Abstract
The Broad-headed snake Hoplocephalus bungaroides is one of Australia’s most endangered vertebrates. Extant populations of H. bungaroides are restricted to several geographically isolated reserves to the north, west, and south of Sydney. We analysed mitochondrial DNA from 184 specimens drawn from across the geographic range of the Broad-headed snake. Phylogenetic analysis demonstrated that H. bungaroides comprises two divergent mitochondrial lineages with a “northern” clade comprising populations west and north of Sydney and a “southern” clade comprising animals in Morton National Park. The two clades differ by an uncorrected genetic distance of 1.7%, which implies a divergence dating to approximately 755,000–850,000 years ago. We complemented our molecular data set with a detailed analysis of morphological variation both between and within the genetic clades. The two H. bungaroides genetic clades are morphologically indistinguishable and show little sexual dimorphism. Our results demonstrate that the populations north and south of this biogeographic split function as two distinct populations with no recent gene flow. There is no reason for separate taxonomic recognition of these two clades, but they do represent distinct evolutionarily significant units (ESUs) that require separate conservation management. In addition, within the northern ESU, populations from Royal National Park, Blue Mountains National Park, Wollemi National Park, and the Sydney Water Catchment supply areas should be considered as separate management units to conserve both evolutionary and ecological processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human land clearing activities have relegated populations of many threatened taxa to small geographically isolated habitat patches (Saunders et al. 1991). Species with poor dispersal ability or highly specialised habitat requirements may be unable to disperse between habitat patches, and consequently, they may face an increased risk of extinction (Goodman 1987; Hobbs and Yates 2003; McKinney 1997). Given limited funding and conflicts between conservation and other land-use activities, it is rarely possible to manage all habitat patches to conserve endangered species. The challenge for conservation biologists is to decide which populations or habitats to protect or manage in order to preserve both ecological and evolutionary processes (Frankel 1974). One solution to this problem is to use genetic techniques to identify evolutionarily significant units for conservation (ESU’s), which in turn can aid in the management of the target species (Moritz 1994a, b; 2002). Molecular techniques can also help to identify management units (demographically distinct populations) that require management to maintain the viability of ESUs (Moritz 1994a, b, 2002). This approach, when combined with knowledge of the biology of the species in question, can allow land managers to identify the most important areas for management and conservation of the target species and their habitats.
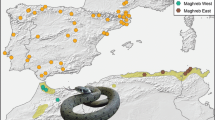
The endangered Australian Broad-headed snake, Hoplocephalus bungaroides, is a medium-sized (to 90 cm long), brightly coloured, nocturnal, venomous snake that only occurs on sandstone rock formations within a 200 km radius of Sydney (Cogger 2000; Fig. 1). In the 1800s, Broad-headed snakes were common in the Sydney region (Krefft 1869), but in the last century they have declined dramatically due to urban development of ridge tops and the collection of sandstone rocks for landscaping urban gardens (Hersey 1980; Shine and Fitzgerald 1989). Today, the Broad-headed snake is locally extinct in the Sydney metropolitan area, and extant populations are restricted to a handful of geographically isolated national parks and water catchment reserves in the areas surrounding Sydney (Shine et al. 1998). Recent surveys suggest that the snake is locally extinct or rare within many national parks and reserves (Goldingay 1998; Newell and Goldingay 2005; Shine et al. 1998; Fig. 1). Extant populations are threatened by the removal of rocks for landscape gardening (Hersey 1980; Shine et al. 1998), the displacement and breakage of rocks caused by reptile collectors (Goldingay and Newell 2000), the collection of snakes for the illegal pet trade (Webb et al. 2002a), and the overgrowth of rock outcrops by encroaching vegetation (Pringle et al. 2003; Webb et al. 2005).
