Abstract
The value of squamous-cell carcinoma antigen (SCC-Ag) as a tumor marker for cervical cancer is controversial because it is not elevated (> 2 ng/mL) in a quarter of patients at diagnosis. Two hundred ninety one IB-IVA cervical squamous cell-carcinoma patients who underwent definitive chemoradiotherapy (CRT) were included in four tertiary institutions. Serum conversion pattern between pre- and post-treatment SCC-Ag levels was categorized into the following three arms: (1) Consistent Seronegative arm (both ≤ 2 ng/mL); (2) Negative Conversion arm (from > 2 ng/mL to ≤ 2 ng/mL); and (3) Consistent Seropositive arm (both > 2 ng/mL). Median follow-up time was 40.3 months. For Consistent Seronegative (N = 67), Negative Conversion (N = 165), and Consistent Seropositive (N = 59) arms, the 3-year recurrence-free survival (RFS) rates were 79.4%, 62.0%, and 48.4% (P < 0.001) and the 3-year overall survival (OS) rates were 86.3%, 80.6%, and 58.7% (P = 0.001), respectively. The serum conversion pattern of SCC-Ag between pre- and post-treatment was the most significant and potent prognostic factor of RFS (P = 0.001) and OS (P = 0.007) on the multivariate analysis. Simply checking whether SCC-Ag level is above or below 2 ng/mL before and after definitive CRT can provide clinicians with a simple rule-of-thumb for prediction of disease outcome in cervical cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 14,000 women are diagnosed with cervical cancer in the United States each year and only two thirds of them remain alive at 5 years. At diagnosis, 45%, 35%, and 15% of them are staged localized, regional, and distant, respectively [1]. However, patients of the same stage do not always result in the same outcome. This makes oncologists worldwide persevere to treat with the best personalized approach for each patient. Tumor marker is a way to get a hint on the characteristics of a specific disease. It can be used not only for screening, diagnosis, and monitoring of disease but may also have prognostic or predictive roles. Tumor markers not only can predict the outcome of a disease itself without treatment but also can identify patients who will benefit from certain treatments.
For squamous-cell carcinoma of the cervix, squamous-cell carcinoma antigen (SCC-Ag) is the most actively explored candidate, which was originally purified from human cervical squamous-cell carcinoma specimens [2]. There are a few aspects to consider before applying the results of tumor marker research to clinical practice [3]. First, the analytic validity in case of SCC-Ag testing is well-established with accurate, reliable, and reproducible results. In addition, the clinical validity of SCC-Ag is supported by a wealth of studies reporting association between elevated SCC-Ag and outcomes such as recurrence-free survival or overall survival [4]. However, the clinical utility, or the reliability of SCC-Ag results used in making clinical decisions for patient management, is another problem. At least a quarter of cervical cancer patients have SCC-Ag levels within normal range at diagnosis [5, 6]. Moreover, the purportedly significant cut-off levels of SCC-Ag range widely in the literature, with pre-treatment cut-offs of 1.1–40 ng/mL and post-treatment cut-offs of 0.9–3.5 ng/mL. These wide range further complicate the clinical use of SCC-Ag in the real practice.
To overcome the dilemma of arbitrary cut-off levels owing to the inconsistency of patient cohorts and treatments among previous studies, we focused on the serum conversion pattern between baseline and post-treatment SCC-Ag in locally advanced cervical cancer treated with definitive chemoradiotherapy (CRT). To the best of our knowledge, this study is the first to apply the concept of serum conversion in SCC-Ag levels. We defined the different obtainable patterns of serum conversion and investigated the impact of serum conversion pattern of SCC-Ag on outcome by demonstrating its association with recurrence and survival in the multi-institutional data.
Methods and materials
Patients and work-up
This study was conducted in accordance with the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria [7]. Patients with biopsy-proven, stage IB2-IVA (by the American Joint Committee on Cancer staging system, 8th edition) squamous-cell carcinoma of the cervix who received definitive concurrent CRT between 2015 and 2019 in four tertiary university hospitals were analyzed. Inclusion criteria were as follows: (1) pathological confirmation of squamous-cell carcinoma, (2) primarily diagnosed as stage IB2-IVA uterine cervical cancer, (3) completed CRT of definitive aim, and (4) acquisition of SCC-Ag levels before initiation of radiotherapy as well as after completion of planned treatment. Patients with distant or extrapelvic disease (including para-aortic nodal metastases) at diagnosis were excluded.
