Abstract
The effects of human-caused fragmentation require further study in landscapes where physical dispersal barriers and natural ecological transitions can be discounted as causes for population genetic structure. We predict that fragmentation can reduce dispersal across such barrier-free landscapes because dispersal also is limited by a perception of risk. Considerable fragmentation has occurred in the Riding Mountain National Park (RMNP) region in Manitoba, Canada, during the past 60 years. We examine data from 13 autosomal microsatellites to determine whether fragmentation is correlated with genetic population structure in wolves (Canis lupus). Moderate and significant differentiation between RMNP and a genetic cluster identified 30 km farther north (F ST = 0.053, 95% CI [0.031–0.073]) is consistent with predicted effects of fragmentation. The RMNP population cluster represents at least seven wolf packs followed weekly by radio tracking during 2003–2006. Distinct mtDNA haplotypes have been identified in the Park and no successful wolf dispersal from RMNP has been documented in several multi-year tracking studies since 1974. Tracking data also indicate that some wolves might be reluctant to leave RMNP. Although the influence of behaviour and local adaptation require investigation, human-caused fragmentation appears to have caused cryptic genetic structure on fine spatiotemporal scales in a vagile species that is: (1) not influenced by physical movement barriers or historical ecological discontinuities in our study area, and; (2) able to live relatively close to humans. The Great Plains is now an intensely human-managed landscape. Detection of cryptic genetic structure could therefore function as an important indicator in conservation management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical barriers such as mountain ranges have been found to limit dispersal and gene flow in plants, amphibians and mammals (e.g. Taberlet et al. 1998), and reduce dispersal in vagile species such as the Canadian lynx (Lynx canadensis) (Rueness et al. 2003). Moreover, human-caused barriers represented by roads with high traffic volume have reduced gene flow in coyotes (Canis latrans) and bobcats (Lynx rufus) (Riley et al. 2006). Recent findings also suggest that a combination of landscape features with low permeability can influence fine-scale genetic structure without dispersal barriers. Such cryptic population structure (see Sacks et al. 2005) has been identified in species such as wolverines (Gulo gulo) (Cegelski et al. 2003; Guillot et al. 2005) and roe deer (Capreolus capreolus) (Coulon et al. 2006).
The spatiotemporal effect of landscape fragmentation on vagile mammals in the absence of physical movement barriers is not well understood. This is in part because such population structure can be attributed in vagile taxa to factors such as diet specialization, natal habitat-biased dispersal (preference for dispersal into familiar habitat) and climate (Hoelzel et al. 1998; Carmichael et al. 2001; Ernest et al. 2003; Rueness et al. 2003; Sacks et al. 2004; Pilot et al. 2006). The influence of human-caused fragmentation requires further study in landscapes where physical dispersal barriers are not present and natural ecological discontinuities (e.g. climate, prey distribution, mountain-lowland transitions) can be discounted as causes for population genetic structure. Such potential influence should be examined in organisms where high gene flow is expected to limit independent evolution within local population units, so that fine-scale spatiotemporal effects can be determined and incorporated into both theoretical planning and applied conservation management. We test the prediction that fragmentation creates genetic population structure due to reduced dispersal and subsequent genetic drift, even across short distances and in landscapes lacking physical barriers to dispersal.
Landscape matrices, areas surrounding reserves and altered by human use, play a critical role in connectivity (Franklin 1993; Noss et al. 1996; Kramer-Schadt et al. 2004). Some forms of human activity interrupt wildlife movement without physically disconnecting habitats, and a combination of landscape features with low permeability can influence fine-scale genetic structure in the absence of dispersal barriers (Coulon et al. 2006). Considerable landscape fragmentation has occurred over the past 60 years in the region surrounding Riding Mountain National Park (RMNP) in southwestern Manitoba, Canada. Agricultural development has removed forest cover to the RMNP edge (McNamee 1993). Several mammalian species have been extirpated and the Park is considered a wilderness “island” within an agricultural region (Carbyn 1980; Noss 1995). We examine genetic population structure in wolves (Canis lupus), a canid that has been present in the region for at least 5,000 years (Goulet 1993). Wolves show high behavioural plasticity in food acquisition (Weaver et al. 1996), and are considered primarily limited by food availability (Haight et al. 1998). They have high rates of gene flow (Vilà et al. 1999) and records of long-range dispersal (several hundred km) exist in the north-central United States, similar landscapes to our study area (Fritts 1983; Gese and Mech 1991; Wydeven et al. 1995). Wolf populations also have a low number of breeding animals (Mech and Boitani 2003) and genetic drift can affect allele frequencies within a few generations (Allendorf and Luikart 2007).
Human-caused extirpation has resulted in significant range reduction for wolves (Leonard et al. 2005) and their persistence in the landscape matrix is sensitive to human tolerance (Carroll et al. 2006). Wolves within European landscapes with a long history of human development show genetic structuring over relatively short distance (<200 km) (Pilot et al. 2006). We examine whether landscape fragmentation over the past 60 years in the form of conversion to a human-dominated agricultural matrix with a dense network of (unfenced) roads has reduced gene flow in a highly mobile species (wolves). The effects of roads on wolf movement are complex and depend on factors such as wolf harvest management, vehicle mortality, and ease of travel (see e.g. Fuller et al. 2003; Whittington et al. 2005). Landscapes such as our study area are not disconnected from a wolf’s perspective, however, as they remain easy to navigate and negotiate. Wolves inhabiting protected areas surrounded by matrix may nonetheless be increasingly isolated from neighbouring wolf populations. Genetic structuring reflecting divergent distribution of gray and eastern wolves (proposed as separate species C. lycaon by Wilson et al. 2000) or Great Lakes wolves (Koblmüller et al. 2009 and references therein) have been reported (Wilson et al. 2009). The possibility that behavioural factors and local habitat adaptations influence the distribution of different types of wolves thus requires further study. Our study area was nevertheless well-connected as recently as 60 years ago. If local habitat adaptations presently favour any wolf type in particular, such a situation is unlikely to have come about without prior human-caused landscape fragmentation.