Distribution map for Hoplocephalus bungaroides in the Sydney basin, in south eastern Australia. The map indicates all recorded localities from museum records, sites from which the snakes are known to be extinct and the sampling areas from which we have tissue samples used in this study. The line on the figure indicates the east-facing sandstone escarpment that is unsuitable for snake thermoregulation and which we consider to be a biogeographic break in H. bungaroides
Despite its endangered status, the demography, ecology and behaviour of the Broad-headed snake is well known. Unlike other elapid snakes, Broad-headed snakes mature late (around 5 years), are long-lived, and females produce small clutches every second or third year (Webb et al. 2002b). These life history traits render populations of this species vulnerable to extinction, and the snake has disappeared from much of its former geographic range (Newell and Goldingay 2005; Shine et al. 1998). In Morton National Park, in the southern part of their range, Broad-headed snakes are restricted to a handful of disjunct plateaus, but long-term mark-recapture studies suggest that this population is small (<1,000 individuals, Webb et al. 2002a). During the cooler months, juvenile and adult Broad-headed snakes thermoregulate under thin rocks and inside crevices on rock outcrops (Webb and Shine 1998), a trait that makes them vulnerable to the removal of rocks for landscaping urban gardens (Shine et al. 1998). In summer, adult Broad-headed snakes disperse from rock outcrops to adjacent eucalypt forests, where they shelter inside tree hollows (Webb and Shine 1997a, b). Although adults can travel long distances during summer, most snakes return to the same rock outcrops where they were first captured (Webb and Shine 1997a, b). Mark-recapture studies suggest that Broad-headed snakes are poor dispersers, with most juveniles settling <1 km from their birth sites (Webb and Shine 1997b; Webb et al. 2002b). The high degree of adult philopatry and poor dispersal of juveniles could influence underlying genetic structuring among populations.
In this study we evaluate phylogeographic structure among the remaining highly disjunct Broad-headed snake populations, and we complement our genetic data with morphological data taken from preserved specimens in museum collections. We use this information to make informed management recommendations to guide future conservation efforts of this critically endangered snake.
Materials and methods
Molecular data
We obtained a total of 184 tissue samples of H. bungaroides from sites throughout the current geographic range of the species including most of the reserves known to harbour populations (Fig. 1). We sampled populations from Morton National Park in the far south of the range, Royal National Park near Sydney, Yengo National Park north of Sydney, and Newnes State Forest northwest of Sydney. Where possible, we included samples from 3 to 4 individuals from each of these four regions to evaluate intra-population level variation (Table 1). We included a large number of samples from Morton National Park, which were obtained as part of a long-term mark-recapture study by two of us (J. K. W and R. S). We also included individuals captured during a recent survey of the distribution and abundance of this species and several samples were from captive individuals with reliable locality information. Because of the endangered status of this species, we used sloughed skins or scale clips to obtain tissue samples, and no animals were killed.
For each sample we targeted an approximately 900 bp DNA fragment of the mitochondrial genome which included the 3′ half of the ND4 gene and most of the tRNA cluster containing the histidine, serine and leucine tRNA genes. The target fragment was amplified using modified primers ND4 and Leu (Arévalo et al. 1994). This region was targeted because work at comparable taxonomic levels in numerous other squamate reptile groups has revealed useful levels of variability and because this gene was particularly useful in a similar study of phylogeographic structure in Hoplocephalus stephensi (Keogh et al. 2003). All laboratory procedures are as in Scott and Keogh (2000). Aligned sequences were translated into amino acid sequences using the vertebrate mitochondrial genetic code. No premature stop codons were observed, so we conclude that all sequences obtained are true mitochondrial copies.
The closely related elapid snake Notechis scutatus was used as an outgroup (Keogh et al. 1998, 2000) and we combined our data with the ND4 data for H. bitorquatus and H. stephensi from Keogh et al. (2003) to provide a comparison of species-level divergence within Hoplocephalus. We used parsimony and Bayesian approaches to analyse the data with PAUP* v4.0b10 (Swofford 2002) and MrBayes (v3.0b4; Huelsenbeck and Ronquist 2001), respectively. For the parsimony analyses we used TBR branch swapping and ran the parsimony analyses five times from random starting points and with random sequence addition to confirm that overall tree space was well searched. For the Bayesian analyses we allowed all parameters to be estimated from the data during the runs. We used the default value of four Markov chains per run and also ran the full analysis four times to make sure overall tree-space was well sampled and to avoid being trapped in local optima. We ran each analysis for a total of 5,000,000 generations and sampled the chain every 100 generations (standard MrBayes settings), resulting in 50,000 sampled trees. We discarded the first 10,000 trees and used the last 40,000 trees to estimate Bayesian posterior probabilities. We used 1,000 unweighted non-parametric parsimony bootstrap replicates and Bayesian posterior probabilities to assess branch support.
