Abstract
The poly(azomethine), TiO2 and poly(azomethine)/TiO2 nanocomposites were prepared and exemplified by Fourier Transform-Infra red spectroscopy, UV–Visible spectroscopy, Powder X-ray diffraction, EDAX, SEM and TEM techniques. Methylene blue, malachite green and Bismarck brown were debased from water using poly(azomethine) (PAZ), titanium di oxide (TiO2), poly(azomethine)/titanium di oxide (PAZ/TiO2) nanocomposites as photo-catalyst in presence of natural sunlight. The deprivation efficiency and reaction kinetics was calculated and the outcome of the photo-catalytic experiments proved that the PAZ/TiO2 nanocomposites reveals excellent photo-catalytic activity and efficient for achromatize the dyestuff present in the waste water than PAZ and TiO2 in presence of normal sunlight. The maximum degradation efficiency 95, 93 and 95% was obtained for PAZ/TiO2 nanocomposites at optimum dosage of catalyst as 500 mg and 50 ppm of methylene blue, malachite green and Bismarck brown dye concentration respectively. The maximum deprivation time was 5 h. After photo-catalytic study the samples were portrayed by FT-IR and UV–Visible spectroscopy. The main aim of this research was to protect our environment from the contamination of water due to the effluence released from dyestuff industries, to resolve this crisis effective nanocomposite were synthesized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Manmade dyes are poisonous and heavy chemicals that can produce concentrated colors are dangerous to the surroundings. These dyes are opposed to bio-decomposition and have pernicious effect on human health. Adsorption, ultra filtration, extraction, oxidation ozone and hydrogen peroxide are some of the conservative technique utilized to eliminate color from industrial runoff noxious materials [1]. Advanced oxidation processes (AOPs) are substitute to all other techniques, of which the photo-catalysis is the most popular one. In current scenario, heterogeneous photo-catalysis technique can direct to the full deterioration of various inorganic, organic dyes in industrialized operations. There are many semiconductor components are available which acts as photo-catalyst such as TiO2, MnO2, ZnO etc. The system depends on the concept of decaying various organic hazardous substances speedily and not a particular one. Among many photo-catalyst element, TiO2 and ZnO are the best photo-catalyst because they have broad band gap [2]. TiO2 is the extensively favored because it is harmless, steady, biologically static, excellent absorption/desorption properties, safety and photo corrosion stability [3]. A TiO2 photo-catalyst has achieved several significant in the ancient times due to its outstanding quality of degrading the huge variety of ecological pollutants such as bacteria, organics, viruses and the subsequent discovery of photo activated for water separating of TiO2.
Lot of Interest has been created on this technique because solar radiation is used as main source for the removal of contaminant from these industrial runoff noxious materials [4]. Investigation in the synthesis of creative photo-catalyst has been provoked by two major problems: (i) the enhancement of the effectiveness in the presence of solar light by changing the consumption of TiO2 placed in the near UV to the visible wavelength range and (ii) the restriction of the active semiconductor elements to promote the removal of the catalyst once the reaction has been completed. Huge hard works have been dedicated to the study of TiO2 material, which directs to several challenging functions in the various fields from photo-voltaics and photo-catalysis to electro chromic and sensors. Innovative physical and chemical characteristics appear while the dimension of the TiO2 turns into lesser and lesser and down to the nanometer size [5]. The outcome of the photo-catalytic deprivation of biphenyl by TiO2 with UV light illumination in the year 1976 has created more interest in the ecological application of the TiO2 as photo-catalyst. Though the band gap of TiO2 (anatose) is 3.2 eV so it can only absorb and be energized by UV illumination with a wavelength below 387 nm UV light accounts for < 5% of solar illumination. The intrinsic property of anatose limits realistic application.
