, 1992; 50 CFR Part 17 1992). A recovery plan was approved in June 1994 (USFWS 1994). The 2008 U.S. Fish and Wildlife Service 5 Year Review of E. telephioides reports that nine locations appear to have been extirpated by development and/or habitat modification during the 5 years prior to the report. Habitat modification was the primary threat identified in the recovery plan and remains the prime issue; specific threats are real estate development and habitat conversion to pine plantations with accompanying mechanical vegetation destruction and soil disruption. The species was assigned a recovery priority number of 2C, which indicates a species with high threat of extinction due to habitat destruction, but with high recovery potential. With the goal of preserving the species as a whole, our objective is to estimate the levels and distribution of genetic diversity within and among populations of E. telephioides distributed throughout its current native range. Our specific questions: (1) Are there significant differences in genetic diversity within and among populations of E. telephioides? (2) Is genetic similarity among populations a function of spatial distances separating them? (3) Is genetic diversity within populations related to population sizes and/or the geographic location of populations (i.e., center of the species’ range, margin of the range, or isolated)?
Materials and methods
Study species
Euphorbia L. (Euphorbiaceae), commonly called spurge, is diverse worldwide, and consists of about 1,600 annual or perennial herbaceous, woody shrub, or tree species (Narbona et al. 2002). There are approximately 107 Euphorbia spp. native to the United States and Canada (http://www.invasive.org/eastern/biocontrol/14LeafySpurge.html). The genus is currently divided into five subgenera (Webster 1967) and several sections and subsections (Bridges and Orzell 2002). Within subsection Inundatae, five Euphorbia taxa are currently recognized as endemic to areas ranging from southern Georgia to southern Florida, with some extending west to southern Mississippi (Bridges and Orzell 2002). All are adapted to recurrent natural fires and are found on sandy outer coastal plain ridges that date to the Pleistocene, Pliocene, or Miocene (Bridges and Orzell 2002). One of these five taxa is the perennial herb Euphorbia telephioides Chapman (telephus spurge) of northwest Florida.
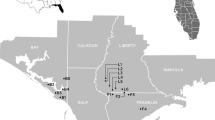
This species is ephemeral; it can be abundant at newly disturbed sites but often is completely absent when sites are revisited a few years later. Even if shoots are not present, plants may persist as large tuberous roots, which help individuals survive harsh conditions and disturbances. Frequent prescribed fire regimes are important for maintenance of flatwoods diversity (Hiers et al. 2007), and where fire management is implemented, healthy stable populations of E. telephioides are maintained (USFWS 2007). Euphorbia telephioides is restricted to Bay, Gulf, and Franklin counties in Florida (Fig. 1). It is unknown whether E. telephioides was once continuously distributed throughout the three counties or whether populations have always been patchily distributed. Currently populations are localized and separated by clear cuts, pine plantations or residential/commercial development. At present the species occurs in about 40 locations, all within 7 km of the Gulf of Mexico. Patch sizes are highly variable ranging, from 20 to >3,000 individuals/population (Table 1). Nearly half of the populations occur on public lands with many remaining populations persisting on private timberland and utility rights-of-way (USFWS 2007). Within this restricted range, E. telephioides occurs in xeric to mesic pine flatwoods and in scrubby pinelands dominated by Aristida stricta (wiregrass) and/or Pinus palustris (longleaf pine) or P. elliottii (slash pine). Uncommonly, E. telephioides grows in wetlands with seepage slope species and in small thick clumps of wiregrass surrounded by pine or cypress (Rountree et al. 2005). It is locally abundant along disturbed sandy roads, and in sites with bedding. It can be found in sporadic abundance in dense grass of unburned scrubby flatwoods (Negrón-Ortiz, pers. observ.) and in upland communities, which have been historically burned on a 2–3 year rotation (J. Huffman, pers. comm.). In general, plants do well on sandy, acidic soil, with no litter, and low organic and moisture content (Peterson and Campbell 2007).