Materials and methods
Study area
The region is located at the Prairie and Boreal Plain ecozone transition (Environment Canada 1993), and includes Duck Mountain Provincial Park (1,424 km2), Duck Mountain Provincial Forest (3,760 km2), and Riding Mountain Biosphere Reserve (15,000 km2). The biosphere reserve encompasses the core 2,974 km2 protected area RMNP and 15 surrounding rural areas with local governments. The area includes numerous lakes and ponds; deciduous, boreal and mixed forest; rough fescue grasslands, and extensive marshes and wetlands (Manitoba Conservation 2004; Parks Canada 2006). Elk (Cervus elaphus), moose (Alces alces), beaver (Castor canadensis), and white-tailed deer (Odocoileus virginianus) are abundant. Other relevant mammals include wolves, black bear (Ursus americanus), coyote, lynx, red fox (Vulpes vulpes), and snowshoe hare (Lepus americanus). The regional climate is continental interior, with cold winters and moderate snow depths (Carbyn 1982). The growing season is variable but averages 72 days (Parks Canada 2004).
Forest cover was almost continuous between RMNP and the Duck Mountains until the 1950s, but only 14% remained by 1991 and intense development in the center effectively severed RMNP from other forested areas (Walker 2001). Agriculture is now the dominant land use and occupies approximately 58% of the area (35% cropland and 23% rangeland), whereas managed public land (including parks) makes up 16% (Parks Canada 2004). Road development within the region around RMNP is extensive, with 30,000 km of roads at a density of 0.7 km of road per km2 (Parks Canada 2004). Figure S1 in the supplementary material shows a satellite image of RMNP and the surrounding human-modified agricultural landscape. The Duck Mountains are considered to be less isolated than RMNP, as provincial forest land is located about 10 km to the northeast and is connected to relatively undeveloped areas of central Manitoba. Wolves occupied the RMNP region until a probable combination of hunting, trapping, land clearing, and poisoning caused a local extirpation around 1900 (Carbyn 1980). However, the species recolonized the region by the 1930s, possibly via dispersal from the forested areas north of RMNP when forest connectivity between the Duck Mountains and RMNP was more apparent (Fritts and Carbyn 1995). The Park population has numbered approximately 70–75 individuals in late winter over the past 5 years (RMNP unpublished data). RMNP wolves have been followed for several multi-year studies since 1974 with no evidence of dispersal between the Park and surrounding areas, despite 13,000 km of ground tracking and altogether >20 years of radio telemetry (Carbyn 1980; Paquet 1992; Stronen 2009). Mitochondrial DNA studies have identified distinct RMNP haplotypes that have not been documented outside the Park (Lehman et al. 1991; Geffen et al. 2004; Stronen et al. 2010). The agricultural landscape supports a large number of white-tailed deer and there are also elk found in the area around RMNP. It is therefore unlikely that prey availability would limit movement into the area surrounding the Park.
Sampling
We analysed samples from 13 wolves (one tissue sample, 12 hair samples) radio-collared by RMNP during 2003–2005. Attempts were made to collar at least two of the younger wolves (the most likely dispersers) in each pack. Wolves were located weekly, weather permitting, using a Cessna 172 aircraft with antennas mounted on the wing struts, and a handheld global positioning system receiver. We furthermore included 45 tissue samples collected throughout Manitoba 1990–2005. These comprised 18 samples from within or near the boundary of RMNP, 12 samples from the Duck Mountains, and 15 samples from surrounding areas, mostly in central Manitoba north and east of RMNP and the Duck Mountains.
Microsatellite DNA analyses
Fine scale population processes can be examined by genotypic arrays in the form of multiple microsatellite loci, which are reshuffled in each generation in sexual species (Taberlet et al. 1999; Sunnucks 2000). Wolves generally live in social and territorial groups of 2–42 animals with mean pack size of 3–11 individuals (Fuller et al. 2003) characterized by long-lived pair bonds (Mech and Boitani 2003), where kinship structuring plays an important role in genetic heterogeneity (vonHoldt et al. 2008). Hence, wolf family groups are likely to represent an underlying level of structure (Wahlund effect) in population genetics studies (Pilot et al. 2006). Importantly, radio-collared wolves were captured in seven different packs. One of these had a territory outside the northwestern boundary of the park and the six other packs were distributed throughout RMNP. Based on weekly radio tracking of these individuals during 2003–2006, we are confident that these wolves represent different packs and provide a representative sample of the RMNP wolf population.
We chose the 13 tetranucleotide microsatellite markers FH2001, FH2010, FH2017, FH2054, FH2088, FH2096, FH2422 (Breen et al. 2001), FH3313, FH3725 (Guyon et al. 2003), PEZ06, PEZ08, PEZ15, PEZ19 (Halverson J. in Neff et al. 1999), and the dinucleotide Y-chromosome marker MS41B (Sundquist et al. 2001). DNA from tissue and hair samples were extracted using a solution of (A) 200 mM NaOH and (B) 200 mM HCl and 100 mM Tris–HCl, pH 8.5 (Sancristobal-Gaudy et al. 2000, and modified by C. Penedo pers. comm.). The product of treatment with solution B is used as a template in the PCR reaction. Five hair roots, or 2 mm3 of tissue, were combined with 100 μl of solution A and heated on a thermocycler for 15 min at 97°C, before addition of 100 μl of solution B. Polymerase Chain Reaction (PCR) conditions optimized for the markers were: 95°C/15 min (denaturation 94°C/30 s, annealing 58°C/90 s, extension 72°C/60 s) × 30 PCR cycles, final extension 60°C/30 min, 15°C/HOLD. A master mix from the Qiagen multiplexing kit was used that contains Taq polymerase enzyme, deoxyribonucleotide triphosphate, Magnesium and buffer, as well as a Q-solution for augmenting amplification of difficult templates. Protocol for a 10-μl reaction is: (1) Qiagen master mix X2 (5 μl); (2) Q-solution 5X (1 μl); (3) Primer mix 2 μM (1 μl, 0.2 μM final concentration); (4) IRD primer 1 μM (0.4 μl, infrared dye, 0.04 μM final concentration), (5) DNA template (1.5 μl, concentration unknown but likely variable among samples) and (6) sterile H2O (1.1 μl). Genotyping was done with a LICOR® 4200 DNA Analyzer System and genotypes scored using LICOR® program GeneImagIR.
