Abstract
MicroRNAs (miRs) are short non-coding RNAs that bind complementary sequences in mRNA resulting in translation repression and/or mRNA degradation. We investigated expression of the reported metastasis-associated miRs-335, 206, 135a, 146a, 146b, 10b, 21, let7a and let7b in normal mucosa, non-metastatic and metastatic colorectal cancer (CRC). Expression of target miRs in micro-dissected paraffin embedded tissues was evaluated in 15 primary tumours with adjacent normal tissue from patients that were disease-free at 4 years (cohort A) and 19 paired primary tumours with corresponding liver metastases (cohort B) by quantitative real-time PCR. Increased expression of miR-21, mir-135a and miR-335 was associated with clinical progression of CRC, while miR-206 demonstrated an opposite trend. The levels of mir-21 did not associate with the expression of PTEN, an important tumour suppressor in CRC and one of many putative targets of miR-21, but interestingly was associated with stage of disease in the PTEN expressing tumours. Surprisingly, let7a, a KRAS-targeting miR, showed elevated expression in metastatic disease compared to normal mucosa or non-metastatic disease, and only in KRAS mutation positive tumors. Finally, a prognostic signature of miR 21,135a, 335, 206 and let-7a for detecting the presence of metastases had a specificity of 87% and sensitivity of 76% for the presence of metastases. In summary, we have shown stage-associated differential expression of five out of nine tested metastasis-associated miRs. We have further found that an analysis of these five miRs expression levels in primary tumors significantly correlates with the presence of metastatic disease, making this a potential clinically useful prognostic tool.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Every year, over a million people worldwide will develop colorectal cancer (CRC) and over half a million will succumb to this disease [1]. Development of metastatic disease is the leading cause of morbidity and mortality associated with this disease [2]. Although considerable advances in understanding the pathogenesis of CRC have occurred over the past 25 years, much is still unknown about the regulators of its metastatic spread. Among the known mechanisms driving metastasis are genetic instability of malignant cells, epithelial–mesenchymal transition, resistance to apoptosis and anoikis, angiogenesis and lymphangiogenesis [3]. Alterations in the expression of oncogenes and tumour suppressors are drivers of this process, regulated by both genetic and epigenetic processes.
Evolving evidence suggests that microRNAs (miRs) play an important role in carcinogenesis and in cancer progression through their ability to affect the expression of genes that can regulate the tumourigenic process [4]. These non-coding small RNA molecules (19–22 nucleotides) regulate gene expression by complementarily binding to the 3′UTR of mRNAs, thereby causing mRNA degradation and/or translation inhibition. The primary transcripts of miRs (pri-miRs) are transcribed via RNA polymerase II, cleaved in the nucleus by Drosha into pre-miRs (70–100 nucleotides) and subsequently processed by Dicer in the cytoplasm to a mature miR product. This mature miR is incorporated in a complex known as miR-containing RNA-induced silencing complex (miRISC) which is the effector complex [5]. Various miRs contribute to cancer pathogenesis through their altered expression due to chromosomal abnormalities, mutation, polymorphisms, transcriptional deregulation, changes in miR biogenesis machinery and epigenetic changes [4].
Investigations into the role of miRs in CRC have been limited and have yielded conflicting results [4, 6–9]. The most robust data comes from miR expression profiles that compared colonic tumors to non-tumour tissue, using whole tumour samples. In Schetter et al. [10], 37 miRs were differentially expressed. However, only miR-21 levels were associated with poor survival. MiR-135a and miR135b were reported to down regulate the expression of the adenomatous polyposis coli gene, thus activating the β-catenin pathway, and their levels were elevated in CRC compared to normal colon mucosa [11]. The let-7 family of miRs has been shown to regulate the human KRAS gene [12], an important tumour oncogene that is a major driver of CRC progression. A number of miRs have been implicated in the regulation of metastasis (“metastamiRs”), in studies performed in mostly in breast [13] but also in colon [14] cancer cell lines. Among others, miR-335, 206 [15], 10b [16], 146a and b [13], let7a [17] and let7b [18] show altered expression that is associated with metastatic spread, and were shown to control metastatic spread in in vivo model systems. Consequently, we explored the role of several metastasis-associated miRs, by evaluating their expression in well defined cohorts of normal colonic tissue, localized CRC and metastatic CRC primary tumours and their paired liver metastases (LM). In this study, we evaluated the expression of various metastasis-associated miRs in micro-dissected samples thereby avoiding contributions of stromal components to miR expression profiles. This allowed a comprehensive study of miR modification in colon epithelial cells along the stages of cancer progression. We demonstrated differential expression of five of the nine tested miRs. A signature comprised of the expression levels of miRs 21,135, 335, 206 and let-7a in the primary tumours significantly correlated with the presence of metastatic disease.