Morphological data
We collected extensive data on the morphology of H. bungaroides to test for any corroborating morphological differentiation between the mitochondrial clades. We examined all H. bungaroides specimens available in the following museum collections: Australian Museum (AM), Queensland Museum (QM), National Museum of Victoria (NMV), South Australian Museum (SAM), and the CSIRO Australian National Wildlife Collection (ANWC). After excluding any museum specimens with dubious or absent locality data, 61 specimens were available for analysis.
We collected data on all the external morphological characters traditionally used in elapid snake systematics. We measured scalation characteristics including nasal–preocular contact (present or absent); number of supralabial, infralabial, preocular, postocular, anterior temporal, and posterior temporal scales; number of dorsal scale rows one head length posterior to the neck, at mid-body, and one head length anterior to vent; and number of ventral and subcaudal scales. We measured snout-vent length (SVL), tail length, head length from the quadrate-articular projection at the rear of the jaw to the tip of the snout, head width at the widest part of the head, mouth length from posterior corner of the mouth and eye diameter.
After excluding characters that were largely invariant (nasal–preocular contact, number of supralabial, infralabial, preocular, postocular, anterior temporal, posterior temporal scales, dorsal scale rows), the remaining variables were natural log transformed prior to analysis to meet the assumptions of the statistical tests we employed. Each specimen was assigned to a genetic clade based on distribution. We then performed an analysis of variance (ANOVA) on each meristic character to test for differences between the genetic clades. For body size variables we used analysis of covariance (ANCOVA) against stable co-variates with clade as the nominal variable. For tail length and head length we used SVL as the covariate and for head width, mouth length and eye diameter we used head length as the covariate. We then pooled all the data on adult specimens and performed the same analyses based on sex to test for sexual size dimorphism.
Results
Molecular data
The edited alignment comprised 783 bp. Within the genus Hoplocephalus, 85 sites were variable and 77 of these were informative under parsimony. Both unweighted parsimony and Bayesian analyses produced the same topology and so in Fig. 2 we show an unweighted parsimony phylogram with both bootstrap values and Bayesian posterior probabilities. As we found in a previous study based on many fewer samples, H. bungaroides and H. stephensi are very closely related with an uncorrected genetic divergence of only 2.41–3.15% between them (Keogh et al. 2003). Hoplocephalus bitorquatus is more distantly related, with an uncorrected genetic distance of 6.91–7.36% between it and H. bungaroides and 7.29–7.77% between it and H. stephensi. H. bungaroides comprises two divergent clades that correspond geographically to two distinct regions surrounding Sydney. The “northern” clade comprises animals from Royal National Park, Newnes State Forest, Yengo National Park and Putty, whereas the “southern” clade comprises animals from Morton National Park, Bugong National Park and Nowra (Fig. 2). The two clades differ by an uncorrected genetic distance of 1.71–1.79%. Within these two clades, mitochondrial DNA variation is extremely low. Based on 175 sequences, the southern clade comprises just two haplotypes, which differ by a single base pair. The northern clade comprises fewer samples (9) but displays four closely related haplotypes which each differ by a single base pair (Fig. 2). Each of these divergent clades is supported by high bootstrap values (95–100%) and posterior probabilities of 100%. While support for the sister group relationship between the two clades is low, the morphological data strongly suggest monophyly (see below).
Parsimony phylogram for the genus Hoplocephalus based on the mtDNA gene ND4 and associated tRNAs. Data for H. stephensi and H. bitorquatus from Keogh et al. (2003). For H. bungaroides sample sizes for each of the six haplotypes (A–F) are noted in brackets (see Table 1), and the northern and southern clades are identified. The network diagram illustrates relative haplotype frequency for H. bungaroides. The numbers above the branches represent uncorrected branch lengths and the numbers below the branch lengths represent parsimony bootstrap values and Bayesian posterior probabilities (bold)
The commonly used rough mitochondrial DNA clock of approximately 2% divergence per million years (Avise 2004) dates the split between the two H. bungaroides clades to approximately 850,000 years ago. This is very similar to an estimate based on modern molecular genetic dating analyses for Australian elapids using multiple mtDNA and nDNA loci (Sanders et al. 2008). That study dated the divergence between the clades comprising N. scutatus and Hoplocephalus (9.5% based on our mtDNA data) at approximately 4.2 mya which equates to approximately 2.25% divergence per million years. Extrapolating from this date to the divergence between the two H. bungaroides clades gives an estimate of approximately 755,000 years ago. However, it is worth noting that this mutation rate is specific to the portions of genes sampled and may vary considerably depending upon the rate of evolution of the sequenced region (e.g. Smith et al. 2007).