Statistical analyses
Amplification of nine makers per individual gives a probability of identity [the probability of sampling identical genotypes, denoted P (ID)] in siblings of between 0.001 and 0.0001 (1 in 1,000 to 10,000) at a heterozygosity level of 0.08 (Waits et al. 2001). We successfully amplified at least 10 markers for each individual, with the exception of a hair sample from one radio-collared female wolf for which only 8 markers were amplified. The Y-chromosome marker MS41b was excluded from genetic diversity analyses to avoid bias in heterozygosity measures. We used GenAlEx (genetic analyses in Excel) version 6 (Peakall and Smouse 2006) to examine spatial autocorrelation across all loci with a test of 999 permutations and 1,000 bootstrap replicates. We tested for gametic disequilibrium and departures from Hardy–Weinberg equilibrium analyses in GENEPOP 4.0.10 (Raymond and Rousset 1995) and GENETIX 4.05.2 (Belkhir et al. 2004), and used the Hardy–Weinberg exact test (Guo and Thompson 1992) in GENEPOP with the Markov chain method. Here, we applied parameter values from Coulon et al. (2006) for a population with expected low genetic differentiation and used global test dememorization number = 10,000, number of batches = 400, and number of iterations of batches = 3,000. We adjusted P values for Hardy–Weinberg and gametic equilibrium tests using Bonferroni correction (Rice 1989) to account for the testing of multiple hypotheses. Estimates for F IS per locus are calculated according to Weir and Cockerham (1984).
Different methods with various underlying models can provide a range of gene flow estimates and thus relative measures of connectivity (Cegelski et al. 2003). It is therefore useful to compare gene flow estimates between approaches that highlight different aspects of our theoretical (the study organism easily moves within the study area) and observed (no dispersal documented) assumptions. We examined genetic structure by comparing results from a clustering analysis based on Bayesian models using STRUCTURE 2.1 (Pritchard et al. 2000) and one approach based on a maximum likelihood method; the Assignment Test (Paetkau et al. 1995; Waser and Strobeck 1998) using ARLEQUIN 2.00 (Schneider et al. 2000). The Assignment Test requires a priori definition of populations and then attempts to assign individuals to these populations. Genotypes are not georeferenced and the approach allows an explicit test of whether landscape fragmentation corresponds with genetic structure. For the Assignment Test we entered the three major sampling locations (Riding Mountains, Duck Mountains, and Central Manitoba) as putative a priori clusters.
The STRUCTURE program does not require a priori definition of populations and we examined genetic structure with K (number of genetic clusters) ranging from 1 to 8. We used the option of population admixture and allowed allele frequencies to be correlated, which are considered the best approach where genetic structure is expected not to be strongly differentiated (Falush et al. 2003). A pilot study using a burn-in period of 100,000 and 1,000,000 iterations gave comparable results as a burn-in period of 10,000 and 1,000,000 iterations, and we therefore determined the most likely number of genetic clusters represented by our data by running five repetitions of K = 1–8 using the latter parameter values. We calculated the probability for each value of K as the average value over the five runs and determined the number of populations using the highest value of ln P(D) (equivalent to L(K), Pritchard et al. 2000) and ΔK (Evanno et al. 2005). We subsequently performed 10 STRUCTURE runs at this value of K using 1,000,000 iterations and a burn-in period of 100,000, and determined the proportion of ancestry (qi) using the run that showed the highest probability and the lowest variance (Fain et al. 2010).
For inferred genetic clusters, we tested for gametic disequilibrium and departures from Hardy–Weinberg equilibrium. We estimated F IS per locus with parameter values from Coulon et al. (2006), with global test dememorization number = 10,000, number of batches = 300, and number of iterations of batches = 5,000. We calculated pairwise population differentiation (F ST) by Theta (Weir and Cockerham 1984) with a test of 1,000 permutations using GENETIX. We subsequently tested for evidence of genetic bottlenecks using BOTTLENECK 1.2.02 (Piry et al. 1999). We followed Weckworth et al.’s (2005) approach for small populations with a two-step model of mutation (TPM) accounting for 5, 10, 20 and 30% of all mutations, and used a significance level of 0.05. Finally, we applied factorial correspondence analysis (FCA) using GENETIX to assess the genotypic distributions of individuals. This approach uses multi-locus profiles to project all individuals in a two or three-dimensional space without a priori designations, using each allele as an independent variable (Roques et al. 2001).
Results
The study area could be panmictic so we tested for Hardy–Weinberg and gametic equilibrium, as well as spatial autocorrelation of alleles, within the whole sample. Values for observed and expected heterozygosity (Ho and He), allelic diversity, and F IS are shown in Table 1, with values significant at the 0.05 level (after Bonferroni correction) marked in bold. Twenty-four of 58 individuals scored as males and we found five alleles at the Y-marker locus MS41b (211, 213, 217, 219 and 223). Overall, 4/14 loci showed Hardy–Weinberg disequilibrium with levels of heterozygosity significantly lower than expected. None of the 78 loci pairs showed gametic disequilibrium after Bonferroni correction for multiple tests. Spatial autocorrelation results (Fig. 1) indicated that kinship was positively correlated up to 60 km, and subsequent values varied around zero. There was an increase for 530–690 km, for which very few samples were available. The results do not suggest obvious isolation by distance on a wider geographical scale.
Spatial autocorrelation across all wolf samples in southwestern Manitoba, Canada. Here r is the autocorrelation (kinship) coefficient, and distance is in kilometres. U and L are upper and lower limits, respectively, for the 95% confidence interval around the null hypothesis of no spatial structure as determined by 999 permutations, whereas the upper and lower error bars show the 95% confidence interval about r as determined by 1,000 bootstrap replicates
According to the Assignment Test, individuals were assigned to the population where they were sampled (Fig. 2a–c). However, a few individuals appear to fit almost as well with other populations rather than that of the sample origin, and likely represent immigrants or their descendants. Three individuals sampled in RMNP had assignment values to RMNP that were only slightly higher than their values for the Duck Mountains (Fig. 2b). Similarly, two RMNP individuals had approximately equal values for the RMNP and the Central Manitoba cluster (Fig. 2c). The STRUCTURE results showed that the highest value of ln P(D) was observed at K = 3 (Fig. 3a), with a slightly lower value for K = 2. The results for ΔK nonetheless suggested that the uppermost level of population structure occurred at K = 2 (Fig. 3b). Thus, K = 2 genetic clusters appeared to be the most parsimonious choice (Pritchard et al. 2000; Evanno et al. 2005). We therefore continued analyses using the K = 2 clusters identified by STRUCTURE. Figure S2, supplementary material, shows assignment results for K = 3.