Materials and methods
Tissue samples
Using The Ottawa Hospital Cancer Centre Colorectal Tumor Bank, two cohorts of colorectal patient tissues collected between 1997 and 2005 were selected for analysis. Cohort A included 15 formalin fixed, paraffin embedded (FFPE) CRC primary tumors with adjacent normal tissue (available for 9) of patients that had at least 4 years of disease free survival (DFS) post-treatment and were considered to have a very low likelihood of recurrence. Cohort B included 19 FFPE CRC primary tumors with matched LM samples. As fluorouracil chemotherapy has been shown to alter miR expression [19], we excluded tumors that had been exposed to neoadjuvant chemotherapy. Clinicopathologic information was obtained from the patient charts and pathology reports. All study materials were obtained following informed patient consent and ethics approval by the Ottawa Hospital Research Ethics Board.
Real-time PCR-based detection of miRs
Formalin fixed paraffin embedded tumour blocks were sectioned at 7 μm thickness and micro dissected by laser-capture-micro dissection (LCM, Arcturus, Mississauga, ON), collecting only cancer cells as defined by morphology, or adjacent normal epithelial cells in blocks with no tumour presence. Collected material was digested by proteinase K and frozen at −70°C until RNA extraction employing the miRNeasy (Qiagen, Mississauga, Ontario, Canada) kit was performed according to the manufacturer’s protocol. The average yield of RNA was approximately 230 ng/slice. RNA extraction and RT-PCR were done in batches of 8–11 tumour samples at a time utilizing 20 ng of RNA per RT reaction and 2 μl of the total 20 μl RT reaction used in a quantitative (Q) PCR. Each value represented in the graphs is the average of three replicates normalized to miR-191 expression. Relative quantitation (RQ) where RT-PCR target expression levels was determined relative to control HCT116 CRC cell line was employed as a reference RNA to control for differences between batches. miRs-103 and 191 were used as internal loading controls [20] and only RNA samples that showed similar miR103/191 ratios were included in this study as an assessment of miR extraction efficiency and fidelity. Q-PCR for all of the miRs evaluated was performed using the Applied Biosystems 7500 real-time PCR system and primers sets purchased from Applied Biosystems (Carlsbad, CA).
Direct sequencing of KRAS
The presence of mutations in the KRAS gene at codons 12 and 13 in exon 2 was assessed. 5 μM sections from paraffin-embedded specimens were subjected to either macro dissection of laser capture micro dissection to ensure that all DNA samples were >80% cancer cell derived. DNA was isolated using QuickExtract DNA extraction kit from Epicentre Biotechnologies (Madison, WI). Exon 2 of the KRAS gene was amplified using nested PCRs (outer primer pair 5′-GAA TGG TCC TGC ACC AGT AA-3′ and 5′-GTG TGA CAT GTT CTA ATA TAG TCA-3′; inner primer pair 5′-TTA TAA GGC CTG AAA ATG ACT GAA-3′ and 5′ TGA ATT AGC TGT ATC GTC AAG GCA CT-3′). PCR products from the second round of the nested PCR were screened for the presence of mutations using high resolution melting in a Corbett Rotorgene 6000. For PCR products with melting curve deflections, the presence and specific nature of mutations was confirmed by dideoxy sequencing (Ottawa Hospital Research Institute Core Sequencing Facility).
PTEN immunohistochemistry
Formalin fixed, paraffin embedded sections were deparaffinized and rehydrated using serial washes in xylene and ethanol. Antigen unmasking solution (Vector laboratories, Burlingame, CA) was used for antigen retrieval as per manufacturer’s protocol. Sections were blocked by normal goat serum, incubated with anti-PTEN Ab-6 (Thermo Scientific, Rockford, IL) and processed with mouse EnVision (Dako, Burlington, Ontario, Canada) and DAB according to manufacturer’s protocols. PTEN staining was scored by a trained pathologist (MD) and was graded along a 0–3 scale using normalized controls. Staining was scored zero when no specific staining was apparent in tumour tissue and positive with PTEN staining evident in tumour cells.