To beat this discrepancy, polymer hybridizing, semiconductor coupling, ion doping and various other procedures were introduced. Conjugative polymer/TiO2 hybrid stuff reveals more effectiveness in deterioration of organic contaminants in presence of both UV light and visible illumination. By means of this blending technique, it was found to be complicated to attain electric contact among the metal oxide particles and the conjugative polymer layer, because the polymer chain might hinder with the correlation produced inside the metal oxide. Entire hybridization might not be achieved using the diffusion technique as the polymer may not be filled because of the inadequate penetration power of the conductive polymer film. Since the conjugative polymers merely placed on the TiO2 nanoparticles, but the boundary linking the TiO2 semiconductor and the conjugative polymer layer does not balance. A steady border for the hetero function composite is extremely advantageous since it promotes the speed of charge injection when accelerates by light or electrical motivation. UV accelerates the TiO2 produce conduction band electrons (e−) and valence band holes (h+) in couples provide surface –mediated reduction–oxidation activities. In recent times, it has been revealed that photo energized TiO2 nanoparticles are qualified of catalyzing the free radical polymerization of alkenes such as in methyl metha acrylate and acrylic acid. This polymerization technique directs to excellent bonding nature for the inorganic stuff. The polymers synthesized by this method have good electronic contact with the inorganic semiconductor segment since the polymerization reaction is narrowly started by charge transfer across the inorganic semiconductor–monomer boundary.
Conjugative polymers and their derivatives are widely engaged in photovoltaic conversion of solar illumination owing to their absorption coefficients in the visible fraction of spectrum, elevated movement of charge carries and outstanding constancy. Recently, there is rising interest in using polythiophenes, polyaniline, polypyrrle and their derivatives to sensitive TiO2 produce polymer/TiO2 nanocomposites. Like this several nanocomposites such as polypyrrole/TiO2 composite particles polythiophene/TiO2 composite particles, polyaniline/TiO2 composite particle, poly (3-hexyl thiophene)/TiO2 nanocomposite were also prepared and its effective deprivation of dye stuffs like methyl orange, methylene blue, rhodamine-B under UV and visible sunlight have been recorded in the past decades [1, 3,4,5,6,7,8,9,10,11,12,13,14,15,16].
Recently derivative of polythiophene, polyterthiophene—type conjugative polymers have quickly achieved significant consideration owing to the pre-subsistence of α-α′-linkages in their monomers, which makes the whole polyterthiophene-type chain grow frequently and directs to remarkable electrochromic and optical properties [11]. Polydopamine (PDA) is a biocompatible and biodegrable polymer and has powerful solar absorption and weak fluorescence upon excitation of UV light owing to its chemical disarray. In terms of its electronic properties, PDA is similar to shapeless organic semiconductor or electronic –ionic hybrid conductors due to its π-system, leads to electrical conduction ability which is significantly exaggerated by temperature, morphology and ecological moisture. PDA/TiO2 nanocomposites were prepared and showed absorption in the visible region, which favors the photo-catalytic activity of TiO2 under visible light and expected the TiO2/PDA nanocomposites exhibit better photo-catalytic activity in the deprivation of methyl orange under sunlight compared with PDA/TiO2 this is owing to the synergetic effect between TiO2 and PDA [12]. The maximum photo-catalytic activity for the deprivation methylene blue aqueous solution under UV illumination by TiO2–graphene nanocomposite was obtained [13]. Phenolic compounds are normally present in the effluents discharged from various industries and other every day feature of existence. They are one of the familiar and hazardous contaminants present in water bodies. Elevated toxicity and pernicious compounds are undesirable and leads to severe consequences on the aquatic ecosystem and human health. The exposure of phenolic compounds also ascribed to their accumulations which widen their occurrence in the ecological sequence which merely gives additional harm to the surroundings it is removed by using TiO2/graphene nano compounds.
By analyzing the previous studies [35,18,19,20,22], there have been no reports on nanocompounds of poly(azomethine) PAZ/TiO2 as a photo-catalyst. So conjugative polymer poly(azomethines) are used to make composite with TiO2 to acts as photo-catalyst. Moreover, the poly(azomethines) present the advantages to be p- and n-doped, due to their reversible oxidation and reduction, correspondingly [23]. Conjugated poly(azomethine)s (PAZ), polyamines or poly (Schiff bases) are an additional fascinating class of conjugative polymers holding nitrogen atom (CH=N) in a polymer backbone is used in this study for producing nanocomposites along with TiO2.
Based on the above concept, in this article, poly(azomethine) PAZ, TiO2 and PAZ/TiO2 nanocomposites were prepared and structural characterization such as FT-IR, UV–Vis, SEM, TEM, XRD and EDAX were conferred. The photocatalytic experiments with poly(azomethine) PAZ, TiO2 and PAZ/TiO2 nanocomposites were examined by elimination of methylene blue, malachite green and bismarck brown present in waste water was carried out in presence natural sunlight. Deprivation efficiency of photocatalyst such as poly(azomethine) PAZ, TiO2 and PAZ/TiO2 nanocomposites were evaluated.
