Abstract
We previously described a lipid-accumulating phenotype of estrogen receptor negative (ER−) breast cancer cells exemplified by the MDA-MB-231 and MDA-MB-436 cell lines. These cells had more lipid droplets, a higher uptake of oleic acid and LDL, a higher ratio of cholesteryl ester (CE) to triacylglycerol (TAG), and higher expression of acyl-CoA:cholesterol acyltransferase 1 (ACAT1) as compared to ER+ MCF-7 breast cancer cells. LDL stimulated proliferation of ER-cells only, and proliferation was reduced by inhibition of ACAT. We hypothesized that storage of exogenous lipids would confer an energetic advantage. We tested this by depriving cells of exogenous lipids and measuring chemotactic migration, an energy-intensive behavior. MDA-MB-231 cells were grown for 48 h in medium with either 5% FBS or 5% lipoprotein-depleted (LD) FBS. Growth in LD medium resulted in visibly reduced lipid droplets and an 85% decrease in cell migration. Addition of LDL to the LD medium dose-dependently restored the ability to migrate in an ACAT-sensitive manner. LDL receptor (LDLR) mRNA was 12-fold higher in MDA-MB-231 cells compared to nontumorigenic ER-MCF-10A breast epithelial cells grown in LD medium. Addition of LDL to the LD medium reduced LDLR mRNA levels in MCF-10A cells only. We asked if ACAT1 activity was associated with the expression of the LDLR in MDA-MB-231 cells. LDLR mRNA in MDA-MB-231 cells was substantially reduced by inhibition of ACAT, demonstrating that high ACAT1 activity permitted higher LDLR expression. This data substantiates the association of lipid accumulation with aggressive behavior in an ER-breast cancer cell line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most cancer deaths are caused by metastasis rather than the primary tumor, and identification of ways to slow or prevent metastatic disease are a priority in cancer research [1]. A high fat diet and obesity signify a worse prognosis in breast cancer patients [2]. The mechanisms involved have not been completely elucidated, but are thought to involve hormones and adipokines secreted by adipose tissue [3] and the insulin axis [4]. One factor that has received little attention is higher levels of circulating lipoproteins and free fatty acids. Both LDL and unsaturated fatty acids have been demonstrated to increase proliferation of estrogen receptor alpha negative (ER−) breast cancer cells [5–7]. It is possible that higher levels of circulating lipoproteins and free fatty acids, which are common in obesity, metabolic syndrome and high fat diets, may themselves promote aggressive characteristics of breast cancer.
A mechanism for the effects of circulating lipids on cancer progression lies in the fact that proliferating cells require a constant supply of lipids for membrane construction, and that these lipids can be obtained in two ways. Proliferating cells can synthesize fatty acids and cholesterol de novo from glucose through upregulation of glycolysis and the enzymes of biosynthesis such as fatty acid synthase and HMG-CoA reductase. Many types of cancer cells, termed the lipogenic phenotype [8], use this strategy and fatty acid synthase is an important new target against cancer. The alternative strategy is to increase the uptake of circulating lipids such as LDL, which supplies both cholesterol and fatty acids, and free fatty acids, and to increase the storage of these lipids as neutral lipids in cytoplasmic lipid droplets. The latter approach is more energy efficient and can be used in hypoxic conditions where oxidative metabolism, needed for production of biosynthetic intermediates, may be compromised. Lipid droplets provide a ready depot of lipids which can be accessed as needed without having to invest energy in biosynthesis. We refer to the second strategy the lipid-accumulating phenotype.
Our interest began with the observation that the triple negative (TN, lacking estrogen receptor alpha (ER), the progesterone receptor, and ERBB2/Her2/neu) breast cancer cell lines MDA-MB-231 and MDA-MB-436 had many more lipid droplets as compared to the ER+ MCF-7 cell line under the same growth conditions. Upon investigating neutral lipid composition and metabolism in these cells, we found that the TN cells had higher concentrations of both triacylglycerol (TAG) and cholesteryl esters (CE), and a greater proportion of CE relative to TAG [9]. Acyl-CoA:cholesterol acyltransferase 1 (ACAT1/SOAT1), the housekeeping enzyme that makes CE from long chain fatty acids and cholesterol, was highly upregulated at both the protein and mRNA level in TN breast cancer cells compared to ER+ cells [9]. In addition, TN cells demonstrated a 3–4 fold higher rate of LDL uptake as compared to ER+ cells [9].