Statistical analysis
Statistical analysis was done using GraphPad Prism software 4.03. Significance of difference between cohorts (normal, cohort A and cohort B) was determined by two tailed unpaired t test, while normal and paired tumours in cohort A and the primaries and LM in cohort B were evaluated by two tailed paired t tests. A one way ANOVA test for variance P value was also performed for the comparison of the normal mucosa, cohort A and the primaries of cohort B.
Results
Patients and tumours characteristics
Two cohorts of patient specimens were chosen from the CRC tumour bank at the Ottawa Hospital Cancer Centre to be evaluated as cohort A (N = 15), which consisted of CRC patients with primary disease lacking metastatic dissemination and cohort B (N = 19), consisting of CRC patients with metastatic disease. Patient characteristics are presented in Table 1. The median age in cohort A was 66.1 years compared with 63.7 years in cohort B. Cohort B was mostly males (84%) unlike cohort A (33% males). Most of the tumors in both groups were of colonic origin (87% in A and 84% in B) and had a T3 depth of invasion (93% in A and 63% in B). All patients but one in cohort A had greater than 5 year DFS. The majority of patients in cohort B had synchronous LM (73%) and the average recurrence free survival (RFS) in this group was 9 months. Of note, a high proportion of patients in cohort B (63%) lacked lymph node involvement [21], which is likely the result of the relatively small sample size utilized in this study. The miRs evaluated in this study included miR-335, 206, 146a, 146b, 10b, 135a, 21, let7a and let7b that have all been demonstrated to play a role in the metastatic process in a number of tumour models (Fig. 1a). An example of a typical micro-dissected FFPE slide showing the intact tissue section (upper), the micro-dissected tumour epithelium (middle) and the residual stroma (lower) is shown in Fig. 1b.
Analysis of miRs in micro dissected CRC specimens. a miRs evaluated in this study and their proposed function according to published literature. b An example of LCM processed sample. Top CRC prior to LCM procedure. Middle the tumour epithelium isolated by LCM. Lower the residual stroma following removal of tumour epithelium by the LCM procedure. c Cycle threshold (Ct) values for miR-103 and miR-191 in the analyzed specimens
miR level normalization
As potential controls of miR abundance, we evaluated the expression of miR-103 and miR-191 in all of the specimens used in this study. The level of expression of these miRs is high and consistent in a wide variety of samples analyzed in other studies and are considered reliable markers of miR abundance [20]. In our sample set miR-191 displayed a more consistent expression profile between samples and, therefore, was used as a miR expression comparator in all of our subsequent analyses (Fig. 1c).
Differential expression of miR-135a, miR-335 and miR-206 in CRC progression
Statistical analyses to determine significant differences in miR expression between normal mucosa to cohort A, normal mucosa to cohort B, normal mucosa to cohort A and B, cohort A to cohort B and cohort B to cohort B mets was performed for all miRs evaluated and only comparisons with P values less than 0.05 are displayed. All other comparisons were not significant. Among the nine miRs that were evaluated, no significant changes in expression were observed for miR-10b, miR-146a or miR-146b levels in CRC progression, comparing normal adjacent colonic tissue, primary tumours that did not metastasize (cohort A), primary tumours with metastases and their corresponding LM (cohort B, Fig. 2a).