2 Materials and methods
2.1 Reagents and materials
Highly uncontaminated chemicals were purchased from in Sigma Aldrich, India they are p-phenylene diamine (99.98), Hexamethylene tetramine (99.98), ethanol(99.98), 4,4′-bis (chloro methyl) biphenyl (99.98), toluene, DMF(99.98), methanol (99.98), titanium tetra chloride, ammonium hydroxide, methylene blue, malachite green and Bismarck brown.
2.2 Experiments
-
Preparation of poly(azomethine)
-
Monomer preparation
-
Synthesis of 4,4′-diformyl biphenyl
Hexamethylene tetramine (6.8, 48 mmol) was dissolved in ethanol (90 ml) and 4,4′-bis (chloro methyl) biphenyl (3.00 g, 12 mmol) was added at 40 °C. The combination was stirred 1.5 h at 45–50 °C and precipitate was collected, washed with ethanol for two times. Then acetic acid (40 ml) 50% was added. Reflux the substance for 10 h and filtrated. The filtrate was cooled overnight. The crystals (Scheme 1) were accomplished.
2.2.1 Synthesis of poly(azomethine)
In a 25 ml of RB flask with a magnetic stirrer a condenser and inlet–outlet dean stark system 0.1536 g (0.5 mmol) of 4,4′-diformyl biphenyl and 0.05456 g (0.5 mmol) of p-phenylene diamine, 7 ml of DMF and 2 ml of toluene were introduced. The reaction mixture was refluxed at the boiling temperature of toluene for 6 h. After cooling at room temperature the reaction mixture was added in a huge amount of methanol and the polymer precipitate (Scheme 2) was filtered and dried.
2.2.2 Synthesis of nano TiO2
TiCl4 was slowly introduced into deionised double distilled water in an ice bath (0 °C) under constant stirring until it was completely dissolved and then 18 ml of 30% ammonium hydroxide was added to this suspension. The formed white titanium hydroxide was allowed to stand for 1 h. Then, the obtained TiO2 nanoparticles were filtered, washed with deionised double distilled water and dried at 100 °C in a vacuum oven for 3 h [15].
2.2.3 Preparation of making polymer nanocomposite
Only a small amount of polymers were taken to prepare the composites. The polymer (250 mg) was dissolved in 100 ml DMF by stirring in 250 ml Stoppard conical flask kept in a shaker and sonicated to obtain a dispersed polymer solution. The polymers were incompletely soluble in DMF and continued finely dispersed after 48 h. The relevant nanoparticles dispersed in acetone were sonicated and instantaneously added to the polymer in DMF under sonication. The polymers were permitted to precipitate out. While precipitating, the nanoparticles got encapsulated into the polymer matrix. The composites thus acquired were filtered and washed systematically with acetone and dried [24].
2.3 Characterization
FT-IR spectroscopy (BRUKER EQUINOX-55), UV–Vis spectroscopy (SHIMADZU), Powder X-ray Diffraction (Scintag-XDS-2000m), Scanning Electron microscope (JSM-7600F Japan), Transmission Electron Microscopy TEM (JEOL model 2100) and EDAX of the sample were recorded for structural characterization.
2.4 Photo-catalytic experiments
The photo-catalytic activities of poly(azomethine) PAZ, TiO2 and PAZ/TiO2 nanocomposites were performed using methylene blue, bismarck brown and malachite green dyes in presence of normal sunlight. Before irradiation the suspension was mixed magnetically for 30 min in absence of light until adsorption–desorption equilibrium was recognized and then suspension were illuminated by light source with stirring. Dye stock solution was produced by 4 mg of dye is soluble in 500 ml of DI water. The different amount of catalyst (PAZ, TiO2 and PAZ/TiO2) was added to remove methylene blue, malachite green and Bismarck brown dyes (MB, MG and BB) and kept in ordinary sunlight. Initial and final concentrations of dye solution were measured by recording absorbance on a double beam UV–Vis spectrophotometer at 516, 624 and 668 nm respectively. The samples were received at different time period and concentration of the dye was calculated using UV–Vis spectroscopy. The photo degradation efficiency R (%) was calculated by the employing the following equation
where C0 symbolize the concentration of dye before irradiation and Ct denotes the concentration of the dye after a definite illumination time correspondingly [16, 25,26,27,28].