Map of E. telephioides populations. The solid triangles indicate locations of the 17 populations. Letters indicate the individual populations (Table 1)
Leaves of telephus spurge are somewhat succulent, an adaptation that decreases evaporation during seasonal water stress. It also has a long tuberous root which probably helps plants survive desiccation and disturbance from fire, mowing, or pine plantation site preparation. Euphorbia telephioides is functionally dioecious (Bridges and Orzell 2002) with staminate and pistillate plants differing in morphology (Negrón-Ortiz, pers. observ.). Flowering is from March through August with terminal umbellate inflorescences modified into reddish-green cyathia (Bridges and Orzell 2002). Ovaries develop into capsules that disperse their seeds explosively. Seeds range in color from silver and gray to tan and brown (Peterson and Campbell 2007).
Study sites and sampling
Field searches were conducted across known localities of telephus spurge during Summer 2008, when most of its leaves were green. Young mature leaf material was sampled from 541 individuals within 17 populations (Table 1; Fig. 1). Sample sites were primarily chosen from protected areas and/or well managed populations with prescribed fire. Two populations were collected from periodically mowed highway rights-of-way, a disturbance that also maintains its populations. Depending on the size and accessibility of the population, 10–51 individuals (mean = 32) were sampled per population (Table 1). Actual population sizes were estimated or were obtained from the Florida Natural Areas Inventories (2007). Sampled individuals were at least two meters apart. Most leaves were sampled from flowering adults but a few were collected from seedlings. Approximately 5–102 cm of leaf tissue was collected from each plant and kept chilled during transport to the University of Georgia.
Enzyme extraction and electrophoresis
Leaves were crushed in chilled mortars with a pestle and a pinch of sea sand to disrupt cellular compartmentalization. Enzymes were extracted from the tissue with a polyvinylpyrrolidone-phosphate extraction buffer (Epperson and Alvarez-Buylla 1997). The resulting slurry containing crude protein extract was absorbed onto 4 × 6 mm wicks punched from Whatman 3 mm chromatography paper. Wicks were stored in microtest plates at −70°C until used for electrophoresis. Wicks were placed in horizontal starch gels (10%) and electrophoresis was performed. Five buffer systems resolved 23 loci of which 21 were polymorphic. Enzymes stained and polymorphic loci (in parentheses) for each buffer system were: (1) system 4, phosphoglucoisomerase (PGI1, PGI2), and UTP-glucose-1-phosphate (UGPP1); (2) system 6, acid phosphatase (ACP1), glutamate dehydrogenase (GDH1), and peroxidase (PER1, PER2); (3) system 8-, fluorescent esterase (FE1, FE2), leucineamino peptidase (LAP1), menadione reductase (MNR1), and triosephosphate isomerase (TPI1, TPI4); (4) system morphaline citrate, isocitrate dehydrogenase (IDH2), and malate dehydrogenase (MDH1, MDH2); (5) system 11, 6-phosphogluconate dehydrogenase (6-PGD1, 6-PGD2), F-1,6-diphosphate (F16-1), and phosphoglucomutase (PGM1, PGM2). All buffer and stain recipes were adapted from Soltis et al. (1983) except UGPP (Manchenko 1994). Buffer system 8- is a modification of buffer system 8 as described by Soltis et al. (1983). Banding patterns were consistent with Mendelian inheritance patterns expected for each enzyme system (Weeden and Wendell 1989).
Data analyses
Genetic diversity measures were estimated using a computer program, LYNSPROG, designed by MD Loveless and AF Schnabel. Measures of genetic diversity were percent polymorphic loci, P; mean number of alleles per polymorphic locus, AP; mean number of alleles per locus, A; genetic diversity, He (the proportion of loci heterozygous per individual under Hardy–Weinberg expectations; Nei 1973). Species values for these parameters were calculated by pooling data from all populations. Population values were calculated for each population and then averaged across all populations. Heterogeneity in allele frequencies among populations was tested by the χ2 method of Workman and Niswander (1970). Observed heterozygosity (Ho) was compared with Hardy–Weinberg expected heterozygosity (He) for each polymorphic locus in each population by calculating Wright’s fixation indices (i.e., inbreeding coefficient; Wright 1922; Wright 1951). Deviations from Hardy–Weinberg expectations were tested for significance using χ2 (Li and Horvitz 1953).