Our data were in agreement with microarray studies curated at oncomine.org showing that in both human breast tumors [10–13] and human breast cancer cells lines [14] ACAT1 is more highly expressed in the types of breast cancer characterized as basal-like, TN, or ER−. This type of breast cancer is highly aggressive and lacks targeted therapies [15]. We wanted to know if high ACAT1 expression and the lipid metabolism differences we observed contributed to the aggressive phenotype of TN breast cancer. Thus we investigated the effect of LDL on proliferation of these breast cancer cell lines, using lipoprotein-depleted (LD) medium as a control, in the presence or absence of an ACAT inhibitor. We confirmed that LDL stimulated proliferation of the ER− cells only, and found that this stimulation was sensitive to ACAT inhibition [9]. In the current work, we continue to explore the hypothesis that high expression of ACAT1 and high uptake of LDL contribute to an aggressive breast cancer phenotype. We examined the effect of LDL on chemotactic migration, a characteristic of aggressiveness, in a TN breast cancer cell line known for high motility, MDA-MB-231. Migration is an energy-intensive process, and we predicted that the energy benefit obtained from a strategy of taking up more exogenous lipids and thereby limiting the need for de novo synthesis of lipids would be translated into a greater ability to migrate.
Because high ACAT1 expression and activity are associated with high LDL uptake in this cell line, we asked if ACAT inhibition would modify the effect of LDL on migration. Our results led us to further explore the relationship between high ACAT1 expression and high LDL uptake. We examined the regulation of the LDL receptor (LDLR) in TN MDA-MB-231 cells and the nontumorigenic breast epithelial cells, MCF-10A, which express ~sixfold lower ACAT1 [9]. MCF-10A cells are also considered TN and may represent a precursor cell type to TN breast cancer [16]. Finally, we asked if important signaling pathways for growth and migration impacted either expression of the LDLR or activity of ACAT.
Materials and methods
Cell culture and treatments
MDA-MB-231 and MCF-10A cells were obtained from ATCC (Manassas, VA) at passages 29 and 97, respectively, and all experiments were done within 20 passages. Cells were cultured in growth medium (for MDA-MB-231, DMEM without phenol red containing 1 g/l glucose, 4 mM l-glutamine, 5% FBS; and 100 units/ml each penicillin and streptomycin; for MCF-10A, DMEM/F12 without phenol red containing 5% FBS, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, 100 ng/ml cholera toxin, 20 ng/ml epidermal growth factor, and 100 units/ml each penicillin and streptomycin) at 37°C in a humidified 5% CO2 atmosphere. Cell culture and Western blotting materials were obtained from Invitrogen (Carlsbad, CA) unless otherwise specified.
When testing the effects of lipids, FBS was replaced with lipid reduced FBS (Thermo Scientific Hyclone, Logan, UT) which contained no lipoproteins. Concentrated LDL (Creative Laboratory Products, Inc., Indianapolis, IN) was diluted 1/50 and tested for cholesterol concentration following manufacturer’s instructions (Wako Diagnostics, Richmond, VA). Aliquots were stored at −80°C and a fresh aliquot was used for each experiment. LDL was diluted in growth medium containing 5% LD FBS (LD medium) and sterile filtered. 250× cholesterol lipid concentrate (CLC, Invitrogen, Carlsbad, CA), a defined media supplement containing cholesterol and fatty acids, was similarly assayed for cholesterol. Ethanolic solutions of oleic acid, linoleic acid (Sigma-Aldrich, St. Louis, MO) or cholesterol oleate (CO, Nu-Chek Prep, Elysian, MN) were added to LD medium and incubated at 37°C for 30 min before adding to cells. Final ethanol concentration did not exceed 0.1%. Oil Red O staining and imaging of cells was done as described previously [17]. PKC inhibitor Gő6983 was obtained from Sigma-Aldrich, St. Louis, MO. MEK inhibitors U0126 and PD98059 were from Calbiochem, Gibbstown, NJ. ACAT inhibitor CP-113818 was a gift from Pfizer, Inc., Groton, CT.