a Log likelihood values for Duck Mountain versus Central Manitoba samples using the Assignment Test (Paetkau et al. 1995; Waser and Strobeck 1998). Filled squares are Duck Mountain samples; open squares are Central Manitoba samples. b Log likelihood values for Duck Mountain versus Riding Mountain samples using the Assignment Test (Paetkau et al. 1995; Waser and Strobeck 1998). Filled squares are Duck Mountain samples; Stars are Riding Mountain samples. c Log likelihood values for Central Manitoba versus Riding Mountain samples using the Assignment Test (Paetkau et al. 1995; Waser and Strobeck 1998). Open squares are Central Manitoba samples, stars are Riding Mountain samples
a STRUCTURE analyses for the number of population clusters (K) for wolves in southwestern Manitoba, Canada, showing mean ln probability for five runs of K = 1–8 population clusters. b STRUCTURE analyses for the number of population clusters (K) for wolves in southwestern Manitoba, Canada, showing the ΔK rate of change between ln probability values for K = 1–8. The modal value indicates the uppermost level of structure for the dataset (Evanno et al. 2005)
We calculated individual membership in the two genetic clusters for individuals with qi > 0.8 (Fig. 4) and determined their geographical distribution (Fig. 5). With exception of one individual sampled on Hecla Island approximately 400 km northeast of RMNP, all individuals with qi > 0.8 had 90% confidence intervals for q that excluded membership (i.e. excluded 0%) in the alternate population. Of the 58 individuals examined 22 were assigned to cluster 1 (primarily constituted of individuals sampled in RMNP), 27 to cluster 2 (mainly composed of individuals sampled outside RMNP), and 9 showed admixed ancestry (qi < 0.8). All individuals assigned to cluster 1 showed q1 > 0.9. Among the 9 admixed wolves and the individual from Hecla Island, one wolf had been amplified at 10 markers, one at 11 markers, four at 13 markers, and four at 14 markers. There was therefore no clear relationship between individual amplification success and results suggesting admixed ancestry.
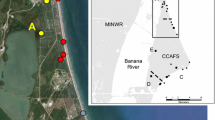
Geographic distribution of individuals for K = 2 wolf population clusters identified by STRUCTURE in southwestern Manitoba, Canada. Cluster 1 (triangles) comprises most individuals sampled in RMNP, whereas cluster 2 (squares) primarily includes individuals sampled outside RMNP. One individual sampled in Sherridon (about 600 km north of RMNP) and one individual sampled in Turtle Mountain Provincial Park (about 200 km south of RMNP), both assigned to cluster 2, were excluded from the map to improve resolution
Three admixed individuals were collected outside RMNP. Two wolves sampled in the Duck Mountains showed q2 = 0.54 and q2 = 0.60, and one wolf sampled approximately 25 km northeast of RMNP showed q2 = 0.53. Of six admixed wolves sampled in RMNP, three had q-values suggesting a relatively high proportion of ancestry from cluster 1 (q1 = 0.70–0.77). The remaining three showed q1 values of 0.61, 0.44, and 0.24. The latter of these was a female captured in January 2004 and monitored in the Park until February 2009. This female and two collared wolves assigned to cluster 1 were killed by humans outside the Park boundary. The movements of at least four other collared wolves, two assigned to cluster 1 and two for which genetic profiling was unsuccessful, showed they travelled widely within the Park but changed direction several times upon encountering the Park boundary (Fig. 6).
We did standard genetic analyses for the two genetic clusters identified by STRUCTURE (Table 2). One locus in cluster 1 and two loci in cluster 2 showed significantly lower heterozygosity than expected. This reduction from the initial Hardy–Weinberg deficit at 4 of 14 loci supports the presence of a Wahlund effect (underlying genetic structure) in the overall sample. Because neither cluster showed gametic disequilibrium, we retained all loci for further testing. We calculated pairwise F ST values for population differentiation using Theta (Weir and Cockerham 1984). Differentiation between the two clusters was moderate (Balloux and Lugon-Moulin 2002) and significant (F ST = 0.053 (95% CI [0.031–0.073]). Test results for BOTTLENECK with a two-step mutation model accounting for 5–30% of all mutations were significant (P = 0.034–0.047) for cluster 1. An FCA plot shows two-dimensional multilocus profiles of individuals identified according to geographical sampling locations (Fig. S3, supplementary material). The first axis represents most of the variation, and the FCA results generally concurred with the findings from STRUCTURE (see Fig. S3 for details).
Discussion
Gene flow and population genetic structure inferred from nuclear DNA
A subtle genetic structure is visible and consistent with fragmentation in the study area. Genetic clusters are separated by a matrix landscape dominated by intensive agricultural development and a dense network of roads. Our results seem to concur with those of Pilot et al. (2006) from an Eastern European landscape without obvious physical barriers to wolf movement.
Genetic diversity was similar to that of wolves in the Canadian Rocky Mountains examined with nine of the same markers, and allelic diversity was relatively high (Thiessen 2007). The presence of closely related individuals likely contributed to the positive F IS values and heterozygosity deficit within clusters, although null alleles, inbreeding, or a Wahlund effect (additional undetected structure) could also have affected our results (Roy et al. 1994; Lucchini et al. 2002; Pilot et al. 2006; Thiessen 2007). STRUCTURE assumes Hardy–Weinberg and gametic equilibrium within each cluster (Pritchard et al. 2000), which may be difficult to fulfill in wolf populations composed of family groups (Pilot et al. 2006; Thiessen 2007; vonHoldt et al. 2008).
A founder effect or bottleneck likely affected the RMNP population (Carbyn 1980) and our results suggest that a bottleneck may have occurred. This could have resulted in significant structure in highly variable loci (Hedrick 1999), particularly if limited gene flow occurred afterward. Genetic drift could also operate in space and time for isolated populations and create a Wahlund effect in both dimensions (Flagstad et al. 2003). Distinguishing relative contributions of bottlenecks and inbreeding toward loss of heterozygosity can be difficult (Eppley et al. 2007) but either situation would suggest a small number of breeders and limited gene flow, which is consistent with our findings.
