Abstract
The Korean Radiation Oncology Group (KROG) assessed the value of Deauville score (DS) on 18F-fluorodeoxyglucose Positron emission tomography-computed tomography (FDG PET/CT) as a predictor of recurrence and survival after rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy in diffuse large B-cell lymphoma (DLBCL). A total of 512 patients with stage I–III DLBCL who received six cycles of R-CHOP with or without radiation therapy (RT) and obtained treatment responses according to PET-CT imagings after R-CHOP ± RT were included. Patients were sorted into two arms; DS 4–5 arm (n = 24) was matched at a 1:2 ratio with DS 1–3 arm (n = 48) using propensity score matching method. After a median follow-up time of 37.2 months, the recurrence-free survival rate (86.6% vs. 66.8%, P = 0.041) and overall survival rate (86.9% vs. 62.2%, P = 0.009) at 5 years were significantly different between the DS 1–3 and DS 4–5 arms. DS 4–5 arm showed higher 5-years locoregional recurrence-free survival (88.8% vs. 74.3%, P = 0.155) and distant failure-free survival (91.1% vs. 84.3%, P = 0.333) than DS 1–3 arm. In the multivariate analysis, DS was still a significant factor for recurrence-free survival [hazard ratio (HR), 3.840 and confidence interval (CI), 1.068–13.806; P = 0.039] and overall survival rates (HR 4.453 and CI 1.274–15.562; P = 0.019). This study showed and validated that Deauville score of 4–5 of PET-CT imaging taken after full-course of R-CHOP chemotherapy with or without RT could predict recurrence-free survival and overall survival in DLBCL patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffuse large B cell lymphoma (DLBCL) is the most common form of aggressive non-Hodgkin lymphoma [1]. The addition of rituximab to the chemotherapy regimen consisting of cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone (R-CHOP) has improved the survival of DLBCL patients in the recent time. The most common predictor for patients with DLBCL is the International Prognostic Index (IPI) [2]. However, the IPI has some limitations. It was established prior to the era of rituximab and could be affected by clinical characteristics before treatment, so there was substantial diversity in each patient [3, 4]. 18F-fluoroDeoxyGlucose Positron Emission Tomography-Computed Tomography (FDG-PET/CT) is regarded as an enhanced imaging modality for the diagnosis and response evaluation for DLBCL patients [5, 6]. The National Comprehensive Cancer Network (NCCN) guidelines recommend that PET/CT scans should be interpreted by the 5-point Deauville score (DS) and Lugano response criteria, on the basis of visual assessment [7, 8].
Although treatment outcomes have improved since the inclusion of rituximab, 30 to 40% of patients with DLBCL still fail to cure completely with R-CHOP alone, leading to further therapeutic interventions [1, 6]. It is important to identify the poor responders to first-line R-CHOP chemotherapy in order to effectively manage the disease. We investigated patients with stage I-III DLBCL in the Korean Radiation Oncology Group (KROG) 17-02 trial. The aim of the current study was to evaluate the prognostic significance and cut-off of DS on the end-of-treatment (EOT) FDG-PET/CT imagings after full-course of R-CHOP ± RT.
Methods and materials
Patients and FDG-PET/CT imaging assessment
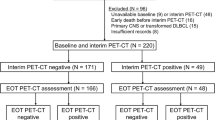
We retrospectively analyzed the data from DLBCL patients enrolled in the KROG 17-02 study. The study collected the data of 512 patients with stage I–III DLBCL (488 patients who had DS 1–3 and 24 patients who had DS 4–5 after R-CHOP) at five institutions from January 2010 to December 2015. The inclusion criteria for this analysis were: (1) histologically proven DLBCL with clinical stage I to III by the Ann Arbor staging system, (2) ECOG performance status 0–2, (3) initial treatment with six cycles of R-CHOP (rituximab, 375 mg/m2; cyclophosphamide, 750 mg/m2; doxorubicin, 50 mg/m2; vincristine, 1.4 mg/m2; and prednisolone, 100 mg), and (4) the presence of FDG-PET/CT imagings before and after completion of R-CHOP chemotherapy with or without radiotherapy. KROG 17-02 was approved by the institutional review board at each participating center and at KROG before enrolling patients. FDG-PET/CT was performed after R-CHOP and before RT, and the response to R-CHOP was evaluated according to the 5-point Deauville scale (DS) on FDG-PET/CT by institutional radiologists [7, 9]. According to previous reports [7, 9], the five-point DS determines FDG uptake in the involved site compared to the mediastinum and liver and yields results of (1) no uptake, (2) uptake ≤ mediastinum, (3) uptake > mediastinum but ≤ liver, (4) uptake moderately higher than the liver, and (5) uptake markedly higher than the liver and/or new lesion. Consolidative radiation therapy (RT) was executed at a median dose of 36 Gy (range, 30–45 Gy) at 1.8 to 2 Gy per fraction one to two months after R-CHOP treatment in 113 (22.1%) of 512 patients.