Relative quantities of miR-10b, miR-146a, miR-135a, miR-335 and miR-206. a Left RQ of miR-10b, middle RQ of miR-146a, right RQ of miR-146b in different stages of tumor progression. Mucosa = normal adjacent colon tissue specimens, cohort A = primary colon tumor specimens from patients that did not develop metastases at 4 years, cohort B primaries = primary colon tumor specimens that developed metastases, cohort B mets = liver metastasis specimens. No significant expression difference between the stages was found. b Left RQ of miR-135, significant up-regulation in both cohorts A and B in comparison to normal mucosa and from cohort A in comparison to cohort B. Right RQ of miR-335, significant up-regulation of this miR when comparing normal mucosa or cohort A to cohort B. c RQ of miR-206, significant down-regulation of this miR when comparing normal mucosa or cohort A to cohort B
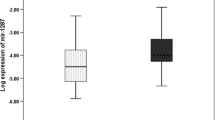
Of interest, increased expression levels of miR-135a were associated with CRC progression. miR-135a levels were significantly elevated in primary tumours derived from patients without metastases (cohort A) when compared with normal adjacent colon tissue (mean RQ 0.405 vs. 0.114; P = 0.032). Similarly, miR-135a was expressed at higher levels in primary tumours that metastasized (cohort B) compared with those without metastatic disease (cohort A) (mean RQ 0.936 vs. 0.405; P = 0.03). The levels of expression were not significantly different when primary tumours were compared with their corresponding LM (Fig. 2b, left). Therefore, miR-135a levels are increased in parallel to the progression of CRC.
Similarly, miR-335 expression levels were significantly elevated in primary tumours that metastasized (cohort B) compared with those that did not (cohort A) (mean RQ 0.8 vs. 0.35; P = 0.015). However unlike miR-135a, the level of expression of miR-335 was not significantly different when normal adjacent colon tissue was compared to primary tumours that did not metastasize (mean RQ 0.36 vs. 0.35). Therefore, miR-335 levels were significantly elevated in CRC primary tumors with a metastatic capacity, retaining similar levels in their metastatic lesions (Fig. 2b, right).
In contrast, expression levels of miR-206 were negatively correlated with CRC progression. Lower expression levels were demonstrated in primary tumours that did not metastasize (cohort A) compared with normal adjacent colon tissue (mean RQ 38.43 vs. 11.03; P = 0.002) (Fig. 2c). Comparisons of miR-206 expression between cohort A and the primary tumours of cohort B (mean RQ 11.03 vs. 8.45), and of those primaries with colorectal LM (mean RQ 8.45 vs. 5.4) demonstrated a similar trend of reduction of miR-206 levels with the progression of disease, but were not statistically significantly. However, an ANOVA test for variance was significant (P = 0.0001) for the comparison of the normal mucosa to cohort A tumours and the primaries of cohort B. We conclude that similar to the reports regarding breast cancer [22], miR-206 levels are lower in transformed colon mucosa than normal mucosa, and tend to be further reduced as CRC progresses.
Up-regulation of miR-21 in CRC progression
As previously demonstrated, increased expression levels of miR-21 were associated with CRC progression (Fig. 3a) [14, 23]. Expression of miR-21 was significantly increased in primary tumours that did not metastasize (cohort A) compared with normal adjacent colon tissue (mean RQ 0.88 vs. 0.52; P = 0.008). Levels of miR-21 were also significantly increased in primary tumours that did metastasize (cohort B) compared with those that did not metastasize (cohort A) (mean RQ 1.98 vs. 0.88; P = 0.024), in accordance to previous reports [10, 23]. No significant difference in expression was found between primary tumours and their matched colorectal LM, but a significant trend was seen of higher levels of miR-21 with disease progression (Fig. 3a, left). Thus, similar to what we observed for miR-135, miR-21 levels are increased along with the progression of CRC. Since miR-21 has been reported to down-regulate PTEN mRNA levels [24], we investigated PTEN expression in our tumour samples by immunohistochemistry (Fig. 3b) and stratified patients based on PTEN expression. We subsequently assessed for levels of miR-21 expression, no significant difference was observed between tumors expressing or not expressing PTEN (data not shown). Interestingly, a significant increase in miR-21 levels was seen along the progression of tumours in the PTEN positive group (ANOVA, P = 0.02), while the differences were not significant in the PTEN negative group (Fig. 3a, right). These results demonstrate the complexity of PTEN regulation as many factors besides miR-21 may target its expression in CRC cells. It can be speculated that when PTEN is expressed in CRC cells, miR-21 up-regulation is required for tumour progression to occur.