Chemotactic migration
Cells were seeded into 6-well plates at 300K cells/well in growth medium. After overnight attachment, the medium was replaced with LD medium overnight. The next morning, fresh treatment media with or without lipid or inhibitors were added and cells were incubated for 48 h. Cells were detached, collected by centrifugation and resuspended in serum-free DMEM for counting. 100K cells in 100 μl serum-free medium were added to transwell chambers (8 μm pore size polycarbonate membrane, Corning, Corning, NY) with 0.3 ml growth medium in the bottom wells. The FBS in the medium served as the chemoattractant. After 6 h, medium and cells were removed from the upper chamber with a cotton swab, and membranes were fixed in buffered 4% paraformaldehyde and stained with Mayer’s hematoxylin. Four adjacent quadrants at the center of each membrane were imaged at ×100 magnification. The cells on the images were counted (cell counts ranged from <10 to 800 per quadrant) and the mean cells/quadrant/membrane was determined. Mean and SD were based on the number of membranes.
ACAT activity in whole cells
Cells at 50% confluence in T25 flasks were treated with inhibitors or vehicle for 24 h in LD growth medium. Fresh media with or without LDL or inhibitors was added along with 2 μCi 3H-cholesterol per flask. After 24 h, cells were detached with trypsin and collected with 5 ml PBS containing 1% fatty acid-free BSA. After centrifugation, the cells were washed with 5 ml PBS. The pellets were suspended in 250 μl PBS, sonicated, and transferred to glass tubes. 1.5 ml methanol, 3 ml chloroform and 0.95 ml 0.88% KCL were added and vortexed to extract lipids. The chloroform layer was evaporated under N2 and then spotted on LK5 TLC plates along with appropriate standards. The plates were developed in hexane:diethyl ether:acetic acid (70:30:1). Bands corresponding to free cholesterol (Rf = 0.18) and CE (Rf = 0.70) were identified using pure standards (cholesterol was from Sigma-Aldrich, St. Louis, MO; cholesteryl nonadecanoate was from Nu-Chek Prep, Elysian, MN) and scraped into scintillation vials. The silica gel was dissolved in 1 ml dichloromethane and 10 ml ScintiVerse fluid and counted on a Beckman LS6000IC scintillation counter. CE formation was represented by % cpm in CE/(cpm in free cholesterol + cpm in CE).
Quantitative real time PCR
Total RNA was isolated with RNA Stat-60 (Tel-test, Friendswood, TX) according to the manufacturer’s protocol. RNA was DNase treated with DNA-free (Ambion, Foster City, CA). RNA (1.0 μg) was reverse transcribed using Affinity Script and with oligo(dT) and random hexamer primers (Stratagene, La Jolla, CA) to generate cDNA. Real-time quantitative PCR was performed with the MX3000P (Stratagene) and 2× Brilliant SYBR Green QPCR Master Mix (Stratagene) for detection of amplified products. Relative mRNA abundance was normalized to 18S rRNA (forward primer 5′-TTA GAG TGT TCA AAG CAG GCC CGA-3′ and reverse primer 5′-TCT TGG CAA ATG CTT TCG CTC TGG-3′). Primers were obtained from Integrated DNA Technologies (Coralville, IA), and designed using the manufacturer’s tools. The following primers were used for LDLR (forward 5′-TGA CAC CGT CAT CAG CAG AGA CAT-3′ and reverse 5′-GAT GCC ATT GGG CCA CTG AAT GTT-3′). Negative controls contained no transcript or reverse transcriptase. RNA from three separate cell pellets for each cell line or condition was analyzed. Relative gene expression was calculated using the method given in Applied Biosystems User Bulletin No. 2 (P/N 4303859B).