Propensity score matching and statistical analyses
To assess the associations between treatment outcomes and Deauville scores of the FDG-PET/CT, we divided the patients into two arms; DS 1–3 and DS 4–5. We conducted propensity-score matching for the enrolled patients. The propensity scores were calculated using a multivariate logistic-regression model based on the following variables; age (< 60 vs. ≥60), ECOG performance status (0–1 vs. 2), clinical stage (I–II vs. III), lesion size (< 5 vs. ≥5, cm), LDH level (< 230 vs. ≥230, IU/L), IPI score (0–1 vs. 2–4), and receipt of radiotherapy. A total of 488 patients in the DS 1–3 arm and 24 patients in the DS 4–5 arm were matched at a 1:2 ratio (n = 48 vs. 24, respectively). The matching model was well-calibrated (Hosmer-Lemeshow test, P = 0.848) with reasonable discrimination (c-index = 0.710).
After 1:2 matching, the patient characteristics were compared with the χ2 test for categorical variables and the t test for continuous variables. The endpoints were recurrence-free survival (RFS) and overall survival (OS) between the two arms. RFS was defined as the interval from the date of last chemotherapy to any locoregional and/or distant failure and OS was defined as the interval from the date of last chemotherapy to death or last follow-up. The survival curves were extracted by Kaplan–Meier analysis and compared with the log-rank test. To evaluate the prognostic factors related to recurrence and survival, multivariate analysis was performed with the Cox proportional hazard method. Chi-squared or Fisher’s exact test was used to evaluate the significance of any correlation between the categorical variables. A P-value of less than 0.05 was considered statistically significant. All analyses were conducted using SPSS Statistics version 12.0 (SPSS Inc., an IBM Company, Chicago, IL).
Results
A total of 72 patients (after 1:2 propensity score matching) were finally analyzed. The median age of the study participants was 57 years (range, 27–80 years). The median lesion size was 5 cm (range 1–12 cm). Among the analyzed patients, 52 received R-CHOP only and 20 received radiotherapy after R-CHOP. The patient characteristics are shown in Table 1. Patient age (P = 0.867), ECOG performance status (P = 0.716), clinical stage (P = 1.000), lesion size (P = 1.000), LDH level (P = 0.450), IPI score (P = 1.000), and RT (P = 0.063) were well-balanced between DS 1–3 arm and DS 4–5 arm after propensity score matching.
After a median follow-up time of 37.2 months (range, 6.0–137.8 months), disease failure including locoregional recurrence (LRR) and distant failure, occurred in 14 patients. Locoregional recurrence occurred in five (10.4%) of 48 patients in the DS 1–3 arm and five (20.8%) of 24 patients in the DS 4–5 arm. Distant failure occurred in four (8.3%) patients in the DS 1–3 arm four (16.7%) patients in the DS 4–5 arm and. Four patients failed at both locoregional and distant sites. The 5-years locoregional recurrence-free survival rates were 88.8% in the DS 1–3 arm and 74.3% in the DS 4–5 arm, respectively (P = 0.155, Fig. 1a). The 5-year distant failure-free survival rates were 91.1% in the DS 1–3 arm and 84.3% in the DS 4–5 arm, respectively (P = 0.333, Fig. 1b). The five-year RFS rates for the DS 1–3 arm and DS 4–5 arm were 86.6% and 66.8%, respectively (Fig. 2a). The five-year OS rates for the DS 1–3 arm and DS 4–5 arm were 86.9% and 62.2%, respectively (Fig. 2b). There were significant differences in RFS (P = 0.041) and OS (P = 0.009) between the two arms.
Table 2 shows the univariate and multivariate analyses of the prognostic factors for recurrence-free survival and overall survival. In the univariate analysis, age, clinical stage, lesion size, LDH level, IPI score, and RT were not significantly associated with RFS and OS. Good performance status (ECOG 0–1) showed improved OS in the univariate analysis (P = 0.032), but not in the multivariate analysis (P = 0.466). In the multivariate analysis, DS was a significant factor for the recurrence-free survival [hazard ratio (HR) 3.840 and confidence interval (CI) 1.068–13.806; P = 0.039] and overall survival (HR 4.453 and CI 1.274–15.562; P = 0.019).