Spatial autocorrelation across loci suggests that kinship is initially positively associated with distance. This can be expected in populations composed of territorial family groups. Aspi et al. (2006) found kinship positively correlated with distances up to 163 km in a continuous Finnish wolf population followed by significant isolation by distance on a limited spatial scale. Overall, the spatial autocorrelation results from our study area do not suggest significant isolation by distance.
Most wolves sampled in RMNP had high assignment to cluster 1. The behaviour of several potentially dispersing wolves (as identified via exploratory movements outside their regular home range) suggested that they were reluctant to cross the Park boundary. Changes in movement behaviour may occur at park boundaries (Paquet et al. 2010), and the possibility that long-lived, social and vagile species may learn to associate human activity and roads with danger (Whittington et al. 2005) require further investigation in landscapes lacking physical barriers to movement.
Four wolves sampled within RMNP were assigned to cluster 2. Two samples, collected in the eastern and central portion of the Park, were from dead animals found in poor physical condition. Their history is unknown but both were relatively young (noted as 1–3 years of age), which reduces the probability of effective dispersal (reproduction in the new location). The westernmost individual was a wolf collared and radio-tracked for several months in RMNP. He was excluded from the FCA plot because of his highly divergent genotype. This individual appears to have been an immigrant (wolves were collared as subadults or adults) or the offspring of immigrants, though another wolf collared in the same pack (overlapping sample in Fig. 5) was assigned to cluster 1. The fourth putative immigrant to RMNP was a male in good body condition found dead on the southern Park boundary. Necropsy revealed he had been killed by other wolves (T. Bollinger pers. comm.). The divergent genotype indicated by the FCA results is consistent with this wolf being an immigrant from outside the primary study area. He had mtDNA haplotype C23, which is common in RMNP and throughout North America (Stronen et al. 2010).
The finding of admixed individuals within and outside the Park suggests that some dispersal and gene flow is taking place in the area. Our observational data suggests that the admixed female followed during 2004–2009 may have reproduced. Although it is uncertain whether she may have dispersed into RMNP prior to her capture, or been born in RMNP to one or two immigrant parents, her case suggests that effective dispersal into RMNP has occurred recently. It is nevertheless important that successful dispersal does not guarantee reproduction (Greenwood 1980; Riley et al. 2006). Several putative dispersers (Fig. 5) were found near the RMNP boundary, which is considered marginal wolf habitat with high risk of human-caused mortality (Carbyn 1980, RMNP unpublished data). These putative dispersers may have been unable to establish territories and reproduce, and might therefore have been ‘queuing’ for space in the Park. Behavioural mechanisms including kin-clustering and subsequent local recruitment seen in territorial birds (Watson et al. 1994; Temple et al. 2006) and mammals (Lambin and Yoccoz 1998) could also make it increasingly difficult for immigrants to get established. Territoriality and a social structure with few breeding animals, combined with high human-caused mortality in the area surrounding RMNP, could thus present additional obstacles for gene flow into isolated reserves.
Expected ecological and evolutionary consequences of landscape fragmentation
The two genetic clusters we identified comprised 22 and 27 individuals, and such group sizes may be consistent with observation of larger wolf family groups (Fuller et al. 2003). Based on our sampling locations and weekly radio-tracking results, we nonetheless feel confident that the RMNP cluster does not reflect a large wolf family group but spatial structuring consistent with a small and increasingly isolated population of RMNP wolves. Biological interpretation of F ST values is difficult and values within the range of 0.05–0.15 are generally considered as moderate (Balloux and Lugon-Moulin 2002). The divergence between the two genetic clusters in our study area is nevertheless notable compared with wolves in the Canadian Rocky Mountains studied with nine of the same markers (Thiessen 2007). Thiessen found similar divergence values (F ST = 0.0306–0.0552) between four populations (n = 92–129) separated by larger geographic distances (>100 km). Previous studies have also shown moderate short-distance genetic structure in mobile species such as wolverines, lynx, and coyotes (Cegelski et al. 2003; Rueness et al. 2003; Sacks et al. 2004; Guillot et al. 2005) in areas without obvious barriers. However, historical ecological discontinuities potentially combined with the shy nature of some species and subsequent low tolerance of humans could not be excluded.
Our results are consistent with the findings of distinct mtDNA haplotypes in the RMNP population that have not been reported outside the Park (Lehman et al. 1991; Geffen et al. 2004; Stronen et al. 2010). mtDNA haplotypes of gray wolves and eastern wolves have been identified in the Duck Mountains, whereas eastern wolves appear rare or absent from RMNP based on samples identified to date (Wilson et al. 2000; Stronen et al. 2010). A mtDNA study including the RMNP region that examined 20 recent samples from RMNP, found 19 individuals with gray wolf haplotypes and one that clustered with New World haplotypes identified in coyotes and eastern wolves (Stronen et al. 2010, GenBank accession numbers HM014451–HM014467).
It is possible that the presence of different wolf types contributes to genetic differentiation between animals found in RMNP and those occurring in the Duck Mountains and central Manitoba. We nonetheless believe that such a situation would constitute a proximate reason for population structuring in our study area. Prey species commonly used by eastern wolves in Algonquin Provincial Park in Ontario such as moose, deer, and beaver (Forbes and Theberge 1996; Loveless 2010) are abundant in RMNP. Wolf body mass for RMNP individuals sampled during 1999–2004 was approximately 36 kg for females (n = 12) and 39 kg for males (n = 8) (Stronen et al. 2010). Hence, it is doubtful that RMNP wolves would physically exclude immigrating eastern wolves. New results also indicate that individuals with a mixture of eastern/Great Lakes and gray wolf genetic material are common in the Great Lakes region (Fain et al. 2010; Wheeldon et al. 2010; vonHoldt et al. 2011). Importantly, we cannot exclude the possibility that local adaptive differences, including predator–prey relationships, could affect wolf genetic structure. Recent findings also suggest habitat discontinuities and foraging behaviour cause genetic differentiation within grey wolves of British Columbia, Canada (Muñoz-Fuentes et al. 2009). Based on the similar habitat and prey species found in RMNP and surrounding areas, we would nonetheless expect eastern-grey admixed individuals to be common in RMNP if dispersal into the Park was frequent. The question therefore remains as to why eastern or eastern-gray admixed wolves appear not to be (effectively) dispersing into RMNP.