Initial work-ups comprised patient history, pelvic examination, colposcopy, serum chemistry, and complete blood-cell count. Pre- and post-treatment SCC-Ag levels from serum samples were obtained. Serum SCC-Ag levels were measured using an immunoradiometric assay (Riakey SCC IRMA Tube, Shinjin Medics Inc., Goyang, Republic of Korea) with one-step non-competitive reaction. This kit detects both SCCA1 and SCCA2 isoforms of the SCC-Ag. The analytical sensitivity of this assay (calculated as 2 standard deviations above the zero standard) is 0.03 ng/mL. The upper limit of normal is 2.0 ng/mL.
Imaging study included chest computed tomography (CT), magnetic resonance imaging (MRI) of the pelvis, and positron emission tomography-CT. Staging was performed according to the American Joint Committee on Cancer staging system, 8th edition. The institutional review board (IRB) approved all procedures of this study (No. KC19RIMI0369).
Treatment and follow-up
All patients received pelvic external-beam radiotherapy based on CT simulation. Pelvic radiotherapy of 45–50.4 Gy (median, 46.8 Gy) was planned to cover target volumes including cervical gross tumor and internal, external, and presacral nodal areas contoured according to the Radiation Therapy Oncology Group guidelines [8]. Intracavitary radiation (ICR) was delivered to patients with patent cervical canals amenable to tandem insertion. High-dose rate ICR of median 30 Gy (range, 12–40 Gy) prescribed to point A in median 6 fractions (range, 3–8 fractions) was delivered following CRT. Boost to the grossly involved lymph node was given up to median 59.4 Gy (range, 55.8–70.4 Gy). Concurrent 6 cycles of weekly cisplatin (30–40 mg/m2) or intravenous cisplatin (75 mg/m2/day) on day 1 and 5-fluorouracil (500 mg/m2/day) on days 2–5 during the first and fifth week was administered with radiotherapy.
Post-treatment serum SCC-Ag levels were measured 1–2 months after completion of radiotherapy. Patients were followed up every 3 months for 2 years, every 6 months for the next 3 years, and every year thereafter. Follow-up studies after completion of CRT included clinical and pelvic examination, colposcopy, PAP smear, abdomino-pelvic CT, pelvic MRI, and biopsy if necessary.
Definition of serum conversion patterns of SCC-Ag
The normal SCC-Ag level was 0–2.0 ng/mL. Thus, serum SCC-Ag level > 2 ng/mL was defined as seropositive. A serum SCC-Ag level ≤ 2 ng/mL was defined as seronegative. Negative conversion was defined as pre-treatment SCC-Ag level > 2 ng/mL reduced to post-treatment SCC-Ag level ≤ 2 ng/mL. Positive conversion was defined as pre-treatment SCC-Ag level ≤ 2 ng/mL increased to post-treatment SCC-Ag level > 2 ng/mL.
Statistical analysis
Survival time was calculated from the date of initiation of radiotherapy. Overall survival (OS) was defined as the time interval from the first day of radiotherapy to the date of death from any cause. Recurrence-free survival (RFS) was defined as the time interval from the first day of radiotherapy to the date of any recurrence or death. Locoregional recurrence-free survival (LRFS) was defined as the time interval from the first day of radiotherapy to the date of first intrapelvic recurrence or death. Distant recurrence-free survival (DRFS) was defined as the time interval from the first day of radiotherapy to the date of extrapelvic recurrence or death. Recurrence and survival rates were estimated with the Kaplan–Meier method and compared using the log-rank test. Prognostic factors for recurrence and survival were evaluated with the Cox proportional hazards regression model in the multivariate analysis. Independent t-test was used to compare continuous variables and χ2 test was used to compare categorical variables. A P-value of < 0.05 was considered statistically significant. Statistical analyses were performed using the IBM SPSS Statistics for Windows version 24 (IBM Corp., Armonk, NY, USA) and the Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Results
A total of 291 patients who satisfied the inclusion criteria were analyzed. The median pre-treatment SCC-Ag level was 6.4 ng/mL (range, 0.1–130.5 ng/mL) at diagnosis. The median post-treatment SCC-Ag level was 1.1 ng/mL (range, 0.1–87.4 ng/mL). Pre-treatment SCC-Ag levels were obtained at diagnosis before initiation of CRT. Post-treatment SCC-Ag levels were acquired at a median of 5.5 weeks (range, 4–8 weeks) after completion of planned treatment.