3 Results and discussion
3.1 Characterisation of photocatalyst
3.1.1 Fourier transform-infra red spectroscopy
A representative FT-IR spectrum of poly(azomethine) (PAZ), TiO2 and PAZ/TiO2 (PNT) nanocomposites are reproduced in (Fig. 1a, b, c). The characteristics band of azomethine or imine linkage (–CH–N) appeared at 1512 cm−1. The absorption band at about 3736 cm−1 is related with the N–H stretching vibration in the aromatic unit. The absorption spectrum at 3555 cm−1 is attributed to the C–H stretching in aromatics. The peaks at 1387 and 1352 cm−1 are endorsed to C=C stretching in benzene ring. The peaks visible at 809, 811, 872 cm−1 are the region for N–H stretching vibration respectively. The absorption peak at 800 cm−1 corresponds to the Ti–O–Ti stretching mode [16] and the same peak was observed in PAZ/TiO2 (PNT) composite but the intensity of that peak is not observed in PAZ spectrum.
The PAZ/TiO2 (PNT) nanocomposites displays related distinctive peaks like PAZ, moreover it shows the quality absorption of Ti–O-Ti at about 800 cm−1 (Fig. 1a). Slight alteration in high peak magnitude and wave numbers may be the outcome of the interaction with PAZ chains and TiO2 particles [29,30,31,32,33,34].
After the photo-catalytic process respective FT-IR spectrum of PAZ and PAZ/TiO2 (PNT) were observed (Fig. 1b, c). The peaks are similar for PAZ only and slight variation due to absorption of methylene blue (PAZMB), malachite green (PAZMG) and bismarck brown (PAZBB) dye. In case of PAZ/TiO2 (PNT) the peaks remains same position like PAZ but there is change in the intensity of peaks, it shows the deprivation efficiency of methylene blue (PNTMB), malachite green (PNTMG) and bismarck brown (PNTBB) is more for PAZ/TiO2 (PNT) than PAZ.
3.1.2 UV–Visible spectroscopy
The UV–Vis spectrum (Fig. 2a) displays the absorption band in the series of 280–290 nm was designated to the π–π* and n–π* transition in the conjugated chain present in the structure of PAZ and PAZ/TiO2 (PNT) nanocomposites. TiO2 has shown strong absorption at 296 nm when TiO2 nanoparticles were incorporated into the polymer matrix, a slight shift in the absorption band of TiO2 from 296 to 300 nm was observed which indicated the formation of PAZ/TiO2 (PNT) nanocomposite (Fig. 2a). Sudden change in the absorption peak at around 350–390 nm is due to the electron transition from the valence band to the conduction band [13]. The peak in the range of 296 nm is present in TiO2 and PAZ/TiO2 (PNT) nanocomposites but not observed in PAZ (Fig. 2a). It was noticed that peak intensity was higher for the entire three spectrums (Fig. 2a) it shows hyperchromic effect which indicated presence of auxochrome group like –NH2 and –O in the structure. Therefore the prepared nanocomposite can be photo stimulated by visible light or existing natural sunlight illumination which has no destructive effect on human health [35,36,37,38,39].
After the photo-catalytic process respective UV–Vis spectrum of PAZ and PAZ/TiO2 were observed (Fig. 2b, c). The appearance of absorption band in the range 500–700 nm PAZ/TiO2 (PNT) nanocomposites is due to the colour absorption of methylene blue (PNTMB) Malachite green (PNTMG) and Bismarck brown (PNTBB) present in water. But, absorption band in the range of 500–700 nm was not observed in PAZ (Fig. 2b) it shows the dye removal of methylene blue (PAZMB), malachite green (PAZMG) and Bismarck brown (PAZBB) is less when compared to PAZ/TiO2 (PNT) nanocomposites (Fig. 2c)
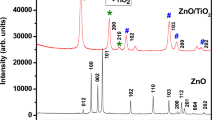
3.1.3 X-ray diffraction
Figures 3, 4 and 5 shows the XRD pattern of PAZ, PAZ/TiO2 and TiO2. Generally polymers exhibited a broad halo in the wide angle region (at about 2θ = 20°) indicating that the polymers were shapeless. The peak at 2θ = 20° also symbolizes the distinctive interval linking the ring planes of aromatic units in neighboring chains or the convenient association with inter-chain distance. Further, the sharp peak centered at 2θ = 25° might be consigned to the spreading from PAZ chains at inter planar interval distance and specify the PAZ had also same degree of crystalline [40].