Analysis of miR-21 and PTEN protein levels. a Left RQ of miR-21 in different stages of tumor progression. Mucosa = normal adjacent colon tissue specimens, cohort A = primary colon tumor specimens from patients that did not develop metastases at 4 years, cohort B primaries = primary colon tumor specimens that developed metastases, cohort B mets = liver metastasis specimens. Significant differences were observed in expression levels of miR-21 with increasing CRC progression. Right analysis of PTEN negative and PTEN positive tumours for miR-21 expression. No correlation between miR-21 and PTEN levels was demonstrated. b Representative examples of PTEN negative and positive tumour IHC
Up-regulation of let7a in CRC metastatic disease
The let-7 family of miRs has been shown to regulate the expression of the KRAS gene [12]. Let-7a expression was significantly increased in CRC primaries of metastatic patients compared to the tumors of non-metastatic patients (mean RQ 0.285 vs. 0.154; P = 0.028; Fig. 4a). Although let-7a levels were increased in the LM compared to primary tumours of cohort B patients, the differences did not reach statistical significance (0.285 vs. 0.375). However, an ANOVA test for variance of the primary non-metastatic tumours, primaries of metastatic tumours and their metastasis demonstrated a significant increase along those three groups (P = 0.01) (Fig. 4a). Since let-7a has been reported to down-regulate KRAS expression, and KRAS is considered as an important driver of CRC progression, this result was counterintuitive.
let-7a levels and KRAS mutation analysis. a RQ of let-7a levels in tumour specimens showed significant differences in expression comparing cohort A to cohort B. b The distribution of KRAS mutations in the tumors bearing such mutations. c One-Way ANOVA test for the difference between cohort A, cohort B primaries and cohort B metastasis is significant in the mutant KRAS tumors, while the same test in the wild type KRAS tumors is non-significant (P value 0.129). d RQ of let-7a levels in tumors with mutant KRAS, wild type KRAS, and those with codon 12 mutations. e No significant differences in let-7b expression observed within these cohorts
To gain further insight into the molecular mechanisms at work, we determined KRAS mutation status in the tumours tested in this study. KRAS mutation status was evaluated on all the available tumours (N = 53; 15 of cohort A, and 19 primaries and 19 metastatic lesions of cohort B). KRAS mutation was detected in 24 (45%); G12D was the most common mutation (8 tumours), followed by G12V (6 tumours), G13D (5 tumours), G12S (3 tumours), G12C (2 tumours) and G12A and G12F (1 tumour each; Fig. 4b). Surprisingly, the increase in let-7a levels in parallel to the progression of tumours was significant only in the set of mutant KRAS tumors (Fig. 4c, ANOVA analysis, P = 0.013). Furthermore, let-7a levels were higher in the mutant KRAS tumours compared to the wild type KRAS tumours (RQ 0.334 vs. 0.217; P = 0.025, Fig. 4d). Recent data indicate that the clinical behavior of KRAS mutations in codon 13 is more indolent compared to those of codon 12 [25]. Interestingly, the tumours with a KRAS mutation in codon 13 (N = 4) had the highest let-7a values (data not shown). After removal of those tumours from the analysis, the difference in let-7a levels between the wild type KRAS and the codon 12 mutants was no longer significant (Fig. 4d). This data adds support to the existing evidence indicating significant differences among different KRAS mutants. However, the increase of let-7a levels seen in the mutant KRAS tumours along the three stages of disease was still significant in the codon 12 mutant specimens (ANOVA analysis, P = 0.02, data not shown).
A single nucleotide polymorphism (SNP) was reported to occur in the 3′UTR of the KRAS gene within the let-7a binding site that affects the efficacy of KRAS down regulation by this miR [26, 27]. As this SNP can nullify the inhibition of KRAS expression by let-7a, we tested the tumors for the presence of this SNP. Only four specimens were found to harbor this SNP, two of them being a pair of primary and metastasis from the same tumour, thus demonstrating a frequency of 8.8% in our set (3/34 tumours). The reported general population frequency of this SNP is 5.8% with a higher frequency in lung cancer patients of 18–20% [26], suggesting the possibility that this SNP might have a minor CRC predisposing effect. Following removal of the specimens harboring the let-7a SNP from our data set, let-7a levels remained elevated along with the progression of disease in the mutant KRAS tumors (mean RQ, cohort A; 0.1884, cohort B primary tumours; 0.2828, cohort B metastases; 0.4261). However, due to the smaller number of tumours analyzed now, the differences were no longer statistically significant (ANOVA P = 0.069). Therefore, let-7a levels increase with tumour stage, and interestingly are elevated in tumours harboring mutant KRAS. We were also interested in the rate of KRAS mutation formation during the process of metastasis. KRAS mutation status concordance between primary and metastasis was the most common situation (79%, 15 out of 19 pairs). In our set of specimens, it seems uncommon for the KRAS gene to acquire a mutation during the progression from a primary to a metastatic CRC.