Partitioning of variation among populations was estimated according to Nei’s (1973) genetic diversity (GST) as well as AMOVA using GenAlEx 6.41 (Peakall and Smouse 2006). Hierarchical genetic structure was determined at three spatial scales: among all populations, among regions (i.e., the three counties) and among populations within regions. Nei’s (1972) genetic distance statistics were calculated for each locus (monomorphic and polymorphic) and mean genetic distance values were calculated for each pair of populations. Jackknifing procedures (Weir 1996) were used to test if pairwise GST comparisons of populations within each county differed significantly from the other counties. Neighbor joining and UPGMA phenograms of genetic distances were generated using PHYLIP (Felsenstein 2005). To test for correspondence between genetic distances and geographic distances a Mantel test was performed (Smouse et al. 1986) using NTSYS-PC (Rohlf 2000). Isolation by distance (IBD) was also calculated by plotting Rousset’s (1997) GST/(1−GST) for each pair of populations against the geographic distance separating them (due to the linear distribution of the species) using IBDWS version 3.16 (Jensen et al. 2005).
Allelic richness was determined both as the total number of alleles observed and as the number of alleles adjusted for population sample size (i.e., rarefaction) using HP-Rare (Kalinowski 2004, 2005). For the estimation of allelic richness, all E. telephioides populations were treated as consisting of 20 alleles because our smallest population contained 10 individuals. The Spearman rank correlation between allelic richness values obtained by rarefaction and actual population sizes were calculated and the significance of the relationship determined.
Results
Twenty-three allozyme loci were resolved for samples from 17 E. telephioides populations. Twenty-one of the 23 loci (91.3%) were polymorphic for the species as a whole and two were monomorphic (TPI2 and TPI3). Levels of genetic diversity were quite high for the pooled (over the 17 populations) species-wide sample and at the mean within population level (Tables 2, 3). In addition to the high proportion of polymorphic loci, the number of alleles per polymorphic locus (APs = 3.81) and for all loci (As = 3.57) were quite high as was genetic diversity (Hes = 0.352) for the pooled species-wide sample. Within populations of E. telephioides, the mean percentage of polymorphic loci (Pp) was 80.7% (range 73.9–87.0%). The number of alleles per polymorphic locus (APp) and per locus (Ap) were also high; mean APp = 2.98 (range 2.50–3.35); mean Ap = 2.59 (range 2.17–2.86). Mean expected heterozygosity within populations (Hep) averaged 0.320 (range 0.279–0.352). Observed heterozygosity (Hop = 0.310) was slightly lower than expected heterozygosity, indicating a small excess of homozygosity relative to Hardy–Weinberg expectations. A total of 83 alleles were observed across all loci and all populations of E. telephioides. The highest number of alleles occurred in populations G (66) and M (66) while the fewest alleles were observed in the two populations with the smallest sample sizes [D (50) and F (51); Table 2]. However, when the sample size effect was removed through rarefaction, the number of alleles/population ranged from 49 to 57 with populations B, G and J2 having the most alleles and D and O having the least (Table 2). Populations D and O are located at the margins of the two clusters of populations to which they belong (Fig. 1). Four unique alleles (mean frequency = 0.022) were observed, with each occurring in a different population (G, J2, K and L). The Spearman rank correlation between allelic richness values obtained by rarefaction and actual population sizes was non-significant (r = 0.07, p > 0.5).
All 21 polymorphic loci had significant allele frequency heterogeneity among the 17 populations. At the broadest geographic scale (i.e., all 17 populations), the proportion of genetic diversity partitioned among populations (GST) is 0.106 and the AMOVA value is 0.11. Because GST and AMOVA values were very similar or identical, we will present only the GST values. Among regions (i.e. Bay, Gulf and Franklin Counties) GST = 0.036 (34% of total) while the GST value among populations within regions was 0.070 (66% of total). Among population differentiation within regions was GST = 0.033 in Bay County, 0.084 in Gulf County and 0.059 in Franklin County. Jackknifing procedures (Weir 1996) indicated that pairwise GST comparisons of populations within Bay County differed significantly (p = 0.01) from pairwise comparisons within Gulf County. However, similar comparisons between Bay and Franklin Counties, and Gulf and Franklin Counties were not significant. The Mantel test showed significant genetic isolation by geographic distance (r = 0.109; p < 0.001). When Rousset’s GST/(1−GST) was plotted against geographic distance separating all possible pairs of populations, IBD was significant (r = 0.454; p < 0.01; Fig. 2). Because significant IBD was observed, further analysis with Structure 2.3 (Pritchard et al. 2000) was not employed; the authors of Structure explicitly state that the underlying Structure model is not well suited to such data and accurate interpretation of the results would be challenging.