Morphological data
Hoplocephalus bungaroides populations show little morphological variation across their entire range. Scale characters associated with the head and the number of dorsal scale rows were largely invariant across the specimens examined. The range of variation in the number of ventral scales and the number of subcaudal scales was minimal and analyses showed no statistically significant differences between the two genetic clades in any of these characters. Similarly, analyses of the body size variables showed no significant differences in relative body proportion measurements between the two genetic clades. Given this homogeneity, we pooled the data to test for sexual size dimorphism across H. bungaroides as a whole. Like many snakes, females have significantly more ventral scales and fewer subcaudal scales than do males, with correspondingly longer tails in males (Table 2). Females were on average larger than males.
Discussion
Australia’s most endangered snake, H. bungaroides, comprises two genetically divergent clades that correspond to distinct regions north and south of Sydney. This genetic divergence was not matched by morphological divergence. The two clades are morphologically homogeneous. Our results have important implications for understanding the evolutionary history of this species, and for future conservation management decisions.
The existence of two distinct genetic clades was unexpected. Molecular dating suggests that the two populations were isolated from each other during the late Pleistocene, approximately 755,000–850,000 years ago. During the Pleistocene, the climate in southeast Australia oscillated from warm, wet periods during interglacials to cold dry conditions during glacial periods (Byrne 2008). In the mid to late Pleistocene, significant changes in vegetation occurred during the glacial and interglacial periods (Dodson 1994; Wagstaff et al. 2001). These changes involved expansion and contraction of more mesic rainforest habitats. However, the available data suggest that there was little influence of Pleistocene climatic cycles on sclerophyll habitats (Byrne 2008). For example, populations of three animal species (two frogs and a glider) and a tree that occur in sclerophyll habitat along Australia’s east coast show little evidence of phylogeographic structure (James and Moritz 2000; Brown et al. 2006; Jones et al. 2006; Burns et al. 2007).
The fact that Broad-headed snakes show significant geographic structuring suggests that Pleistocene climatic cycling may have restricted dispersal. Interestingly, the break between the southern and northern clade occurs in a narrow geologically distinct region where volcanic soils cover the sandstone plateau (Branagan and Packham 2000), resulting in few exposed sandstone rock outcrops that could act as ‘stepping stones’ for juvenile snake dispersal (Webb and Shine 1997b). In this region, a narrow east-facing sandstone escarpment runs west of Wollongong in the north through to Kangaroo Valley in the south, but the cliffs are swathed in tall closed forests that shade rocks and make them unsuitable for snake thermoregulation (Pringle et al. 2003). Previous field studies have shown that during the cooler months, Broad-headed snakes select sun-exposed rocks on west, north-west, or north-facing slopes or cliffs as diurnal thermoregulatory sites (Webb and Shine 1998). These sites allow snakes to attain high body temperatures at dusk, when the snakes ambush nocturnal lizards (Webb and Shine 1998). Both the Broad-headed snake and one of its main prey items, the velvet gecko, Oedura lesueurii, are rarely found under rocks on heavily timbered east-facing rock outcrops (Pringle et al. 2003). Hence, it is likely that this region formed a natural barrier to snake dispersal, particularly during colder glacial periods when the snakes would have required exposed areas on north or west facing exposed slopes for thermoregulation.
Our results reveal two evolutionarily significant units within the geographic range of the Broad-headed snake. Interestingly, genetic diversity was highest in the northern clade and lowest in the southern clade. This finding may reflect the greater geographic area covered by the northern clade. The presence of two distinct evolutionarily significant units (ESUs) within H. bungaroides has important implications for the management of this species. First, Broad-headed snakes are absent from several conservation reserves in the northern region where they were detected several decades ago (Newell and Goldingay 2005; Shine et al. 1998), which suggests that management is urgently needed to halt further declines. Second, extant Broad-headed snake populations are restricted to habitat fragments surrounded by a matrix unsuitable for snakes (urban areas, cleared lands, and pine plantations). Given the poor dispersal ability of this species (Webb and Shine 1997a), it is unlikely that snakes will disperse between adjacent conservation reserves in the future. For example, the population in Royal National Park is separated from western populations by urban areas and a major four-lane highway, both of which are potential barriers to snake dispersal (Foreman and Alexander 1998; Mader 1984; Roe et al. 2006). Each of these conservation reserves harbours viable populations of H. bungaroides and should be designated a separate management unit (MU) for conservation (Moritz 2002). Within the northern ESU, populations from Royal NP, Blue Mountains NP, Wollemi NP, and the Sydney Water Catchment supply areas also meet this criterion, and require careful management to prevent extinction of H. bungaroides. We also need more survey work to identify areas where Broad-headed snakes occur, particularly in the northern region that contains large expanses of protected habitat (e.g., Wollemi National Park).