Our results indicate that human-caused fragmentation of a landscape without physical barriers to movement can reduce gene flow and cause cryptic genetic population structure in highly mobile organisms on fine spatiotemporal scales. We found significant genetic structure in a vagile species that is: (1) not influenced by barriers or historical ecological discontinuities in our study area and; (2) able to live relatively close to humans if shown tolerance (Fuller et al. 2003). Ecological or behavioural factors (including prey distribution and natal habitat-biased dispersal) might now influence gene flow. However, such potential influences are unlikely to have become established without prior fragmentation. We believe our findings are significant because they suggest that human-caused fragmentation can have more profound consequences for gene flow than previously thought. This influence can act relatively quickly, which seems consistent with rapid responses to human-induced landscape change reported over recent years (Ashley et al. 2003). Long-term monitoring is needed to establish whether differentiation between population clusters may be increasing.
Human tolerance of wolves is often limited. Many local residents believe there are ‘too many wolves’ (Stronen et al. 2007), whereas effective dispersal in the landscape matrix remains low. Our research in the RMNP-region demonstrates that both situations can occur simultaneously, which has important implications for long-term conservation of carnivores. The Great Plains is now an intensely human-managed landscape (Guertin et al. 1997) and similar results may be found for other vagile species. Conservation planning for wide-ranging and low-density species in uninterrupted landscapes modified by human development should therefore consider more conservative predictions of gene flow to isolated sites.
References
Allendorf FW, Luikart G (2007) Conservation and the genetics of populations. Blackwell Publishing, Malden
Ashley MV, Wilson MF, Pergarms ORW, O’Dowd DJ, Gende SM, Brown JS (2003) Evolutionary enlightened management. Biol Conserv 111:115–123
Aspi J, Roininen E, Ruokonen M, Kojola I, Vilà C (2006) Genetic diversity, population structure, effective population size and demographic history of the Finnish wolf population. Mol Ecol 15:1561–1576
Balloux F, Lugon-Moulin N (2002) The estimation of population differentiation with microsatellite markers. Mol Ecol 11:155–165
Belkhir KP, Borsa P, Chikhi L, Raufaste N, Bonhomme F (2004) GENETIX 4.05, logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome, Populations, Interactions. CNRS UMR 5171, Université de Montpellier II, Montpellier
Breen M, Jouquand S, Renier C et al (2001) Chromosome-specific single-locus FISH probes allow anchorage of an 1800-marker integrated radiation-hybrid/linkage map of the domestic dog genome to all chromosomes. Genome Res 11:1784–1795
Carbyn LN (1980) Ecology and management of wolves in Riding Mountain National Park, Manitoba. Final report, Large Mammal System Studies, Report No. 10, September 1975–March 1979. Canadian Wildlife Service, Edmonton
Carbyn LN (1982) Incidence of disease and its potential role in the population dynamics of wolves in Riding Mountain National Park, Manitoba. In: Harrington FH, Paquet PC (eds) Wolves of the world: perspectives of behaviour, ecology and conservation. Noyes Publications, Park Ridge, pp 106–115
Carmichael LE, Nagy JA, Carter NC, Strobeck C (2001) Prey specialization may influence patterns of gene flow in wolves of the Canadian Northwest. Mol Ecol 10:2787–2798
Carroll C, Philips MK, Lopez-Gonzales CA, Schumaker NH (2006) Defining recovery goals and strategies for endangered species: the wolf as a case study. Bioscience 56:25–37
Cegelski CC, Waits LP, Anderson NJ (2003) Assessing population structure and gene flow in Montana wolverines (Gulo gulo) using assignment approaches. Mol Ecol 12:2907–2918
Coulon A, Guillot G, Cousson J-F et al (2006) Genetic structure is influenced by landscape features: empirical evidence from a roe deer population. Mol Ecol 15:1669–1679
Environment Canada (1993) Canada: terrestrial ecoregions. http://atlas.nrcan.gc.ca/site/english/maps/archives/5thedition/environment/ecology/mcr4164#download. Accessed May 2007
Eppley SM, Taylor PJ, Jesson LK (2007) Self-fertilization in mosses: a comparison of heterozygosity deficiency between species with combined versus separate sexes. Heredity 98:38–44
Ernest HB, Boyce WM, Bleich VC, May B, Stiver SJ, Torres SG (2003) Genetic structure of mountain lion (Puma concolor) populations in California. Conserv Genet 4:353–366
Evanno G, Regaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Fain SR, Straughan DJ, Taylor BF (2010) Genetic outcomes of wolf recovery in the western Great Lakes states. Conserv Genet 11:1747–1765
Falush D, Stephens M, Pritchard JK (2003) Inference on population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Flagstad Ø, Walker CW, Vilà C, Sundqvist A-K, Fernholm B, Hufthammer AK, Wiig Ø, Kojola I, Ellegren H (2003) Two centuries of the Scandinavian wolf population: patterns of genetic variability and migration during an era of dramatic decline. Mol Ecol 12:869–880
Forbes GJ, Theberge JB (1996) Response by wolves to prey variation in central Ontario. Can J Zool 74:1511–1520
Franklin JF (1993) Preserving biodiversity: species, ecosystems, or landscapes? Ecol Appl 32:202–205
Fritts SH (1983) Record dispersal by a wolf from Minnesota. J Mammal 64:166–167
Fritts SH, Carbyn LN (1995) Population viability, nature reserves, and the outlook for gray wolf conservation in North America. Restor Ecol 3(1):26–38
Fuller TK, Mech LD, Cochrane JF (2003) Wolf population dynamics. In: Mech LD, Boitani L (eds) Wolves: behaviour, ecology and conservation. University of Chicago Press, Chicago, pp 161–191