The XRD pattern of TiO2 shows diffraction peaks at 2θ values of 25.53, 38.34, 48.38, 54.61, 55.64, 63.21, 69.21, 69.14, 75.42 corresponding to the (101), (004), (200), (105), (211), (204), (116) and (215) which could be attributed to the anatase phase in the TiO2 (JCPDS-87-0598) [15]. In Figs. 4 and 5 the major diffraction peaks of PAZ/TiO2 composites are parallel to those of clean TiO2 particles. This concludes that the PAZ placed on the exterior of TiO2 particles and the crystalline structure of TiO2 was not influenced by the alteration of poly(azomethine) PAZ. The addition of PAZ species did not change the crystalline phases of TiO2. However the intensity of the peaks of TiO2 is lower in the case of PAZ/TiO2 nanocomposite than that of nano TiO2 suggesting that the TiO2 particles are uniformly embedded in the polymer matrix of PAZ/TiO2. Therefore, the XRD patterns of composites propose a flourishing merging of nano- TiO2 in PAZ composites [23].
3.1.4 Morphological studies
The structural modification of TiO2, poly(azomethine)PAZ and PAZ/TiO2 nanocomposites were exemplified by TEM method and the outcomes are exhibited in Fig. 7. As shown in the Fig. 7d, e the nature of the TEM images revealed that all nanoparticles are spherical in shape with uniform structure. The morphology of the composites is related to that of precise TiO2 (Fig. 7g, h). However the constituent part in the composites tends to aggregate and lump tightly than precise TiO2. This might be owing to the PAZ (Fig. 7a, b) chains performing as folder in the composite, which hold together or lump the composite particle together. A same outcome was found in another research article [40]. Moreover, the TiO2 particles (dark shaded nanoparticle) are determined that they get captured in polymer (light shaded) matrix. These outcomes expose that the TiO2 particles are not merely mixed up or merged with the polymer, signifying that the TiO2 particles are implanted in the polymer matrix, which is fairly in conformity with the outcomes of the XRD analysis [12].
The selected area electron diffraction (SAED) pattern of PAZ/TiO2 and TiO2 (Fig. 7c, f, i) also confirms the binding of composites material together [40].
The SEM image of TiO2 nanoparticles (Fig. 6e, f) with a diameter of nearly 10 nm and a crystalline structure confirm the SEM images of PAZ/TiO2 nanocomposite (Fig. 6c, d) with low and high exaggeration images correspondingly. Surface alteration of TiO2 (Fig. 6e, f) particles by poly(azomethine) PAZ (Fig. 6a, b) chain show minor change in the morphology of PAZ/TiO2 nanocomposite (Fig. 6c, d).
The EDAX spectrum (Fig. 6g) proves the presence of Ti and O in the catalyst PAZ/TiO2 nanocomposite (Fig. 7).
3.1.5 Photo-catalytic behavior of PAZ/TiO2 nanocomposites under normal sunlight
The photo-catalytic deprivation of methylene blue, malachite green and bismarck brown using poly(azomethine) PAZ, TiO2 and PAZ/TiO2 (PNT) as a catalyst under sunlight irradiation were investigated. By varying the dosage of photo-catalyst such as 100,200,300,400 and 500 mg at different time intervals (1–5 h) are shown in the Figs. 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 and 22 at constant concentration (50 ppm) of dye.
The variation of decomposition vs irradiation time of methylene blue (Figs. 8, 9, 10, 11, 12), malachite green (Figs. 13, 14, 15, 16, 17) and Bismarck brown (Figs. 18, 19, 20, 21, 22) dye solutions are represented.
The maximum removal of Methylene blue (95%), Malachite green (93%) and Bismarck brown (95%) dye in water is achieved at 500 mg of PAZ/TiO2 (PNT) at 50 ppm of dye concentration under ordinary sunlight radiation in the duration of 5 h are shown in Table 1.
The rate constants of photo-catalytic deprivation of methylene blue (MB), malachite green (MG) and Bismarck brown (BB) dye was determined (Ct/Co vs. time; Figs. 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22) using the following equation [11,12,13,14]
KApp (min−1) is the first order kinetics of rate constants. The rate constant for photo-catalytic deprivation composition of methylene blue (MB), malachite green (MG) and Bismarck brown (BB) dyes were regulated to be k = 0.2803 min−1, k = 0.3232 min−1 and k = 0.3762 min−1 correspondingly.