Western blotting for ERK1/2 activation
Cells were seeded into 6-well plates in growth medium until 30–40% confluent. Medium was then changed to growth medium with 5% FBS, 5% LD FBS, or no serum (SF). Inhibitors were added and cells incubated for 24 h. Cells were washed with PBS and lysed with MPER lysis buffer (Pierce) containing Complete protease inhibitor cocktail (Roche) and 100 mM NaF. Protein was determined by bicinchoninic acid assay. Lysates were diluted in Laemmli loading buffer and electrophoresed on 4–12% bis–tris gels. Proteins were transferred to 0.2 μm nitrocellulose membranes. Blots were blocked with 10% Western Blocking Reagent (Roche) in TBST prior to exposure to antibody diluted in 5% Western Blocking Reagent. Polyclonal antibodies to phospho-p42/44 MAPK and p42/44 MAPK were obtained from Cell Signaling Technology, Danvers, MA. Bands were detected with anti-species HRP conjugate and ECL reagent (GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Statistical analysis
Data are represented as means and SDs. Differences between means were determined by one-way ANOVA with post hoc testing using Dunnett’s method to compare the treatments with the control. The level of significance was set at α = 0.05.
Results
Treatment with LD medium eliminated most cytoplasmic lipid droplets
MDA-MB-231 cells were incubated for 48 h in growth medium with either 5% FBS or 5% LD FBS. The cells were then fixed and stained with Oil Red O and hematoxylin. Lipid deprivation conditions depleted the cells of visible lipid droplets (Fig. 1).
Treatment with LD medium decreased migration
Chemotactic migration of MDA-MB-231 cells was reduced 85% when cells were grown for 48 h in medium containing LD FBS compared to standard FBS (Fig. 2a). Because LDL is a complex mixture of CE, TAGs, proteins and possible other factors, we tested LDL and individual lipids for their effects on migration (Fig. 2b). Compared to LD medium alone, supplementation with LDL, linoleic acid, oleic acid or CLC (which contains a ~2:1 ratio of saturated to monounsaturated fatty acids (our analysis, data not shown)) in LD medium for 48 h resulted in increased migration. CO had a slight but not significant effect. A higher concentration of CO did not increase the effect (data not shown), however we do not exclude that longer duration or repeated treatments with CO might have an effect on migration.
Effect of media lipids on migration of MDA-MB-231 cells. a Cells were grown for 48 h in medium containing FBS or LD FBS and then assayed for migration as described. Data are mean and SD of three experiments. ***P < 0.001 compared to FBS b Cells were grown for 48 h in LD medium supplemented with various lipids: OA 10 μM oleic acid, LA 10 μM linoleic acid, LDL 10 μg/ml LDL cholesterol, CLC cholesterol lipid concentrate, 11 μM cholesterol, CO 5 μM cholesterol oleate. Cells were assayed for migration as described. Data are mean and SD of three experiments. ***P < 0.001 compared to NONE
ACAT inhibition reduced migration
To test if cholesterol esterification was a necessary component of LDL-induced migration of MDA-MB-231 cells, we used the ACAT inhibitor CP-113818. Cells were treated with increasing doses of LDL or fatty acids in LD medium in the presence or absence of 10 μM CP-113818 for 48 h prior to migration assay. ACAT inhibition reduced migration at all doses of LDL (Fig. 3a). In a similar manner, cells treated with oleic acid or linoleic acid in LD medium exhibited reduced migration when cholesterol esterification was blocked (Fig. 3b).