Geffen E, Anderson MJ, Wayne RK (2004) Climate and habitat barriers to dispersal in the highly mobile grey wolf. Mol Ecol 13:2481–2490
Gese EM, Mech LD (1991) Dispersal of wolves (Canis lupus) in northeastern Minnesota, 1969–1989. Can J Zool 69:2946–2955
Goulet GD (1993) Comparison of temporal and geographical skull variation among Nearctic modern, Holocene and Late Pleistocene gray wolves (Canis lupus) (and selected Canis). MSc Thesis, University of Manitoba, Winnipeg
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Guertin DS, Easterling WE, Brandle JR (1997) Climate change and forests in the Great Plains. Bioscience 47:287–295
Guillot G, Estoup A, Mortier F, Cosson J-F (2005) A spatial statistical model for landscape genetics. Genetics 170:1261–1280
Guo SW, Thompson EA (1992) Performing the exact test of Hardy–Weinberg proportion for multiple alleles. Biometrics 48:361–372
Guyon R, Lorentzen TD, Hitte C et al (2003) A 1-Mb resolution radiation hybrid map of the canine genome. Proc Natl Acad Sci USA 100:5296–5301
Haight RG, Mladenoff DL, Wydeven AP (1998) Modeling disjunct gray wolf populations in semi-wild landscapes. Conserv Biol 12:879–888
Hedrick PW (1999) Highly variable loci and their interpretation in evolution and conservation. Evolution 53:313–318
Hoelzel AR, Dahlheim M, Stern SJ (1998) Low genetic variation among killer whales (Orcinus orca) in the eastern north Pacific and genetic differentiation between foraging specialists. J Hered 89:121–128
Koblmüller S, Nord M, Wayne RK, Leonard JA (2009) Origin and status of the Great Lakes wolf. Mol Ecol 18:2313–2326
Kramer-Schadt S, Revilla E, Wiegand T, Breitenmoser U (2004) Fragmented landscapes, road mortality and patch connectivity: modelling influences on the dispersal of Eurasian lynx. J Appl Ecol 41:711–723
Lambin X, Yoccoz NG (1998) The impact of population kin-structure on nestling survival in Townsend voles (Microtus townsendii). J Anim Ecol 67:1–16
Lehman N, Eisenhawer A, Hansen K, Mech D, Peterson RO, Gogan PJP, Wayne RK (1991) Introgression of coyote mitochondrial DNA into sympatric North American gray wolf populations. Evolution 45:104–119
Leonard JA, Vilà C, Wayne RK (2005) Legacy lost: genetic variability and population size of extirpated US grey wolves (Canis lupus). Mol Ecol 14:9–17
Loveless K (2010) Foraging strategies of eastern wolves in relation to migratory prey and hybridization. MSc Thesis, Trent University, Peterborough
Lucchini V, Fabri E, Marucco F, Ricci S, Boitani L, Randi E (2002) Noninvasive molecular tracking of colonizing wolf (Canis lupus) packs in the western Alps. Mol Ecol 11:857–868
Manitoba Conservation (2004) Duck Mountain Provincial Park. http://www.gov.mb.ca/conservation/parks/popular_parks/duck_mtn/info.html. Accessed June 2007
McNamee K (1993) From wild places to endangered spaces: a history of Canada’s national parks. In: Dearden P, Rollins R (eds) Parks and protected areas in Canada: planning and management. Oxford University Press, Toronto, pp 17–44
Mech LD, Boitani L (2003) Introduction. In: Mech LD, Boitani L (eds) Wolves: behaviour, ecology and conservation. University of Chicago Press, Chicago, pp xv–xvii
Muñoz-Fuentes V, Darimont CT, Wayne RK, Paquet PC, Leonard JA (2009) Ecological factors drive genetic differentiation in British Columbia gray wolves. J Biogeogr 31:1516–1531
Neff MW, Broman KW, Mellersh CS et al (1999) A second-generation genetic linkage map of the domestic dog, Canis familiaris. Genetics 151:803–820. doi:10.1073/pnas.0831002100
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Noss RF (1995) Maintaining ecological integrity in representative networks. World Wildlife Fund Canada and World Wildlife Fund United States
Noss RF, Quigley HB, Hornocker MG, Merrill T, Paquet PC (1996) Conservation biology and carnivore conservation in the Rocky Mountains. Conserv Biol 10:949–963
Paetkau D, Calvert W, Stirling I, Strobeck C (1995) Microsatellite analysis of population structure in Canadian polar bears. Mol Ecol 4:347–354
Paquet PC (1992) Prey use strategies of sympatric wolves and coyotes in Riding Mountain National Park, Manitoba. J Mammal 73:337–343
Paquet PC, Alexander S, Donelon S, Callaghan C (2010) Influence of anthropogenically modified snow conditions on movement and predatory behaviour of gray wolves. In: Musiani M, Boitani L, Paquet PC (eds) The world of wolves: new perspectives on ecology behaviour and policy. University of Calgary Press, Calgary, pp 157–173
Parks Canada (2004) Riding Mountain National Park: ecological integrity statement. http://www2.parkscanada.gc.ca/pn-np/mb/riding/plan/plan3_e.asp. Accessed June 2007
Parks Canada (2006) Riding Mountain National Park: natural heritage. http://www2.parkscanada.gc.ca/pn-np/mb/riding/natcul/natcul1_E.asp. Accessed June 2007
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pilot M, Jedrzejewski W, Branicki W et al (2006) Ecological factors influence population genetic structure of European grey wolves. Mol Ecol 15:4533–4553
Piry S, Luikart G, Cornuet J-M (1999) BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90:502–503
Pritchard JK, Stephens M, Donnely P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) Genepop (version 1.2): population genetics software for exact tests and ecumeniscism. J Hered 86:248–249
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Riley SPD, Pollinger JP, Sauvajot RM et al (2006) A southern California freeway is a physical and social barrier to gene flow in carnivores. Mol Ecol 15:1733–1741
Roques S, Sevigny J-M, Bernatchez L (2001) Evidence for broadscale introgressive hybridization between two redfish (genus Sebastes) in the North-west Atlantic: a rare marine example. Mol Ecol 10:149–165
Roy MS, Geffen E, Smith D, Ostrander EA, Wayne RK (1994) Patterns of differentiation and hybridization in North American wolf like canids, revealed by analysis of microsatellite loci. Mol Biol Evol 11:553–570