3.2 Mechanism of photo-catalytic activity
In a normal semiconductor photo-catalytic design, electrons of the photo catalyst are energized from the valence band lower energy level to the conduction band higher energy level by the absorbing illumination, an electron energy level conversion happens which directs to the creation of electron–hole pairs. At this point, 2 divergent passages are produced: the created electron hole (h+) influencing with another particle is able to be utilized for oxidation reactions and the energized electrons (e−) are able to contribute for reduction reactions (Fig. 23).
A conjugated structure consists of linked π-orbitals by means of delocalized electrons are responsible for absorbing radiation from sunlight. Because of this, conjugated polymers are large organic molecules their structures are described as different single and multiple bonds with overlapping π-orbitals in their back bone which leads to delocalization. Numerous resources such as PEDOT, polyaniline and polypyrrole have been examined as possible visible light active photo-catalysts. Recently, clean detectable luminous active conjugated polymer photo catalysts have been established, including polyazomethines and polyphenylenes were communicated for H2evolution, however the polymer materials were typically energetic in presence of UV–Visible radiation with moderate study [40].
4 Conclusion
In this study, the photo-catalytic deprivation of the dye stuff from textile industries by PAZ, TiO2 and PAZ/TiO2 nanocomposites as catalyst was examined. The experiments were carried out using different catalyst by varying parameters such as the dosage variation of photo-catalyst and reaction time. The decolonization times was decreased by increasing the amount of PAZ/TiO2 nanocomposites in the range of the test conducted in 100, 200, 300, 400 and 500 mg of methylene blue, malachite green and bismarck brown. The optimum dosage of catalyst was found to be 0.500 g. The optimum time of the tests in those dosage variation tests of PAZ, TiO2 and PAZ/TiO2 nanocomposites was 5 h.
This practice may be employed effectively in the treatment of fabric dye waste matter which are hazardous to the environment, as this synthesized photo catalyst is economically feasible compared to other oxidative process. The present study demonstrates that the synthesized PAZ, TiO2 and PAZ/TiO2 nanocomposites could be used as efficient photocatalyst using the natural sunlight which contributes towards the remediation of pollution.
References
Ozbay, B., Genc, N., Ozbay, I., Baghaki, B., Zor, S.: Photocatalytic activities of polyaniline-modified TiO2 and ZnO under visible light: an experimental and modeling study. Clean Technol. Environ. Policy 18(8), 2591–2601 (2016)
Mostafaei, A., Zolriasatein, A.: Synthesis and characterisation of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. 22(4), 273–280 (2012)
Zhu, Y., Dan, Y.: Photocatalytic activity of poly(3-hexylthiophene)/titanium dioxide composites for degrading methyl orange. Sol. Energy Mater. Sol. Cells 94, 1658–1664 (2010)
Marigan, J., Lopez Munoz, M.J., van Grieken, R., Aguado, J.: Photocataytic decolorization and mineralization of dyes with nanocrystalline TiO2/SiO2 materials. Ind. Eng. Chem. Res. 46, 7605–7610 (2007)
Barakat, N.A.M., Kanjawal, M.A., Al-Deyab, S.S., Chronakis, I.S.: Influence of silver—doping on the crystal structure, morphology and photocatalytic activity of TiO2 Nanofibers. Mater. Sci. Appl. 2, 1188–1193 (2011)
Mahyar, A., Behnajady, M.A., Modirshahla, N.: Enhanced photocatalytic degradation of CI basic violet 2 using TiO2- SiO2 composite nanoparticles. Photochem. Photobiol. 87, 795–801 (2011)