The draft recovery plan for the Broad-headed snake lists captive breeding and return of captive-bred progeny to the wild as a possible conservation strategy (NSW Department of Environment and Climate Change, unpublished draft plan). Part of this plan involves releasing juveniles to areas where the species is currently extinct. Clearly, this strategy will need to carefully consider the genetic identity of adults used for breeding, and should ensure that captive bred progeny are released to the same region where the adults were obtained. However, unless factors causing the decline of H. bungaroides are ameliorated, the release of captive-born progeny to the wild is likely to fail (Caughley and Gunn 1996; Fischer and Lindenmayer 2000). Given that bush-rock removal has occurred throughout the northern geographic range of H. bungaroides, there is little point translocating animals to rock-denuded outcrops. Clearly, we need to develop methods to restore degraded habitats prior to translocating animals. One new method that is promising in this respect is the deployment of artificial concrete rocks that mimic the colour, shape, crevice structure, and thermal profile of natural rocks. These rocks were deployed in degraded rock outcrops, and were rapidly colonised by velvet geckos, and later, by Broad-headed snakes (Croak et al. 2009).
In conclusion, our study shows that extant Broad-headed snake populations display significant geographic structuring. This present day pattern probably resulted from changes in dispersal and/or habitat use that occurred in response to past climatic changes. Given that future changes in climate and vegetation are predicted to occur in the next century, we might expect future range contractions and local extinctions to occur in this sandstone-dependent species. Bioclimatic modelling to examine how this species responds to changes in climate, and how best to mitigate such effects, should be given a high priority if we are to conserve this iconic species.
References
Arévalo E, Davis SK, Sites J (1994) Mitochondrial DNA sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in Central Mexico. Syst Biol 43:387–418. doi:10.2307/2413675
Avise JC (2004) Molecular markers, natural history and evolution, 2nd edn. Sinauer Associates Inc., Sunderland
Branagan DF, Packham GH (2000) Field geology of New South Wales, 3rd edn. Department of Mineral Resources, Sydney
Brown M, Cooksley H, Carthew SM, Cooper SJB (2006) Conservation units and phylogeographic structure of an arboreal marsupial, the yellow-bellied glider (Petaurus australis). Aust J Zool 54:305–317. doi:10.1071/ZO06034
Burns EL, Eldridge MDB, Crayn DM, Houlden BA (2007) Low phylogeographic structure in a wide spread endangered Australian frog Litoria aurea (Anura: Hylidae). Conserv Genet 8:17–32. doi:10.1007/s10592-006-9143-8
Byrne M (2008) Evidence for multiple refugia at different time scales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography. Quat Sci Rev 27:2576–2585. doi:10.1016/j.quascirev.2008.08.032
Caughley G, Gunn A (1996) Conservation biology in theory and practise. Blackwell Science, Cambridge
Cogger HG (2000) Reptiles and amphibians of Australia, 6th edn. Reed New Holland, Sydney
Croak BM, Pike DA, Webb JK, Shine R (2009) Using artificial rocks to restore non-renewable shelter sites in human-degraded systems: colonization by fauna. Restor Ecol. doi:10.1111/j.1526-100X.2008.00476.x
Dodson JR (1994) Quaternary vegetation history. In: Groves RH (ed) Australian vegetation. Cambridge University Press, Cambridge, pp 37–56
Fischer J, Lindenmayer DB (2000) An assessment of the published results of animal relocations. Biol Conserv 96:1–11. doi:10.1016/S0006-3207(00)00048-3
Foreman RTT, Alexander LE (1998) Roads and their major ecological effects. Annu Rev Ecol Syst 29:207–231. doi:10.1146/annurev.ecolsys.29.1.207
Frankel OH (1974) Genetic conservation: our evolutionary responsibility. Genetics 78:53–65
Goldingay R (1998) Between a rock and a hard place: conserving the Broad-headed snake in Australia’s oldest National Park. Proc Linn Soc N S W 120:1–10