Effect of ACAT inhibitor CP-113818 on migration of MDA-MB-231 cells. a Cells were treated for 48 h in LD medium with increasing doses of LDL ± 10 μM CP-113818, then assayed for migration as described. Data are mean and SD of three experiments. b Cells were treated for 48 h in LD medium with oleic acid (OA) or linoleic acid (LA) ± 10 μM CP-113818, then assayed for migration. Data are mean and SD of three experiments. ***Different from NONE, *different from DMSO
LDLR is highly expressed in MDA-MB-231 cells
It is generally accepted that some cancer cells have higher than normal uptake of LDL [18–20] and we showed that TN breast cancer cells had higher uptake of LDL compared to ER+ breast cancer cells [9]. We asked if mRNA expression of the LDLR in TN MDA-MB-231 aggressive cancer cells was elevated compared to the immortalized but nontumorigenic MCF-10A breast epithelial cells when both were grown in LD medium to 70–80% confluence. We found that mRNA expression of the LDLR in MDA-MB-231 cells was higher (fold change 11.7, range 7.27–18.7) compared to MCF-10A cells (1.00, range 0.77–1.30) (P = 0.02). In addition, while the MCF-10A cells demonstrated down regulation of the LDLR expression when LDL was provided, consistent with normal homeostasis, the MDA-MB-231 cells did not (Table 1). ACAT inhibition, however, resulted in lower expression of the LDLR mRNA in both cell lines. This suggests that high ACAT activity is a perturbation of cholesterol homeostasis in favor of higher LDLR expression.
Effect of PKC and MEK inhibition on LDLR, cholesterol esterification and migration
We showed that ACAT1 (mRNA and protein) and the LDLR (mRNA) are more highly expressed in the aggressive TN MDA-MB-231 breast cancer cells as compared to the nontumorigenic MCF-10A breast epithelial cells under similar conditions ([9] and above). The question of how these gene expressions are controlled remains open. We asked if inhibition of the important signaling molecules PKC and MEK, previously shown to inhibit cholesterol esterification in transformed cells [21], would have similar effects in MDA-MB-231 cells. The compound Gő6983 inhibits PKC isozymes α, β, γ, δ, and ζ. PKC inhibition in cells provided LDL led to lower LDLR mRNA expression in the MDA-MB-231 cells only (Table 1). Two different MEK inhibitors also reduced LDLR expression in MDA-MB-231 cells (Table 1).
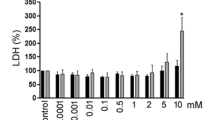
To examine the effect of these inhibitors on migration and cholesterol esterification, cells were treated for 48 h with LD media + 10 μg/ml LDL cholesterol in the presence or absence of inhibitors. All inhibitors reduced migration, with dose dependence seen for MEK inhibitors (Fig. 4a). PKC inhibition, but not MEK inhibition, dose-responsively reduced cholesterol esterification (Fig. 4b). ERK1/2 activation, which was constitutive in this cell line, was not affected by PKC inhibition (Fig. 4c).
Effect of PKC and MEK inhibitors on migration and cholesterol esterification of MDA-MB-231 cells. a Migration of cells treated for 48 h with LD medium containing 10 μg/ml LDL cholesterol in the presence or absence of inhibitors (μM concentrations of inhibitors are indicated). Data are mean and SD of three experiments. **P < 0.01, ***P < 0.001 compared to DMSO. b Cells were incubated for 24 h in LD medium ± inhibitors. Medium was changed to fresh LD medium ± LDL and inhibitors with a 3H-cholesterol tracer. After 24 h, cholesterol esterification was determined as described. Concentrations of inhibitors were: CP-113818, 10 μM; Gő6983, 2 and 5 μM; U0126, 10 μM; PD98059, 50 μM. Data are mean and SD of three experiments. ***P < 0.001 compared to DMSO. c Western blots showing activation of ERK1/2 in MDA-MB-231 cells after 24 h in growth medium containing either FBS, LD FBS, or no FBS (SF) ± inhibitors
Discussion
Here we extend our previous work with evidence that LDL impacts not only proliferation, but energetically costly migration in an in vitro model of breast cancer. TN MDA-MB-231 breast cancer cells exhibited depletion of lipid droplets and decreased migration when incubated in LD medium for 48 h. Supplementation of LD medium with LDL or free fatty acids or a mixture of free cholesterol and free fatty acids (CLC) dose responsively restored migration.