Nei’s genetic identity values ranged from 0.810 (A and N) to 0.984 (M and O) with mean Ī = 0.932 and indicated that populations N (Ī = 0.884), A (Ī = 0.899) and D (Ī = 0.912) had the lowest mean genetic identities with the other 16 populations (Table 3). Populations E, I and G (Ī = 0.950–0.951), had the highest mean genetic identities with the other populations. Neighbor joining and UPGMA phenograms of genetic distances showed little agreement, probably because of the high levels of genetic identity among many populations. Both phenograms indicated that populations A, B, and C from the more disjunct Bay County are more genetically similar to one another than to other populations and that M and O from Franklin County are tightly clustered. Both also revealed a similarly close relationship between H1 and H2.
Discussion
Euphorbia telephioides maintains a very high level of allozyme genetic diversity relative to other Euphorbia spp. (Park and Elisens 1997, Park 2004) and other plant species. The average seed plant species has values for Ps and Hes of 50.5% and 0.149, respectively (Hamrick and Godt 1989) while E. telephioides values are approximately twice as high (Table 4). More specifically, short-lived herbaceous perennial plants average Ps = 41.3% and Hes = 0.116. Its values also exceed the mean reported for rare and common species surveyed by Cole (2003; Table 4). Genetic diversity values for E. telephioides rank it within the highest 10% of all plant species that have been analyzed (Hamrick and Godt 1989). Such high genetic diversity values may reflect an allopolyploidy event in E. telephioides, since the patterns of inheritance observed are consistent with disomic inheritance. Reviews of the allozyme literature have demonstrated that recent polyploid species tend to have more genetic diversity than diploids within the same genus (Hamrick et al. 1979; Soltis and Soltis 1993). While the ploidy of E. telephioides is not known, the chromosome number and size have been examined for 136 Euphorbia spp. which are quite variable, with polyploidy observed in 40% of the species (Hans 1973). The low inbreeding coefficient within these 17 populations is consistent with the dioecious mating system of this species.
Genetic differentiation (GST = 0.106) among populations of E. telephioides is lower than that observed for other herbaceous outcrossing perennial plant species (GST > 0.200; Hamrick and Godt 1989) or rare species (GST = 0.212; Cole 2003). The eleven populations in Gulf County are more different from one another (GST = 0.084) than populations within Franklin County (0.059) or Bay County (0.033). Gulf County populations may represent older, more stable populations and thus have had more time to diverge from one another. This is supported by the observation that the four unique alleles were found in four Gulf County populations (G, J2, K, and L). It is also possible, however, that the higher GST values seen among Gulf County populations are due to greater geographic separation from one another. Bay County populations were probably founded by multiple propagules from the same source population/s and may represent remnants of a single continuous population. The same may be true of Franklin County populations although they are more genetically differentiated than Bay County populations. Peripheral populations do not have reduced genetic diversity and in fact the two populations with the highest Hep values (B and N) occur in Bay and Franklin Counties which represent the periphery of the current species range. While there is some IBD (Fig. 2), which is primarily the result of regional differences, the low among population partitioning of genetic variation indicates that high rates of gene flow have historically occurred between populations of this species. This is probably due to the relatively short geographic distances between its populations within counties and the more continuous distribution of E. telephioides in the relatively recent past.
The evenness of allelic richness provides additional evidence that E. telephioides was probably more continuously distributed in Bay, Gulf and Franklin Counties and has only recently become patchily distributed. Our analyses indicate that variation in sample size influences allelic richness, as expected. However, the relationship between the allelic richness after rarefaction and actual sizes of the natural populations was non-significant indicating that historical population sizes have been larger than current census sizes would indicate.