The serum conversion pattern between pre- and post-treatment SCC-Ag levels of the patients included in this study was categorized into the following three arms: (1) the Consistent Seronegative arm including patients who had been seronegative before treatment and remained seronegative after treatment; (2) the Negative Conversion arm including patients who had been seropositive before treatment and became seronegative after treatment; and (3) the Consistent Seropositive arm including patients who had been seropositive before treatment and remained seropositive after treatment. There was no positive conversion observed in our cohort.
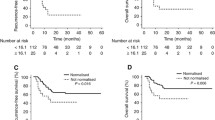
At baseline, 224 (77%) were seropositive. 165 patients underwent negative conversion, thus leaving 232 (79.7%) patients seronegative after treatment. There were 23% (N = 67), 56.7% (N = 165), and 20.3% (N = 59) patients included in the Consistent Seronegative, Negative Conversion, and Consistent Seropositive arms, respectively (Fig. 1). Two thirds of the patients (66.7%) were stage IIB (Table 1). Approximately two thirds (67.4%) had large tumors sized ≥ 4 cm. When the patient and treatment characteristics are compared among the three arms, the Consistent Seropositive arm tended to include larger tumors (P = 0.002) and higher stage (P = 0.057) than the Consistent Seronegative and Negative Conversion arms (Table 2).
Median follow-up time was 40.3 months (range, 5.8–70.8 months). At the time of analysis, there were total 61 recurrences, including 35 locoregional, 17 paraaortic, and 37 distant. The median SCC-Ag level at tumor recurrence was 3.3 ng/mL (range, 0.5–115 ng/mL). The 3-year LRFS (P = 0.003), DRFS (P = 0.002), RFS (P < 0.001), and OS (P = 0.001) rates were all significantly different among the three serum conversion arms (Fig. 2). The 3-year RFS rates were significantly higher in the order of Consistent Seronegative (N = 67), Negative Conversion (N = 165), and Consistent Seropositive (N = 59) arms (79.4%, 62.0%, and 48.4%; P < 0.001). The 3-year OS rates were significantly higher in the order of Consistent Seronegative (N = 67), Negative Conversion (N = 165), and Consistent Seropositive (N = 59) arms (86.3%, 80.6%, and 58.7%; P = 0.001).
For all patients, the 3-year LRRFS, DRFS, and RFS rates were 71.8%, 72.6%, and 68.8%, respectively. Fifty-nine patients had expired at the time of analysis and the 3-year OS rate was 78.1%.
Factors associated with RFS were serum conversion pattern of SCC-Ag (P < 0.001) and tumor size (P = 0.006) on the multivariate analysis. Serum conversion pattern of SCC-Ag was most significantly related to RFS, with the Negative conversion arm (hazard ratio [HR] of 1.74 and 95% confidence interval [CI] 1.13–3.28) and the Consistent Seropositive arm (HR of 3.74 and 95% CI 1.83–7.63), as compared to the Consistent Seronegative arm. Likewise, factors associated with OS were serum conversion pattern of SCC-Ag and tumor size in both the univariate and multivariate analyses. Serum conversion pattern of SCC-Ag had the strongest association with OS (P = 0.007), of which the Consistent Seropositive arm had a HR of 3.43 (95% CI 1.46–8.08) compared to the Consistent Seronegative arm.