In normal cells, lipoproteins provide cholesterol and fatty acids from the diet. LDL contains a core of TAG and CE, whose constituent fatty acids supply the essential fatty acids (such as linoleic acid and its metabolite, arachidonic acid, an important eicosanoid precursor) to cells. The demonstration that linoleic and oleic acid (which can be endogenously synthesized) stimulated migration approximately equally supports that the decrease in migration in LD medium is not due to a lack of essential fatty acids. The fact that we could not demonstrate an effect of CE on migration may be due simply to insolubility in cell culture medium. We expect that the cholesterol supplied by LDL through the normal LDL uptake pathway and stored in lipid droplets plays an equally important role in the ability of these cells to migrate.
Our hypothesis that cholesterol esterification (one method of neutral lipid accumulation) is involved in migration was supported by our finding that inhibition of ACAT reduced migration stimulated by exogenous lipids (LDL or fatty acids). Because the primary activity of ACAT is esterification of cholesterol to long chain fatty acids, we considered the contribution of ACAT to intracellular cholesterol homeostasis in TN MDA-MB-231 breast cancer cells. We showed that control of intracellular cholesterol is perturbed in the direction of cholesterol accumulation in these cells. In normal homeostasis, the free cholesterol concentration in the endoplasmic reticulum membrane, as a sensor of cell cholesterol availability, influences the activation of the transcription factor that controls the expression of the LDLR and hence LDL uptake [22]. In MDA-MB-231 cells, addition of LDL did not reduce LDLR expression as it did in the nontumorigenic MCF-10A cells, but ACAT inhibition was able to substantially reduce LDLR expression. This indicates that the high expression and activity of ACAT1 in the MDA-MB-231 cells, by esterifying free cholesterol and removing it from the cholesterol sensing mechanism, allows a continued high expression of the LDLR. A similar situation was noted in SV40 transformed rodent fibroblasts, which exhibited a 5- to 9-fold increase in intracellular CEs, a twofold increase in LDLR number, and insensitivity to LDL-induced down regulation of LDLR compared to nontransformed cells [23].
ACAT activity was previously found to be important in U87 glioma cells and NIH-3T3 cells expressing a constitutively active cholecystokinin 2 receptor, where inhibition of cholesterol esterification reduced proliferation and invasion [21]. Our data provides evidence of a similar role for ACAT1 in MDA-MB-231 breast cancer cells.
ACAT1/SOAT1 was recently identified as differentially over-expressed in the prognostically useful “claudin-low” designation of a subtype of breast cancer cell lines which includes MDA-MB-231 [24]. In fact, over-expression of ACAT1 in cancer compared to normal cells was seen in multiple analyses across a wide variety of cancer types, including brain, breast, cervical, head and neck, kidney and leukemia (oncomine.org, [25]) suggesting a more general role for high ACAT1 expression in cancer.
It remains unclear what mediates the high expression of the LDLR in some cancer cells, although a growth pathway might be suspected. The Ras/Raf-1/MEK/ERK pathway is an important oncogenic pathway due to the presence of Ras mutations in many human malignancies [26]. Sustained activation of ERK1/2 in the MDA-MD-231 cell line such as we noted (Fig. 4) was found by others to be critical for cell motility and invasiveness [27]. The degree of ERK1/2 activation correlated with the expression level of the LDLR in HepG2 cells stably expressing an inducible form of oncogenic Raf-1 kinase [28]. Our results were consistent with an involvement of this pathway, as we found that in MDA-MB-231 cells MEK inhibition resulted in lower expression of the LDLR and reduced migration but no effect on cholesterol esterification. PKCα is a critical mediator of migration in MDA-MB-231 breast cancer cells [29], and PKCζ was shown to be involved in both invasion and cholesterol esterification in U87 glioma cells [21]. In our study, an inhibitor of PKC (including PKCα and PKCζ isozymes) reduced expression of the LDLR, cholesterol esterification and migration while only LDLR expression and migration were inhibited by ERK1/2 inhibition. These results are different from Paillasse et al. [21], who showed that PKCζ are involved in cholesterol esterification through activation of ERK1/2. However, different cell types may utilize different pathways and it will require further investigation to completely understand the regulation of the LDLR and cholesterol esterification in breast cancer cells.