In contrast let-7b, which is another member of this important miR family that shows significant sequence homology to let-7a with similar gene targets including KRAS [18], did not exhibit differential expression in these patient samples (Fig. 4e). This data demonstrates the specificity of changes in miR expression in these patient cohorts.
Potential miR prognostic signature of CRC metastatic disease
Adding the normalized values of miRs 21, 135a, 335 and let 7a, and subtracting the normalized value of miR206 created a miR prognostic signature. miR RQ values, corrected by miR191, were normalized relative to the maximal and minimal values of each miR [(RQ value of miR) − (minimal RQ of that miR)/(maximal − minimal RQ of that miR)]. Signature values of the 15 tumours of cohort A and the 19 primary tumours of cohort B are presented (Fig. 5). T test of the differences between the signature values was significant with a P value of 0.0004 (two tailed, unpaired t test). Using a “leave one out” design, and a median of the signature values as the cutoff, this signature was able to predict correctly the outcome of 28 out of 34 tumours (82%). The specificity of this signature for the detection of tumours that had metastasized was 87%, while the sensitivity was 76%.
Discussion
In the present study, we have shown that previously suggested metastasis-associated miRs [13] are also important in the progression and metastatic potential of CRCs. Using human colorectal tissue, we have shown differential expression of miR-135a, 206, 21, 335 and let-7a along the progression of CRC. Exploration of a miR prognostic signature suggests that miR profiles may compliment clinicopathologic variables in predicting the risk of metastasis in resected colorectal tumours.
The mechanisms by which some of these differentially expressed miRs are involved in CRC pathogenesis has been suggested by prior investigations. miR-135 has been shown to suppress expression of the tumour suppressor gene APC, inducing Wnt pathway signaling, possibly contributing to the development of colorectal adenomas and carcinomas [11]. Further, a study exploring miR expression in exfoliated colonocytes from feces has shown significantly higher miR-135 in patients with CRC compared with healthy volunteers [28]. Consistent with these reports, we present further evidence that miR-135 is higher in primary colorectal tumours compared with normal adjacent tissues and higher in tumours that have the ability to metastasize. Over-expression of miR-206 has shown pro-apoptotic properties through inhibition of notch3 signaling, also inhibiting cell migration, focus formation [29] and proliferation [30]. Moreover, miR-206 was inversely related to c-met expression in rhabdomyosarcoma with inhibition of miR-206 promoting cell proliferation and migration [31]. Consistent with these reports, down-regulation of miR-206 in our study was associated with a more malignant phenotype, suggesting a tumour suppressor role for miR-206 may also exist in CRC.
The tumour suppression genes PTEN, Pdcd4 and tropomyosin 1 (TPM1), matrix metalloproteinase regulators and cell cycle regulators have been suggested as targets of miR-21 [9, 14, 24, 32–34]. Clinically, higher mir-21 over-expression was reported in a variety of cancers, and in CRC has been associated with more advanced disease, poor survival and poor outcome [10]. Our findings validate this, as miR-21 expression increased along with tumors’ metastatic potential. Correlating miR-21 levels with PTEN IHC in our specimens suggests that miR-21 may regulate PTEN only in a subset of PTEN expressing tumors. miR-21 was the only miR found to correlate with clinical outcome in a recent CRC study [10]. However in contrast to this previous study that analyzed miRs isolated from whole tumour tissue, we evaluated miR expression specifically in tumour epithelium. Indeed, high stromal expression of miR-21 in stage II colon cancer has been associated with shorter DFS [7]. miR-21 is up regulated in the epithelial malignant compartment as well as in the stromal components as part of the progression of CRC. Thus, changes in the expression levels of the miRs we have discovered as associated with CRC progression may not have been detected in previous studies as they can be differentially expressed in the stromal compartment, which may mask the ability to detect differences in the tumour epithelium. This observation stresses the need to analyze the malignant component and the components of the cancer’s microenvironment separately.