Discussion
Our results showed that patients with Deauville scores of 4–5 from FDG-PET/CT imaging assessment after standard R-CHOP chemotherapy had significantly poorer recurrence-free survival and overall survival outcomes than patients with Deauville scores of 1–3. PET/CT in DLBCL possesses prognostic value for predicting response and treatment outcomes [3]. Interim PET/CT (iPET/CT), conducted after two to four cycles of chemotherapy has significant prognostic importance for RFS and OS in patients with DLBCL [3, 10, 11]. In the current study, EOT PET/CT was also performed after R-CHOP with six cycles similar to other studies [12, 13]. For EOT PET/CT, reports on the prognostic value have been controversial [11]. Jerusalem et al. [14] reported that EOT PET/CT was a very useful modality with a higher diagnostic and prognostic value which could distinguish tumors from fibrosis. According to Yoo et al. [15], iPET/CT might be unnecessary and omitted because their study found no difference in survival outcomes as a result of iPET/CTs. The prognostic efficacy of iPET/CT may be controversial but EOT PET/CT has a crucial prognostic value in lymphoma treatment [4].
Many studies on PET/CT in non-Hodgkin’s lymphoma have used diverse assessment criteria [7, 16]. The studies suggested using visual assessment criteria, such as standardized uptake value, metabolic tumor volume, or DS, etc. [7, 16, 17]. The International Harmonization Project response criteria categorized complete response (CR), partial response (PR), stable disease (SD), and relapsed disease or progressive disease (PD) reflecting PET/CT and CT response [18]. Recent studies reported that DS predicted outcomes more effectively than IHP criteria when interpreting response in FDG-PET/CT imagings [19].
Different treatment outcomes can be indicated depending upon which score is used as a cutoff point in the DS [20,21,22,23]. While DS 1–2 are considered negative and DS 4–5 are positive and result in the escalation of therapy, DS 3 is considered negative in conservative readings and positive in sensitive readings [24]. However, sometimes, DS 3 may be considered an insufficient response, counted as positive, and result in de-escalation of therapy [8, 24, 25]. There is uncertainty in reading DS scores of 3. The International Conference on Malignant Lymphomas Imaging Working Group described DS 3 as “probably” representing a complete metabolic response, while DS 1 and 2 were clearly defined [26]. In the current study, patients who had achieved DS 1,2,3 after R-CHOP got together as a good prognostic group since there was no significant difference in the overall survival rate among them. In the whole collective data, ECOG performance status (P = 0.116), clinical stage (P = 0.381), lesion size (P = 0.545), LDH level (P = 0.366), IPI score (P = 0.460), and RT (P = 0.551) except for age (P = 0.045) were not statistically different between DS 1–2 and DS 3 arms. When we categorized patients into the DS 1–3 and DS 4–5 arms, the RFS and OS between the two arms were significantly different (86.6% vs. 66.8%, P = 0.041 and 86.9% vs. 62.2%, P = 0.009, respectively). Thus, our results supported that DS 3 was a good prognostic group after chemotherapy for patients with DLBCL.
A complete response assessment is associated with better clinical outcomes compared to partial responses [12, 27, 28]. A residual mass with positive FDG-PET/CT finding after completion of therapy for DLBCL indicates the possibility of viable tumor and is associated with a high risk of disease progression or relapse, therefore, additional treatment should strongly be considered. Studies [12, 14, 28, 29] conducted before the introduction of Deauville scores described positive FDG-PET/CT scans as those with increased activity in a focal or diffuse area compared to normal anatomy. The current multi-institutional study verified that Deauville scores are important for evaluating the positivity of FDG-PET/CT imagings after treatment in the rituximab era and supports these previous reports.
In conclusion, DS 4–5 of FDG-PET/CT imagings after standard R-CHOP with or without radiation predicted poor recurrence-free survival and overall survival in DLBCL patients. This study also concluded that DS 3 could be included in the good prognosis group. For poor responders with DLBCL who had DS 4–5 after standard R-CHOP, further treatments, such as second-line chemotherapy or stem cell transplantation should be considered.