Discussion
The factor most significantly associated with RFS and OS in our data was serum conversion pattern of SCC-Ag. Survival rates decreased in the order of Consistent Seronegative, Negative Conversion, and Consistent Seropositive arms. Although tumor size was also related to outcome, the association was independent of SCC-Ag level. Previous studies assessing the value of SCC-Ag in cervical cancer often include heterogeneous population of patients who had been treated with either surgery or RT with or without chemotherapy [9, 10]. However, removing the tumor by surgery and keep having the tumor after delivery of chemoradiation need to be approached separately in terms of SCC-Ag because it is secreted from the tumor cells. Recent studies with radiotherapy including CRT cohorts reported statistically significant association between pre-treatment SCC-Ag and DFS or OS. However, several studies failed to demonstrate a significant correlation between pre-treatment SCC-Ag and OS in cervical cancer after radiotherapy [11,12,13,14,15]. Post-treatment SCC-Ag has not been studied as extensively as pre-treatment SCC-Ag and yet some reported that post-CRT SCC-Ag, not pre-CRT SCC-Ag was related to RFS and OS [16,17,18,19,20]. Although these studies suggest that SCC-Ag may play a role in predicting outcome, the wide cut-off levels and a constant proportion of patients with normal levels at diagnosis hinder direct clinical application of published results.
SCC-Ag is a tumor antigen which is produced during the squamous formation of epithelial tissue. It is increased when the neoplastic transformation occurs in the cervical squamous epithelium [21]. SCC-Ag is comprised of two isoforms, the neutral SCCA1 (SERPINB3) and the acidic SCCA2 (SERPINB4), both of which belong to the ovalbumin family of serine protease inhibitors [22, 23]. Over 90% of the residues are identical in SCCA1 and SCCA2, which results in identical secondary structures [24]. Murakami et al. reported that both SCCA1 and SCCA2 protect tumor cells from radiation-induced apoptotic cell death [25]. Based on the observation that SCCA1 functioned as a prosurvival factor by neutralizing lysosomal proteases released during cell stress, Markovina et al. tested the role of SCCA1 in response to radiation in cervical tumor cell lines [15]. They first showed that serum SCC-Ag levels correlated with intra-tumoral SCC-Ag by immunohistochemistry. Then they demonstrated that knockout of SERPINB3 decreased cell survival after radiation in cervical tumor cell lines. When the SCCA1-expressing vector was transfected in to the cell lines, increased radio-resistance was observed.
Because SCC-Ag promotes resistance to radiation in tumor cells, the serum conversion pattern of SCC-Ag before and after radiation is biologically more apt to predict tumor outcome in contrast with SCC-Ag levels measured at single time points, either at before or after treatment. By integrating whether pre-treatment SCC-Ag was elevated with whether post-treatment SCC-Ag was normalized, the serum conversion pattern may reflect not only the treatment-naïve disease nature but also the response to radiotherapy.
Locoregional recurrence occurs in up to a quarter of cervical cancer patients and over 10% experience distant recurrence after definitive CRT as demonstrated in randomized trials of cisplatin-based CRT [26,27,28,29]. We consider that the biology underlying these constant proportion of patients nonresponsive to therapy can be better represented by this novel categorization with serum conversion pattern of SCC-Ag which provides a risk stratification such as consistent seropositive as high risk, consistent seronegative as low risk, and negative conversion as intermediate risk for poor outcome. To the best of our knowledge, this is the first study to report serum conversion in the context of SCC-Ag. Adopting the concept of serum conversion and distinct categorization according to its pattern underscores the originality of this study.
The main limitation of this study is that despite continuous decrement of SCC-Ag up to 3 months after treatment the majority of post-treatment samplings were aggregated between 5 to 6 weeks post-treatment in our data, which is why we were unable to analyze in depth the optimal timing for post-treatment serum sampling. There had been previous attempts to assess the value of serially sampled SCC-Ag. Again, patients included in those studies are heterogeneous and most underwent radical surgery [30]. Analysis of serial SCC-Ag in the true sense, obtained from diagnosis through definitive CRT and during follow-up is lacking in locally advanced cervical cancer treated with definitive CRT, which leaves it an area of further investigation that we plan to initiate shortly. Also, caution is advised when interpreting the serum conversion of SCC-Ag because cases with aggressive biologic features independent of SCC-Ag cannot be completely ruled out even in the consistent seronegative subgroup [31, 32].
In conclusion, serum conversion pattern of SCC-Ag pre- and post-chemoradiotherapy was significantly associated with recurrence and survival in locally advanced cervical cancer. Our data suggest that simply checking whether the level of SCC-Ag is above or below 2 ng/mL before and after treatment can provide clinicians with a convenient tool for prediction of tumor outcome.