Rueness EK, Stenseth NC, O’Donoghue M et al (2003) Ecological and genetic spatial structuring in the Canadian lynx. Nature 425:69–72
Sacks BN, Brown SK, Ernest HB (2004) Population structure of California coyotes corresponds to habitat-specific breaks and illuminate species history. Mol Ecol 13:1265–1275
Sacks BN, Mitchell BR, Williams CL, Ernest HB (2005) Coyote movements and social structure along a cryptic genetic subdivision. Mol Ecol 14:1241–1249
Sancristobal-Gaudy M, Renand G, Amigues Y, Boscher M-Y, Levéziel H, Bibé B (2000) Traçabilité individuelle des viands bovines à l’aide de marqueurs génétiques. INRA Prod Anim 13:269–276
Schneider S, Roessli D, Excoffier L (2000) Arlequin ver. 2.00: a software for population genetics data analyses. Genetics and Biometry Laboratory, University of Geneva, Geneva
Stronen AV (2009) Dispersal in a plain landscape: wolves in southwestern Manitoba, Canada. PhD Dissertation, University of New Brunswick, Fredericton
Stronen AV, Brook RK, Paquet PC, McLachlan SM (2007) Farmer attitudes toward wolves: implications for the role of predators in managing disease. Biol Conserv 135:1–10
Stronen AV, Forbes GJ, Sallows T, Goulet G, Musiani M, Paquet PC (2010) Wolf body mass, skull morphology, and mitochondrial DNA haplotypes in the Riding Mountain National Park region of Manitoba, Canada. Can J Zool 88:496–507
Sundquist AK, Ellegren H, Olivier M, Vilà C (2001) Y-chromosome haplotyping in Scandinavian wolves (Canis lupus) based on microsatellite markers. Mol Ecol 10:1959–1966
Sunnucks P (2000) Efficient genetic markers for population biology. Trends Ecol Evol 15:199–203
Taberlet P, Fumagalli L, Wust-Saucy A-G, Cosson J-F (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7:453–464
Taberlet P, Waits LP, Luikart G (1999) Noninvasive genetic sampling: look before you leap. Trends Ecol Evol 14:323–327
Temple HJ, Hoffman I, Amos W (2006) Dispersal, philopatry and intergroup relatedness: fine-scale genetic structure in the white-breasted thrasher (Ramphocinclus branchyurus). Mol Ecol 15:3449–3458
Thiessen CD (2007) Population structure and dispersal of wolves in the Canadian Rocky Mountains. MSc Thesis, Department of Biology, University of Alberta, Edmonton
Vilà C, Amorim IR, Leonard JA et al (1999) Mitochondrial DNA phylogeography and population history of the grey wolf Canis lupus. Mol Ecol 8:2089–2103
vonHoldt BM, Stahler DR, Smith DW et al (2008) The genealogy and genetic viability of reintroduced Yellowstone grey wolves. Mol Ecol 17:252–274
vonHoldt BM, Pollinger JP, Earl DA et al (2011) A genome-wide perspective on the evolutionary history of enigmatic wolf-like canids. Genome Res. Advance online publication 12 May 2011. doi:10.1101/gr.116301.110
Waits LP, Luikart G, Taberlet P (2001) Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol Ecol 10:249–256
Walker DJ (2001) Landscape complexity and vegetation dynamics in Riding Mountain National Park, Canada. PhD Dissertation, University of Manitoba, Winnipeg
Waser PM, Strobeck C (1998) Genetic signatures of interpopulation dispersal. Trends Ecol Evol 13:43–44
Watson A, Moss R, Parr R, Mountford MD, Rothery P (1994) Kin landownership, differential aggression between kin and non-kin, and population fluctuations in red grouse. J Anim Ecol 63:39–50
Weaver JL, Paquet PC, Ruggiero LF (1996) Resilience and conservation of large carnivores in the Rocky Mountains. Conserv Biol 10:964–976
Weckworth BV, Talbot S, Sage GK, Person DK, Cook J (2005) A signal for independent coastal and continental histories among North American wolves. Mol Ecol 14:917–931
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analyses of population structure. Evolution 38:1358–1370
Wheeldon TJ, Patterson BR, White BN (2010) Sympatric wolf and coyote populations of the western Great Lakes region are reproductively isolated. Mol Ecol 19:4428–4440
Whittington J, Cassady St. Clair C, Mercer G (2005) Spatial responses of wolves to roads and trails in mountain valleys. Ecol Appl 15:533–543
Wilson PJ, Grewal S, Lawford ID et al (2000) DNA profiles of the eastern Canadian wolf and the red wolf provide evidence for a common evolutionary history independent of the gray wolf. Can J Zool 78:2156–2166
Wilson PJ, Grewal SK, Mallory FF, White BN (2009) Genetic characterization of hybrid wolves across Ontario. J Hered 100:S80–S89
Wydeven AP, Schultz RN, Thiel RP (1995) Monitoring of a recovering gray wolf population in Wisconsin, 1979–1991. In: Carbyn LN, Fritts SH, Seip DR (eds) Ecology and conservation of wolves in a changing world. Canadian Circumpolar Institute, Occasional Publication No. 35, pp 147–156
Acknowledgments
We thank G. Pflueger, S. Jaward, staff from Parks Canada and Manitoba Conservation, the Duck Mountain Trappers’ Association, trappers from northern Manitoba, and other residents for contributing to sample collection and tracking of radio-collared wolves. Microsatellite analyses were done at GenServe Laboratories at the Saskatchewan Research Council, Saskatoon. A. Arndt, D. Berezanski, D. Bergeson, L. Jesson, D. Keppie, B. Mann, A. McDevitt, E. Navid, Y. Plante, S. Woodley, and two anonymous reviewers provided valuable help and advice on analyses and earlier versions of the manuscript. We gratefully acknowledge funding from Parks Canada, World Wildlife Fund Canada, the Sustainable Development Innovations Fund at Manitoba Conservation, and Louisiana Pacific Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gloria Goulet is retired from Canadian Wildlife Service, Environment Canada
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stronen, A.V., Forbes, G.J., Paquet, P.C. et al. Dispersal in a plain landscape: short-distance genetic differentiation in southwestern Manitoba wolves, Canada. Conserv Genet 13, 359–371 (2012). https://doi.org/10.1007/s10592-011-0290-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-011-0290-1