Abbreviations
- CI:
-
Confidence interval
- DLBCL:
-
Diffuse large B-cell lymphoma
- DS:
-
Deauville score
- ECOG:
-
Eastern Cooperative Oncology Group
- EOT:
-
End-of-treatment
- FDG:
-
18F-fluorodeoxyglucose
- HR:
-
Hazard ratio
- IPI:
-
International prognostic index
- LRR:
-
Locoregional recurrence
- PET/CT:
-
Positron emission tomography-computed tomography
- R-CHOP:
-
Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone
- RT:
-
Radiotherapy
- RFS:
-
Recurrence-free survival
- OS:
-
Overall survival
References
Feugier P, Van Hoof A, Sebban C et al (2005) Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 23:4117–4126
International Non-Hodgkin's Lymphoma Prognostic Factors Project (1993) A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 329:987–994
Kong Y, Qu L, Li Y et al (2016) Predictive significance of a new prognostic score for patients with diffuse large B-cell lymphoma in the interim-positron emission tomography findings. Medicine (Baltimore) 95:e2808
Mamot C, Klingbiel D, Hitz F et al (2015) Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol 33:2523–2529
Juweid ME, Stroobants S, Hoekstra OS et al (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 25:571–578
Tilly H, Dreyling M (2009) Diffuse large B-cell non-Hodgkin's lymphoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(Suppl 4):110–112
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: b-cell lymphomas [Internet]. Fort Washington, PA: National Comprehensive Cancer Network; c2018 [cited 2018 Oct 2]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
Meignan M, Gallamini A, Haioun C et al (2010) Report on the Second International Workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk Lymphoma 51:2171–2180
Mikhaeel NG, Hutchings M, Fields PA et al (2005) FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol 16:1514–1523
Zhu Y, Lu J, Wei X et al (2013) The predictive value of interim and final [18F] fluorodeoxyglucose positron emission tomography after rituximab-chemotherapy in the treatment of non-Hodgkin's lymphoma: a meta-analysis. Biomed Res Int 2013:275805
Spaepen K, Stroobants S, Dupont P et al (2001) Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin's lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol 19:414–419
Takasaki H, Yamamoto W, Ishii Y et al (2015) Post-treatment PET-CT findings may predict the prognosis of DLBCL with a Bulky Mass. Indian J Hematol Blood Transfus 31:346–351
Jerusalem G, Beguin Y, Fassotte MF et al (1999) Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood 94:429–433
Yoo C, Lee DH, Kim JE et al (2011) Limited role of interim PET/CT in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol 90:797–802
Malek E, Sendilnathan A, Yellu M et al (2015) Metabolic tumor volume on interim PET is a better predictor of outcome in diffuse large B-cell lymphoma than semiquantitative methods. Blood Cancer J 5:e326
Kostakoglu L, Chauvie S (2018) Metabolic tumor volume metrics in lymphoma. Semin Nucl Med 48:50–66
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Fallanca F, Alongi P, Incerti E et al (2016) Diagnostic accuracy of FDG PET/CT for clinical evaluation at the end of treatment of HL and NHL: a comparison of the Deauville Criteria (DC) and the International Harmonization Project Criteria (IHPC). Eur J Nucl Med Mol Imaging 43:1837–1848
Filippi AR, Piva C, Levis M et al (2016) Prognostic role of pre-radiation therapy (18)F-Fluorodeoxyglucose positron emission tomography for primary mediastinal B-cell lymphomas treated with R-CHOP or R-CHOP-like chemotherapy plus radiation. Int J Radiat Oncol Biol Phys 95:1239–1243
Ham JS, Kim SJ, Choi JY et al (2016) The prognostic value of interim and end-of-treatment PET/CT in patients with newly diagnosed peripheral T-cell lymphoma. Blood Cancer J 6:e395
Kostakoglu L, Goldsmith SJ, Leonard JP et al (2006) FDG-PET after 1 cycle of therapy predicts outcome in diffuse large cell lymphoma and classic Hodgkin disease. Cancer 107:2678–2687
Pinnix CC, Dabaja B, Ahmed MA et al (2015) Single-institution experience in the treatment of primary mediastinal B cell lymphoma treated with immunochemotherapy in the setting of response assessment by 18fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys 92:113–121
Barrington SF, Qian W, Somer EJ et al (2010) Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 37:1824–1833
Dann EJ (2012) PET/CT adapted therapy in Hodgkin disease: current state of the art and future directions. Curr Oncol Rep 14:403–410
Barrington SF, Mikhaeel NG, Kostakoglu L et al (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048–3058
Coiffier B (1999) How to interpret the radiological abnormalities that persist after treatment in non-Hodgkin's lymphoma patients? Ann Oncol 10:1141–1143
Juweid ME, Wiseman GA, Vose JM et al (2005) Response assessment of aggressive non-Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol 23:4652–4661
Chung JH, Na K, Kim IH (2018) Benefit of volumetric-modulated arc therapy over three-dimensional conformal radiotherapy for stage I-II extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue in the stomach: a dosimetric comparison. Radiat Oncol J 36(4):332–340
Acknowledgements
The statistical analyses performed in this article were advised by Catholic Medical Center Clinical Research Coordinating Center.
Funding
There is no funding relevant to this work to be declared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Institutional Review Board (VC17RESI0046).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, J.W., Oh, D., Eom, KY. et al. The prognostic value of PET/CT evaluation with Deauville score on the recurrence and survival in diffuse large B-cell lymphoma: a multi-institutional study of KROG 17-02. Clin Exp Metastasis 37, 125–131 (2020). https://doi.org/10.1007/s10585-019-09992-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-019-09992-z