Abbreviations
- SCC-Ag:
-
Squamous-cell carcinoma antigen
- CRT:
-
Chemoradiotherapy
- RFS:
-
Recurrence-free survival
- OS:
-
Overall survival
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- LRFS:
-
Locoregional recurrence-free survival
- ICR:
-
Intracavitary radiation
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Kato H, Torigoe T (1977) Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer 40:1621–1628
McShane LM, Hayes DF (2012) Publication of tumor marker research results: the necessity for complete and transparent reporting. J Clin Oncol 30:4223–4232
Charakorn C, Thadanipon K, Chaijindaratana S, Rattanasiri S, Numthavaj P, Thakkinstian A (2018) The association between serum squamous cell carcinoma antigen and recurrence and survival of patients with cervical squamous cell carcinoma: a systematic review and meta-analysis. Gynecol Oncol 150:190
Bolli J-AN, Doering DL, Bosscher JR, Day TG Jr, Rao C, Owens K, Kelly B, Goldsmith J (1994) Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol 55:169–173
Micke O, Prott F, Schäfer U, Tangerding S, Pötter R, Willich N (2000) The impact of squamous cell carcinoma (SCC) antigen in the follow-up after radiotherapy in patients with cervical cancer. Anticancer Res 2000; 20: 5113–5115. National Academy of Clinical Biochemistry. Anticancer Res 20:5113–5115
Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG (2018) Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. JNCI 110:803–811
Lim K, Small WJ, Portelance L, Creutzberg C, Jurgenliemk-Schulz IM, Mundt A, Mell LK, Mayr N, Viswanathan A, Jhingran A, Erickson B, De los Santos J, Gaffney D, Yashar C, Beriwal S, Wolfson A, Taylor A, Bosch W, El Naqa I, Fyles A (2011) Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys 79:348–355
Guo Q, Zhu J, Wu Y, Wen H, Xia L, Wu X, Ju X (2020) Predictive value of preoperative serum squamous cell carcinoma antigen (SCC-Ag) level on tumor recurrence in cervical squamous cell carcinoma patients treated with radical surgery: a single-institution study. Eur J Surg Oncol 46:131–138
Lee YY, Choi CH, Sung CO, Do IG, Huh S, Song T, Kim MK, Kim HJ, Kim TJ, Lee JW, Kim BG, Bae DS (2012) Prognostic value of pre-treatment circulating monocyte count in patients with cervical cancer: comparison with SCC-Ag level. Gynecol Oncol 124:92–97
Hong JH, Tsai CS, Chang JT, Wang CC, Lai CH, Lee SP, Tseng CJ, Chang TC, Tang SG (1998) The prognostic significance of pre- and posttreatment SCC levels in patients with squamous cell carcinoma of the cervix treated by radiotherapy. Int J Radiat Oncol Biol Phys 41:823–830
Ohno T, Nakayama Y, Nakamoto S, Kato S, Imai R, Nonaka T, Ishikawa H, Harashima K, Suzuki Y (2003) Measurement of serum squamous cell carcinoma antigen levels as a predictor of radiation response in patients with carcinoma of the uterine cervix. Cancer 97:3114–3120
Ogino I, Nakayama H, Okamoto N, Kitamura T, Inoue T (2006) The role of pretreatment squamous cell carcinoma antigen level in locally advanced squamous cell carcinoma of the uterine cervix treated by radiotherapy. Int J Gynecol Cancer 16:1094–1100
Jeong BK, Choi DH, Huh SJ, Park W, Bae DS, Kim B-G (2011) The role of squamous cell carcinoma antigen as a prognostic and predictive factor in carcinoma of uterine cervix. Radiat Oncol J 29:191–198
Markovina S, Wang S, Henke LE, Luke CJ, Pak SC, DeWees T, Pfeifer JD, Schwarz JK, Liu W, Chen S, Mutch D, Wang X, Powell MA, Siegel BA, Dehdashti F, Silverman GA, Grigsby PW (2018) Serum squamous cell carcinoma antigen as an early indicator of response during therapy of cervical cancer. Br J Cancer 118:72–78
Ohara K, Tanaka Y, Tsunoda H, Nishida M, Sugahara S, Itai Y (2002) Assessment of cervical cancer radioresponse by serum squamous cell carcinoma antigen and magnetic resonance imaging. Obstet Gynecol 100:781–787