Conservation implications
In conclusion, while E. telephioides is a rare species that is federally listed as threatened, it possesses high levels of genetic variation and relatively low genetic structure. This indicates that there have been historically high levels of gene flow among historically more continuous populations. The patchy distribution of contemporary populations may represent a recent contraction of the species and reflect the extensive disturbance of the landscape in recent decades (Brody 2008). This is supported by the fact that rare species generally have less genetic variation and more genetic structure than their more common congeners (Cole 2003). These factors indicate that E. telephioides would be a good candidate for recovery (i.e., removal from the federal list as stipulated by Sect. 4 of the Endangered Species Act). The design of a conservation plan and choice of populations that should be prioritized (i.e., secure protection, ex situ collection, critical habitat designation, management) should consider the levels and distribution of genetic variation. Our data indicate that even though 66% of the among population genetic variation is partitioned among populations within the three regions (i.e., counties), among county genetic differentiation is substantial. This indicates that for the augmentation or reintroduction of populations of E. telephioides, care should be taken to obtain propagules from populations within each county rather than from other counties. Individuals should be selected that will introduce a representative cross section of the genetic diversity within that county. This strategy should insure that much of the genetic diversity at more adaptive genes will also be included in the propagules used to augment or restore populations of E. telephioides. For example, because populations A, D, and N are the most dissimilar from other populations, propagules from these populations should be utilized. But careful consideration must also be given to the microhabitats of the source and target populations. The relatively low GST values observed, particularly within each county, would indicate that one should be able to restore or enrich populations from any source within the county in question. However, it should be remembered that variation at allozyme loci is considered relatively neutral, thus, may not represent patterns of adaptive variation among populations. The plant evolution literature indicates that plant populations are usually locally adapted to varying microhabitat conditions (i.e., soil types, shade versus sun, moisture levels, etc.). Thus, it would be wise when developing conservation and recovery strategies to avoid mixing individuals from obviously different microhabitats.
References
Bridges EL, Orzell SL (2002) Euphorbia (Euphorbiaceae) section Tithymalus subsection Inundatae in the southeastern United States. Lundellia 5:59–78
Brody SD (2008) Ecosystem planning in Florida: solving regional problems through local decision-making. MPG Books Ltd, Bodmin, Cornwall. Great Britain
Cole CT (2003) Genetic variation in rare and common plants. Annu Rev Ecol Evol Syst 34:213–237
Delcourt HR, Delcourt PA (1991) Quaternary ecology: a paleoecological perspective. Chapman and Hall, New York
Dobzhansky Th (1937) Genetics and the origin of species. Columbia University Press, New York
Epperson BK, Alvarez-Buylla ER (1997) Limited seed dispersal and genetic structure in life stages of Cecropia obtusifolia. Evolution 51:275–282
Felsenstein J (2005) PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle
Fisher RA (1930) The genetical theory of natural selection. Oxford University Press, UK
Florida natural areas inventory (FNAI) (2007) Telephus spurge element of occurrence spatial data. Florida
Gentry AH (1986) Endemism in tropical versus temperate plant communities. In: Soule ME (ed) Conservation biology, the science of scarcity and diversity. Sinauer Associates, Sunderland, pp 153–181
Hamrick JL, Godt MJW (1989) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics, breeding and genetic resources. Sinauer Associates, Sunderland, pp 46–63
Hamrick JL, Linhart YB, Mitton JB (1979) Relationships between life-history characteristics and electrophoretically detectable genetic variation in plants. Ann Rev Ecol Syst 10:173–200
Hans AS (1973) Chromosomal conspectus of the Euphorbiaceae. Taxon 22:591–636