How neutral lipid accumulation contributes to migration is unclear. Because migration, like proliferation, is an energy-intensive process, the energy benefit derived from uptake of circulating fatty acids and cholesterol as a substitute for de novo biosynthesis may be used to drive migration. Although de novo cholesterol synthesis is active in MDA-MB-231 cells, we are proposing a beneficial trade-off of increased uptake for decreased synthesis. This is supported by our previous demonstration that under identical conditions cholesterol synthesis in the TN MDA-MB-231 cells was only about 40% of the synthesis in ER+ MCF-7 cells, while LDL uptake was ~fourfold higher [9]. A similar distinction was made in early work between Ehrlich ascites tumor cells, where the intracellular accumulation of CE was obtained from exogenous lipids, and Morris hepatoma cells, where most of the intracellular cholesterol was derived from a high rate of de novo synthesis [30]. A sample of gene expression data from human breast tumors shows that genes involved in lipid uptake and storage (ACAT1/SOAT1, ADFP/PLIN2, and LDLR) are negatively, while genes involved in lipid synthesis (FASN, SREBF1, and HMGCR) are positively correlated to ERα (ESR1) (Fig. 5).
Pearson correlation coefficients (r) for the mRNA expression of lipid metabolizing genes with ERα (ESR1) from two studies of human breast tumors [10, 12]. Normalized gene expression data were retrieved from the NCBI Geoprofiles database. ADFP/PLIN2 (adipophilin); FASN (fatty acid synthase), SREBF1 (steroid response element binding protein 1); HMGCR (3-hydroxy-3-methylglutaryl-CoA reductase). # P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001
It is also important to consider the possible role of cytoplasmic lipid droplets in cancer cells that may go beyond mere storage of neutral lipids. Lipid droplets are attached to microtubules [31, 32] and move to the farthest reaches of cytoplasmic protrusions in breast cancer cells (our own observations). They are involved in lipid trafficking within the cell [33], interact with other organelles such as mitochondria and peroxisomes [34], and provide a site for lipid signaling [35]. Oleic acid, which readily promotes the formation of lipid droplets, was recently shown to stimulate motility of MDA-MB-231 cells [36, 37]. Whether and how lipid droplets in a cell may contribute to migration is a possible new area for investigation.
In summary, we showed that provision of lipids to the culture medium is required for migration of the highly motile TN breast cancer cell line MDA-MB-231. Further, we demonstrated that pharmacological inhibition of cholesterol esterification reduced migration in the presence of exogenous lipids. Tumors with a similar phenotype, characterized by high expression of ACAT1 and LDLR, might also depend on exogenous lipids for their aggressive behavior. Higher circulating lipids would support the growth and metastasis of these types of tumors, which could be targeted with ACAT inhibitors, lipid lowering therapies or diet.