Goldingay RL, Newell DA (2000) Experimental rock outcrops reveal continuing habitat disturbance for an endangered Australian snake. Conserv Biol 14:1908–1912. doi:10.1046/j.1523-1739.2000.99458.x
Goodman D (1987) The demography of chance extinction. In: Soule ME (ed) Viable populations for conservation. Cambridge University Press, Cambridge, pp 11–34
Hersey F (1980) Broad-headed snake, Hoplocephalus bungaroides. In: Haegl C (ed) Endangered animals of New South Wales. National Parks and Wildlife Service, Sydney, pp 38–40
Hobbs RJ, Yates CJ (2003) Turner review no. 7. Impacts of ecosystem fragmentation on plant populations: generalising the idiosyncratic. Aust J Bot 51:471–488. doi:10.1071/BT03037
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. doi:10.1093/bioinformatics/17.8.754
James CH, Moritz C (2000) Intraspecific phylogeography in the sedge frog Litoria fallax (Hylidae) indicates pre-Pleistocene vicariance of an open forest species from eastern Australia. Mol Ecol 9:349–358. doi:10.1046/j.1365-294x.2000.00885.x
Jones ME, Shepherd M, Henry RJ, Delves A (2006) Chloroplast DNA variation and population structure in the widespread forest tree, Eucalyptus grandis. Conserv Genet 7:691–703. doi:10.1007/s10592-005-9104-7
Keogh JS, Shine R, Donnellan S (1998) Phylogenetic relationships of terrestrial Australo-Papuan elapid snakes based on cytochrome b and 16S rRNA sequences. Mol Phylo Evol 10:67–81. doi:10.1006/mpev.1997.0471
Keogh JS, Scott IAW, Scanlon JD (2000) Molecular phylogeny of viviparous Australian elapid snakes: affinities of ‘Echiopsis’ atriceps (Storr, 1980) and ‘Drysdalia’ coronata (Schlegel, 1837), with description of a new genus. J Zool 252:317–326. doi:10.1111/j.1469-7998.2000.tb00626 (Lond)
Keogh JS, Scott IAW, Fitzgerald M, Shine R (2003) Molecular phylogeny of the Australian venomous snake genus Hoplocephalus and conservation genetics of the threatened H. stephensii. Conserv Genet 4:57–65. doi:10.1023/A:1021823423944
Krefft G (1869) The snakes of Australia; an illustrated and descriptive catalogue of all the known species. T Richards, Govt. Printer, Sydney
Mader HJ (1984) Animal habitat isolation by roads and agricultural fields. Biol Conserv 29:81–96. doi:10.1016/0006-3207(84)90015-6
McKinney ML (1997) Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu Rev Ecol Syst 28:495–516. doi:10.1146/annurev.ecolsys.28.1.495
Moritz C (1994a) Defining evolutionarily significant units for conservation. Trends Ecol Evol 9:373–375. doi:10.1016/0169-5347(94)90057-4
Moritz C (1994b) Applications of mitochondrial DNA analysis in conservation: critical review. Mol Ecol 3:401–411. doi:10.1111/j.1365-294X.1994.tb00080.x
Moritz C (2002) Strategies to protect biological diversity and the evolutionary processes that sustain it. Syst Biol 51:238–254. doi:10.1080/10635150252899752
Newell DA, Goldingay RL (2005) Distribution and habitat assessment of the Broad-headed snake Hoplocephalus bungaroides. Aust Zool 33:168–179
Pringle RM, Webb JK, Shine R (2003) Canopy structure, microclimate, and habitat selection by a nocturnal snake, Hoplocephalus bungaroides. Ecology 84:2668–2679. doi:10.1890/02-0482
Roe JH, Gibson J, Kingsbury BA (2006) Beyond the wetland border: estimating the impact of roads for two species of water snakes. Biol Conserv 130:161–168. doi:10.1016/j.biocon.2005.12.010
Sanders KL, Lee MSY, Leijs R, Foster R, Keogh JS (2008) Molecular phylogeny and divergence dates for Australasian elapids and sea snakes (Hydrophiinae): evidence from seven genes for rapid evolutionary radiations. J Evol Biol 21:882–895. doi:10.1111/j.1420-9101.2008.01525.x
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32. doi:10.1111/j.1523-1739.1991.tb00384.x
Scott IAW, Keogh JS (2000) Conservation genetics of the endangered grassland earless dragon Tympanocryptis pinguicolla (Reptilia: Agamidae) in Southeastern Australia. Conserv Genet 1:357–363. doi:10.1023/A:1011542717349