KRAS mutation status has been shown to be important in the pathogenesis of CRC [35] and the let-7 miR family has been shown to down-regulate KRAS through binding of the 3′UTR of the KRAS gene [12]. Our finding of increased expression of let-7a in CRC specimens bearing KRAS mutations is counterintuitive. Indeed, this difference becomes non-significant when only the codon 12 mutations are considered. However, these results indicate lack of a selective pressure for lower let-7a levels when KRAS is mutant. It can be speculated that let-7a has a tumour-promoting impact that remains to be discovered. Regarding miR-335, in contrast to a proposed metastasis suppressing role of this miR in breast cancer [30], we demonstrated its elevated expression with increased metastatic potential in CRC. The biologic impact of specific miRs is probably very much dependant on the cell type and the transcription level of potential target mRNA species.
To the best of our knowledge, this is the first report investigating miR differences between primary CRC and the corresponding LM using micro dissected specimens. A comparison of miR-21 and miR-143 expression in whole CRC samples and corresponding LM has been reported by Kulda et al. [6] demonstrating higher expression of miR-21 in LM compared with normal colonic tissue, similar to our results in micro dissected cells. We have also found a trend towards increased expression of let-7a and lower expression of miR-206 in LM compared with primary tumours and normal colon tissue. Further studies with larger numbers of primary tumours and paired LM are required to explore the possibility of differential expression of miRs between primary CRC and metastatic disease. As well, the direct contribution of these miRs to CRC progression were not evaluated in this study and will require the elucidation of the gene targets regulated by these miRs.
In an attempt to create a novel prognostic tool, we combined the significantly altered miRs into a prognostic miR signature. By a leave-one-out validation this signature has potential applicability, but validation using a different cohort is required. Others have shown the potential role of prognostic miR signatures in lung cancer, gastric cancer, cervical cancer, and kidney cancer [36–40]. Considering the stability of miRs, the prognostic and possibly diagnostic and predictive use of miRs seem promising.
In summary, we have shown that previously described metastasis-associated miRs are also important in CRC and correlate with the metastatic capacity of primary CRC. This study adds further evidence for the potential of utilizing specific miR targeted therapeutics as inhibitors of CRC metastatic spread.
Abbreviations
- miR:
-
MicroRNA
- CRC:
-
Colorectal cancer
- APC:
-
Adenomatous polyposis coli
- FFPE:
-
Formalin fixed paraffin embedded
- DFS:
-
Disease free survival
- LM:
-
Liver metastasis
- LCM:
-
Laser-capture-micro dissection
- RFS:
-
Recurrence free survival
- SNP:
-
Single nucleotide polymorphism
- PTEN:
-
Phosphatase and tensin homolog
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Kurkjian C, Murgo AJ, Kummar S (2008) Treatment of recurrent metastatic colon cancer in the age of modern adjuvant therapy. Clin Colorectal Cancer 7(5):321–324
Eccles SA, Welch DR (2007) Metastasis: recent discoveries and novel treatment strategies. Lancet 369(9574):1742–1757
Iorio MV, Croce CM (2009) MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 27(34):5848–5856
Williams AE (2008) Functional aspects of animal microRNAs. Cell Mol Life Sci 65(4):545–562
Kulda V, Pesta M, Topolcan O et al (2010) Relevance of miR-21 and miR-143 expression in tissue samples of colorectal carcinoma and its liver metastases. Cancer Genet Cytogenet 200(2):154–160
Nielsen BS, Jorgensen S, Fog JU et al (2011) High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis 28(1):27–38
Schee K, Fodstad O, Flatmark K (2010) MicroRNAs as biomarkers in colorectal cancer. Am J Pathol 177(4):1592–1599
Wang P, Zou F, Zhang X et al (2009) microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res 69(20):8157–8165
Schetter AJ, Leung SY, Sohn JJ et al (2008) MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA 299(4):425–436
Nagel R, le Sage C, Diosdado B et al (2008) Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res 68(14):5795–5802
Johnson SM, Grosshans H, Shingara J et al (2005) RAS is regulated by the let-7 microRNA family. Cell 120(5):635–647
Hurst DR, Edmonds MD, Welch DR (2009) Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res 69(19):7495–7498