Shoubin, X.U., Jiang, L., Haigang, Y.A., Yuanqing, S.O., Yi, D.A.: Structure and photocatalytic activity of polythiophene/TiO2 composite particles prepared by photoinduced polymerization. Chin. J. Catal. 32(4), 536–545 (2011)
Zhang, L., Liu, P., Su, Z.: Preparation of PANI- TiO2 nanocomposites and their solid-phase photocatalytic degradation. Polym. Degrad. Stab. 91, 2213–2219 (2006)
Gu, D.E., Yang, B.C., Hu, Y.D.: A novel method for preparing V-doped titanium dioxide photocatalysts with high photocatalytic activity under visible light irradiation. Catal. Lett. 118, 254–259 (2007)
Jamal, R., Osman, Y., Rahman, A., Ali, A., Zhang, Y., Abdiyim, T.: Solid–state synthesis and photocatalytic activity of polyterthiophene/TiO2 nanocomposites. Materials 7, 3786–3801 (2014)
Zhou, X., Jin, B., Luo, J., Xu, X., Zhang, L., Li, J., Guan, H.: Dramatic visible light photocatalytic degradation due to the synergetic effects of TiO2 and PDA nanospheres. RSC Adv. 6, 64446–64449 (2016)
Loryuenyong, V., Charoensuk, J., Charupongtawitch, R., Usakulwattana, A., Buasri, A.: Kinetics of photocatalytic degradation of methylene blue by TiO2–graphene nanocomposite. J. Nanosci. Nanotechnol. 16, 296–302 (2016)
Al-kandari, H., Abdullah, A.M., Mohammed, A.M., Al-Kandari, S.: Enhanced photocatalytic degradation of a phenolic compounds mixture using a highly efficient TiO2/reduced grapheme oxide nanocomposite. J. Mater. Sci. 51(18), 8331–8345 (2016)
Govindhan, P., Pragathiswaran, C.: Synthesis and characterization of TiO2 @ SiO2–Ag nanocomposites towards photocatalytic degradation of rhodamine B and methylene blue. J Mater. Sci. 27(8), 8778–8785 (2016)
Jayanthikalaivani, G., Suja, S.K.: TiO2 (rutile) embedded inulin-A versatile bio-nanocomposite for photocatalytic degradation of methylene blue. Carbohydr. Polym. 142, 51–60 (2016)
Hua-Yue, Z., Ru, J., Yu-Jiang, G., Yong-Qian, F., Ling, X., Guang-Ming, Z.: Effect of key operational factors on decolorization of methyl orange during H2O2 assisted CdS/TiO2/polymer nanocomposite thin films under simulated solar light irradiation. Sep. Purif. Technol. 74, 187–194 (2010)
Eskizeybek, V., Sari, F., Gulce, H., Gulce, A., Avci, A.: Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl. Catal. B 119, 197–206 (2012)
Gulce, H., Eskizeybey, V., Haspulat, B., Sari, F., Gulce, A., Avce, A.: Preparation of a new polyaniline/CdO nanocomposite and investigation of its photocatalytic activity: comparative study under UV light and natural sunlight irradiation. Ind. Eng. Chem. Res. 52(32), 10924–10934 (2013)
Nishizawa, K., Okada, M., Watanable, E.: New preparation method of visible light responsive titanium dioxide photocatalytic films. Mater. Sci. Appl. 5, 112–123 (2014)
Schwab, M.G., Hamburger, M., Shu, J., Spies, H.W., Wang, K., Antonietti, M., Feng, X., Mullen, K.: Photocatalytic hydrogen evolution through fully conjugated polyazomethine networks. Chem. Commun. 46(47), 8932–8934 (2010)
Li, L., Hadt, R., Yao, S., Lo, W.Y., Cai, Z., Wu, Q., Pandit, B., Chen, L.X., Yu, L.: Photocatalysts based on cobalt—chelating conjugated polymers for hydrogen evolution from water. Chem. Mater. 28(15), 5394–5399 (2010)
Yopendra, K., Naik, S., Mahadevan, K.M., Madhusudhana, N.: A comparative study of photocatalytic activities of two different synthesized ZnO composite against coralene red F3BS dye in presence of natural solar light. Int. J. Environ. Sci. Res. 1(1), 11–15 (2011)
Perji, E., Ghimpu, L., Hitruc, G., Harabagiu, V., Bruma, M., Marin, L.: Organic-inorganic hybrid nanomaterials based on inorganic oxides and mesomorphic polyazomethine. High Perform. Polym. 27(5), 546–554 (2015)
Tripathi, S.M., Tiwari, D., Ray, A.: Electrical Conductivity of polyazomethine nanocomposite. Indian J Chem 53A, 1505–1512 (2014)