Hiers JK, O’Brien JJ, Will RE, Mitchell RJ (2007) Forest floor depth mediates understory vigor in xeric Pinus palustris ecosystems. Ecol Appl 17:806–814
Jensen JL, Bohonak AJ, Kelley ST (2005) Isolation by distance, web service. BMC Genetics 6: 13. v.3.16 http://ibdws.sdsu.edu/
Kalinowski ST (2004) Counting alleles with rarefaction: private alleles and hierarchical sampling designs. Conserv Genet 5:539–543
Kalinowski ST (2005) HP-Rare 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes 5:187–189
Li CC, Horvitz DG (1953) Some methods of estimating the inbreeding coefficient. Am J Hum Genet 5:107–117
Manchenko GP (1994) Handbook of detection of enzymes on electrophoretic gels. CRC Press, Ann Arbor
Narbona E, Ortiz PL, Arista M (2002) Functional andromonoecy in Euphorbia Euphorbiaceae). Ann Bot 89:571–577
Nei M (1972) Genetic distance between populations. American Naturalist 106:283–292
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Park KR (2004) Comparisons of allozyme variation of narrow endemic and widespread species of Far East Euphorbia (Euphorbiaceae). Botanical Bulletin of Academia Sinica 45:221–228
Park KR, Elisens WJ (1997) Isozyme and morphological evidence within Euphorbia section Tithymalopsis (Euphorbiaceae). Int J Plant Sci 158:465–475
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peterson CL, Campbell CC (2007) Seed collection and research on eight rare plants species of the Florida Panhandle region. USFWS grant agreement 401815G173
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rohlf FJ (2000) Numerical taxonomy and multivariate analysis system version 2.1. Exeter Publishing, New York
Rountree A, Tobe J, Busalacchi J (2005) Report: threatened and endangered species survey. Shallow Reed Site, Gulf County
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Slapcinsky JL, Gordon DR, Menges ES (2010) Responses of rare plant species to fire in Florida’s pyrogenic communities. Nat Areas J 30:4–19
Smouse PE, Long JC, Sokal RR (1986) Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst Zool 35:627–632
Soltis DE, Soltis PS (1993) Molecular data and the dynamic nature of polyploidy. Crit Rev Plant Sci 12:243–273
Soltis DE, Haufler CH, Darrow DC, Gastony GJ (1983) Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedules. Am Fern J 73:9–27
U.S. Fish and Wildlife Service (1994) Recovery plan: recovery plan for four plants of the lower Apalachicola region, Florida: Euphorbia telephioides (telephus spurge), Macbridea alba (white birds-in-a-nest), Pinguicula ionantha (Godfrey’s butterwort), and Scutellaria floridana (Florida skullcap). U.S. Fish and Wildlife Service southeast region. Panama City Field Office, Panama City
U.S. Fish and Wildlife Service (2007) Euphorbia telephioides (telephus spurge) 5-year review: summary and evaluation. U.S. Fish and Wildlife Service southeast region. Panama City Field Office. Panama City
Walter KS, Gillett HJ (1998) 1997 IUCN Red List of Threatened Plants. Compiled by the world conservation monitoring centre. IUCN:The World Conservation Union, Gland, Switzerland and Cambridge, UK. pp xiv+862
Webster GL (1967) Genera of Euphorbiaceae in the Southeastern Unites States. J Arnold Arboretum 48:303–430
Weeden NF, Wendell JF (1989) Genetics of plant isozymes. In: Soltis DE, Soltis PS (eds) Isozymes in plant biology. Dioscorides Press, Portland, pp 46–72
Weir BS (1996) Genetic data analysis II. Sinauer Associates, Inc., Sunderland
Workman PL, Niswander JD (1970) Population studies on southwestern Indian tribes. II. Local genetic differentiation in the Papago. Am J Hum Genet 22(24):49
Wright S (1922) Coefficients of inbreeding and relationship. Am Nat 56:330–338
Wright S (1951) The genetical structure of populations. Ann Eugen 15:323–354
Acknowledgments
The authors thank C. Deen and the Hamrick lab personnel for assistance in the lab as well as three anonymous reviewers for their helpful comments. Funding for the project was provided by the U.S. Fish and Wildlife Service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trapnell, D.W., Hamrick, J.L. & Negrón-Ortiz, V. Genetic diversity within a threatened, endemic North American species, Euphorbia telephioides (Euphorbiaceae). Conserv Genet 13, 743–751 (2012). https://doi.org/10.1007/s10592-012-0323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-012-0323-4