Asangani IA, Rasheed SA, Nikolova DA et al (2008) MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27(15):2128–2136
Tavazoie SF, Alarcon C, Oskarsson T et al (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451(7175):147–152
Ma L, Teruya-Feldstein J, Weinberg RA (2007) Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449(7163):682–688
Zhang W, Winder T, Ning Y et al (2011) A let-7 microRNA-binding site polymorphism in 3′-untranslated region of KRAS gene predicts response in wild-type KRAS patients with metastatic colorectal cancer treated with cetuximab monotherapy. Ann Oncol 22(1):104–109
King CE, Wang L, Winograd R et al (2011) LIN28B fosters colon cancer migration, invasion and transformation through let-7-dependent and -independent mechanisms. Oncogene 30(40):4185–4193
Rossi L, Bonmassar E, Faraoni I (2007) Modification of miR gene expression pattern in human colon cancer cells following exposure to 5-fluorouracil in vitro. Pharmacol Res 56(3):248–253
Peltier HJ, Latham GJ (2008) Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA 14(5):844–852
Chang GJ, Rodriguez-Bigas MA, Skibber JM et al (2007) Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst 99(6):433–441
Negrini M, Calin GA (2008) Breast cancer metastasis: a microRNA story. Breast Cancer Res 10(2):203
Slaby O, Svoboda M, Fabian P et al (2007) Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology 72(5–6):397–402
Meng F, Henson R, Lang M et al (2006) Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 130(7):2113–2129
De Roock W, Jonker DJ, Di Nicolantonio F et al (2010) Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA 304(16):1812–1820
Chin LJ, Ratner E, Leng S et al (2008) A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res 68(20):8535–8540
Christensen BC, Moyer BJ, Avissar M et al (2009) A let-7 microRNA-binding site polymorphism in the KRAS 3′ UTR is associated with reduced survival in oral cancers. Carcinogenesis 30(6):1003–1007
Koga Y, Yasunaga M, Takahashi A et al (2010) MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res (Phila) 3(11):1435–1442
Song G, Zhang Y, Wang L (2009) MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J Biol Chem 284(46):31921–31927
Adams BD, Cowee DM, White BA (2009) The role of miR-206 in the epidermal growth factor (EGF) induced repression of estrogen receptor-alpha (ERalpha) signaling and a luminal phenotype in MCF-7 breast cancer cells. Mol Endocrinol 23(8):1215–1230
Yan D, Dong Xda E, Chen X et al (2009) MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem 284(43):29596–29604
Gabriely G, Wurdinger T, Kesari S et al (2008) MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol 28(17):5369–5380
Zhang JG, Wang JJ, Zhao F et al (2010) MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta 411(11–12):846–852
Zhu S, Si ML, Wu H et al (2007) MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 282(19):14328–14336
Vogelstein B, Fearon ER, Hamilton SR et al (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319(9):525–532
Hu X, Schwarz JK, Lewis JS Jr et al (2010) A microRNA expression signature for cervical cancer prognosis. Cancer Res 70(4):1441–1448
Hu Z, Chen X, Zhao Y et al (2010) Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol 28(10):1721–1726
Juan D, Alexe G, Antes T et al (2010) Identification of a microRNA panel for clear-cell kidney cancer. Urology 75(4):835–841
Li X, Zhang Y, Ding J et al (2010) Survival prediction of gastric cancer by a seven-microRNA signature. Gut 59(5):579–585
Yanaihara N, Caplen N, Bowman E et al (2006) Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9(3):189–198
Acknowledgments
Research support from the Canadian Institute of Health Research (J.D.), the Ontario Institute for Cancer Research (J.D.) and Ortho-Biotech Canada (J.D.) is greatly appreciated. The authors would like to thank Ms. Colleen Crane for her technical support. Presented in part at the 2010 European Society of Medical Oncology Meeting, Milan, Italy.
Conflict of Interest
All authors declare no conflict of interests with respect to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
First two authors contributed equally to this study.
Rights and permissions
About this article
Cite this article
Vickers, M.M., Bar, J., Gorn-Hondermann, I. et al. Stage-dependent differential expression of microRNAs in colorectal cancer: potential role as markers of metastatic disease. Clin Exp Metastasis 29, 123–132 (2012). https://doi.org/10.1007/s10585-011-9435-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9435-3