Shine R, Fitzgerald M (1989) Conservation and reproduction of an endangered species: the Broad-headed snake, Hoplocephalus bungaroides (Elapidae). Aust Zool 25:65–67
Shine R, Webb JK, Fitzgerald M, Sumner J (1998) The impact of bush-rock removal on an endangered snake species, Hoplocephalus bungaroides (Serpentes, Elapidae). Wildl Res 25:285–295. doi:10.1071/WR97022
Smith SA, Sadlier RA, Bauer AM, Austin CC, Jackman T (2007) Molecular phylogeny of the scincid lizards of New Caledonia and adjacent areas: evidence for a single origin of the endemic skinks of Tasmantis. Mol Phylo Evol 43:1151–1166. doi:10.1016/j.ympev.2007.02.007
Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland
Wagstaff BE, Kershaw AP, O’Sullivan PB, Harle KJ, Edwards J (2001) An Early to Middle Pleistocene palynological record from the volcanic crater of Pejark Marsh, Western Plains of Victoria, southeastern Australia. Quat Int 83–85:211–232. doi:10.1016/S1040-6182(01)00041-6
Webb JK, Shine R (1997a) Out on a limb: conservation implications of tree-hollow use by a threatened snake species (Hoplocephalus bungaroides: Serpentes, Elapidae). Biol Conserv 81:21–33. doi:10.1016/S0006-3207(96)00160-7
Webb JK, Shine R (1997b) A field study of spatial ecology and movements of a threatened snake species, Hoplocephalus bungaroides. Biol Conserv 82:203–217. doi:10.1016/S0006-3207(97)00032-3
Webb JK, Shine R (1998) Using thermal ecology to predict retreat-site selection by an endangered snake species. Biol Conserv 86:233–242. doi:10.1016/S0006-3207(97)00180-8
Webb JK, Brook BW, Shine R (2002a) Reptile collectors threaten Australia’s most endangered snake, the Broad-headed snake Hoplocephalus bungaroides. Oryx 36:170–181. doi:10.1017/S0030605302000248
Webb JK, Brook BW, Shine R (2002b) What makes a species’ vulnerable to extinction? Comparative life history traits of two sympatric snakes. Ecol Res 17:59–67. doi:10.1046/j.1440-1703.2002.00463.x
Webb JK, Pringle RM, Shine R (2005) Canopy removal restores habitat quality for an endangered snake in a fire suppressed landscape. Copeia 2005:893–899. doi:10.1643/0045-8511(2005)005[0894:CRRHQF]2.0.CO;2
Acknowledgments
We thank Mark Fitzgerald and Jai Thomas for carrying out surveys for H. bungaroides, and Peter Harlow, David Pike and Simon Clulow for kindly providing tissue samples and locality data. Gary Daly and Ross Wellington provided expert advice on the distribution of Broad-headed snakes. We thank Les Mitchell, Phil Craven and Bruce Grey from the Department of Environment and Conservation (Nowra NPWS) for organising helicopter surveys and for their assistance in the field. Meagan Ewings (DEC Hurstville) helped organise surveys and assisted us during field work. We thank the curators and collection managers of all the Australian natural history museums who lent us the specimens in their care: Allen Greer and Ross Sadlier (Australian Museum), Jeanette Covacevich and Patrick Couper (Queensland Museum), John Coventry (National Museum of Victoria), Mark Hutchinson and Adrienne Edwards (South Australian Museum), and John Wombey (CSIRO Australian National Wildlife Collection). All research was carried out according to the University of Sydney Animal Care and Ethics Committee guidelines under a scientific licence from the New South Wales National Parks and Wildlife Service (Licence S10029). The research was supported financially by the Australian Research Council (grants to S. Keogh and R. Shine and a fellowship to J. Sumner), the University of Sydney (Sesqui Fellowship to J. Webb), the Australian Academy of Science (Margaret Middleton Award to J. Webb), and the Department of Environment and Climate Change NSW (grant to R. Shine).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sumner, J., Webb, J.K., Shine, R. et al. Molecular and morphological assessment of Australia’s most endangered snake, Hoplocephalus bungaroides, reveals two evolutionarily significant units for conservation. Conserv Genet 11, 747–758 (2010). https://doi.org/10.1007/s10592-009-9863-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-009-9863-7