Gopalappa, H., Yogendra, K., Mahadevan, K.M., Madhusydhana, N.: A comparative study on the solar photocatalytic degradation of brilliant red azo dye by CaO and CaMgO2 nanoparticles. Int. J. Sci. Res. 1(2), 91–95 (2012)
Rani, S., Aggarwal, M., Kumar, M., Sharma, S., Kumar, D.: Removal of methylene blue and rhodamine B from water by zirconium oxide/grapheme. Water Sci 30(1), 51–60 (2016)
Sabbaghi, S., Doraghi, F.: Photocatalytic degradation of methylene blue by ZnO/SnO2 nanocomposite. J. Water Environ. Nanotechnol 1(1), 27–34 (2016)
Shahabuddin, S., Muhamad Sariah, N., Mohamad, S., Joon Ching, J.: SrTiO3 Nanocube-doped polyaniline nanocomposites with enhanced photocatalytic degradation of methylene blue under visible light. Polymers 8, 27 (2016)
Petronella, F., Truppi, A., Ingrosso, C., Placido, T., Striccoli, M., Curri, M.L., Agostino, A., Comparelli, R.: Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 281, 85–100 (2017)
Pan, B., Xie, Y., Zhang, S., Lv, L., Zhang, W.: Visible light photocatalytic degradation of RhB by polymer—Cds Nanocomposites: role of the host functional groups. Appl. Mater. Interfaces 4(8), 3938–3943 (2012)
Mzoughi, M., Anku, W.W., Oppong, S.O.B., Shukla, S.K., Agorku, E.S., Govender, P.P.: Neodymium doped ZrO2-graphene oxide nanocomposites: a promising photocatalyst for photodegradation of Eosin Y dye. Adv. Mater. Lett. 7(12), 946–950 (2016)
Hassena, H.: Photocatalytic degradation of methylene blue by using Al2O3/Fe2O3 nanocomposite under visible light. Modern Chem Appl. 4(1), 1–5 (2016)
Pathanaia, D., Gupta, D., Al-Muhtaseb, A.H., Sharma, G., Kumar, A., Nausad, M., Ahamed, T., Alshehri, S.M.: Photocatalytic degradation of highly toxic dyes using chitosan-g-poly(acrylamide)/ZnS in presence of solar irradiation. J. Photochem. Photobiol. A 329, 61–68 (2016)
Rajendran, S., Khan, M.M., Gracia, F., Qin, J., Gupta, V.K., Arumainathan, S.: Ce 3+ -ion—induced visible light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci. Res. 5, 31641 (2016)
Abdiriyum, T., Ali, A., Jamal, R., Osman, Y., Zhang, Y.: A facile solid-state heating method for preparation of poly(3,4-ethylenedioxythiophene)/ZnO nanocomposite and photocatalytic activity. Nano Res. Lett. 9, 89 (2014)
Madhusudhana, N., Yogendra, K., Mahadevan, K.M., Naik, S.: Photocatalytic degradation of Coralene Dark red 2B azo dye using calcium zincate nanoparticle in presence of natural sunlight: an aid to environmental remediation. Int J Chem Eng Appl. 2(4), 294–298 (2011)
Madhusudhaa, N., Yogendra, K., Mahadevan, K.M.: Photocatalytic degradation of violet GL2B azo dye by using calcium aluminate nanoparticle in presence of solar light. Res. J. Chem. Sci. 2(5), 72–77 (2012)
Whang, T.J., Hsieh, M.T., Chen, H.H.: Visible-light photocatalytic degradation of methylene blue with laser—induced Ag/ZnO nanoparticles. Appl. Surf. Sci. 258, 2796–2801 (2012)
Nosrati, R., Olad, A., Maramifar, R.: Degradation of ampicillin antibiotic in aqueous solution by ZnO/polyaniline nanocomposite as photocatalyst under sunlight irradiation. Environ. Sci. Pollut. Res. 19, 2291–2299 (2012)
Sadavarte, N.V., Avadhani, C.V., Wadgaonkar, P.P.: Synthesis and characterization of new organosoluble aromatic polyamides and polyazomethines containing pendent pentadecyl chains. High Perform. Polym. 23(7), 494–505 (2011)
Acknowledgements
The authors thank the management and principal of Hindusthan college of Engineering and Technology to carry out the work in a successful manner.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pradeeba, S.J., Sampath, K. & Ramadevi, A. Photo-catalytic degradations of methylene blue, malachite green and Bismarck brown using poly(azomethine)/TiO2 nanocomposite. Cluster Comput 22 (Suppl 2), 3893–3909 (2019). https://doi.org/10.1007/s10586-018-2505-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10586-018-2505-4