Hirakawa M, Nagai Y, Inamine M, Kamiyama K, Ogawa K, Toita T, Murayama S, Aoki Y (2008) Predictive factor of distant recurrence in locally advanced squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy. Gynecol Oncol 108:126–129
Kawaguchi R, Furukawa N, Kobayashi H, Asakawa I (2013) Posttreatment cut-off levels of squamous cell carcinoma antigen as a prognostic factor in patients with locally advanced cervical cancer treated with radiotherapy. J Gynecol Oncol 24:313–320
Ryu HK, Baek JS, Kang WD, Kim SM (2015) The prognostic value of squamous cell carcinoma antigen for predicting tumor recurrence in cervical squamous cell carcinoma patients. Obstet Gynecol Sci 58:368–376
Salvatici M, Achilarre MT, Sandri MT, Boveri S, Vanna Z, Landoni F (2016) Squamous cell carcinoma antigen (SCC-Ag) during follow-up of cervical cancer patients: Role in the early diagnosis of recurrence. Gynecol Oncol 142:115–119
Maruo T, Yoshida S, Samoto T, Tateiwa Y, Peng X, Takeuchi S, Motoyama S (1998) Factors regulating SCC antigen expression in squamous cell carcinoma of the uterine cervix. Tumor biology 19:494–504
Suminami Y, Kishi F, Sekiguchi K, Kato H (1991) Squamous cell carcinoma antigen is a new member of the serine protease inhibitors. Biochem Biophys Res Commun 181:51–58
Remold-O’Donnell E (1993) The ovalbumin family of serpin proteins. FEBS Lett 315:105–108
Schneider SS, Schick C, Fish KE, Miller E, Pena JC, Treter SD, Hui SM, Silverman GA (1995) A serine proteinase inhibitor locus at 18q21. 3 contains a tandem duplication of the human squamous cell carcinoma antigen gene. Proc Natl Acad Sci 92:3147–3151
Murakami A, Suminami Y, Hirakawa H, Nawata S, Numa F, Kato H (2001) Squamous cell carcinoma antigen suppresses radiation-induced cell death. Br J Cancer 84:851
Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S (1999) Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J med 340:1144–1153
Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, Clarke-Pearson DL, Liao SY (1999) Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 17:1339–1348
Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG (2004) Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol 22:872–880
Stehman FB, Ali S, Keys HM, Muderspach LI, Chafe WE, Gallup DG, Walker JL, Gersell D (2007) Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol 197:503.e501-506
Ye S, Sun X, Kang B, Wu F, Zheng Z, Xiang L, Lesenechal M, Heskia F, Liang J, Yang H (2020) The kinetic profile and clinical implication of SCC-Ag in squamous cervical cancer patients undergoing radical hysterectomy using the Simoa assay: a prospective observational study. BMC Cancer 20:138
Liu J, Li Y, Chen X, Xu X, Zhao H, Wang S, Hao J, He BA-O, Liu S, Wang J (2020) Upregulation of miR-205 induces CHN1 expression, which is associated with the aggressive behaviour of cervical cancer cells and correlated with lymph node metastasis. BMC Cancer 20:1029
Huh SJ, Nishimura T, Park W, Onishi H, Ahn YC, Nakamura K (2020) Current status and comparison of national health insurance systems for advanced radiation technologies in Korea and Japan. Radiat Oncol J 38:170–175
Acknowledgements
The statistical analyses performed in this article were advised by Catholic Medical Center Clinical Research Coordinating Center.
Funding
There is no funding relevant to this work to be declared.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Institutional Review Board (No. KC19RIMI0369).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, SW., Hong, J.H., Yu, M. et al. Serum conversion pattern of SCC-Ag levels between pre- and post-chemoradiotherapy predicts recurrence and metastasis in cervical cancer: a multi-institutional analysis. Clin Exp Metastasis 38, 467–474 (2021). https://doi.org/10.1007/s10585-021-10115-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-021-10115-w