Abbreviations
- CE:
-
Cholesteryl ester
- LD:
-
Lipoprotein depleted
- TAG:
-
Triacylglycerol
- TN:
-
Triple negative
References
Sleeman J, Steeg PS (2010) Cancer metastasis as a therapeutic target. Eur J Cancer 46:1177–1180
Patterson RE, Cadmus LA, Emond JA, Pierce JP (2010) Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas 66:5–15
Cleary MP, Grossmann ME (2009) Minireview: obesity and breast cancer: the estrogen connection. Endocrinology 150:2537–2542
Renehan AG, Frystyk J, Flyvbjerg A (2006) Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab 17:328–336
Rotheneder M, Kostner GM (1989) Effects of low- and high-density lipoproteins on the proliferation of human breast cancer cells in vitro: differences between hormone-dependent and hormone-independent cell lines. Int J Cancer 43:875–879
Chajes V, Mahon M, Kostner GM (1996) Influence of LDL oxidation on the proliferation of human breast cancer cells. Free Radic Biol Med 20:113–120
Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y (2003) Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem 278:31861–31870
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Antalis CJ, Arnold T, Rasool T, Lee B, Buhman KK, Siddiqui RA (2010) High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast Cancer Res Treat 122:661–670
Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S (2006) X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell 9:121–132
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009
Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, Duss S, Nicoulaz AL, Brisken C, Fiche M, Delorenzi M, Iggo R (2005) Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 24:4660–4671
van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10:515–527
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363:1938–1948
Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J (2008) Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 68:989–997
Antalis CJ, Arnold T, Lee B, Buhman KK, Siddiqui RA (2009) Docosahexanoic acid is a substrate for ACAT1 and inhibits cholesteryl ester formation from oleic acid in MCF-10A cells. Prostaglandins Leukot Essent Fatty Acids 80:165–171
Vitols S, Gunven P, Gruber A, Larsson O (1996) Expression of the low-density lipoprotein receptor, HMG-CoA reductase, and multidrug resistance (Mdr1) genes in colorectal carcinomas. Biochem Pharmacol 52:127–131
Haeffner EW, Hoffmann CJ, Stoehr M, Scherf H (1984) Cholesterol-induced growth stimulation, cell aggregation, and membrane properties of ascites tumor cells in culture. Cancer Res 44:2668–2676
Vitols S, Gahrton G, Ost A, Peterson C (1984) Elevated low density lipoprotein receptor activity in leukemic cells with monocytic differentiation. Blood 63:1186–1193
Paillasse MR, de Medina P, Amouroux G, Mhamdi L, Poirot M, Silvente-Poirot S (2009) Signaling through cholesterol esterification: a new pathway for the cholecystokinin 2 receptor involved in cell growth and invasion. J Lipid Res 50:2203–2211
Brown MS, Goldstein JL (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331–340
Chen JK, Li L, McClure DB (1988) Altered low density lipoprotein receptor regulation is associated with cholesteryl ester accumulation in Simian virus 40 transformed rodent fibroblast cell lines. In Vitro Cell Dev Biol 24:353–358
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12:R68
Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM (2004) ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6:1–6
Roberts PJ, Der CJ (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26:3291–3310
Krueger JS, Keshamouni VG, Atanaskova N, Reddy KB (2001) Temporal and quantitative regulation of mitogen-activated protein kinase (MAPK) modulates cell motility and invasion. Oncogene 20:4209–4218
Kapoor GS, Atkins BA, Mehta KD (2002) Activation of Raf-1/MEK-1/2/p42/44(MAPK) cascade alone is sufficient to uncouple LDL receptor expression from cell growth. Mol Cell Biochem 236:13–22
Abeyweera TP, Chen X, Rotenberg SA (2009) Phosphorylation of alpha6-tubulin by protein kinase Calpha activates motility of human breast cells. J Biol Chem 284:17648–17656
Brenneman DE, McGee R, Spector AA (1974) Cholesterol metabolism in the Ehrlich ascites tumor. Cancer Res 34:2605–2611
Bostrom P, Rutberg M, Ericsson J, Holmdahl P, Andersson L, Frohman MA, Boren J, Olofsson SO (2005) Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler Thromb Vasc Biol 25:1945–1951
Welte MA (2009) Fat on the move: intracellular motion of lipid droplets. Biochem Soc Trans 37:991–996
Zehmer JK, Huang Y, Peng G, Pu J, Anderson RG, Liu P (2009) A role for lipid droplets in inter-membrane lipid traffic. Proteomics 9:914–921
Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, Gilpin C, Chapman KD, Anderson RG, Goodman JM (2006) An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol 173:719–731
Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP (2008) Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res 68:1732–1740
Navarro-Tito N, Soto-Guzman A, Castro-Sanchez L, Martinez-Orozco R, Salazar EP (2010) Oleic acid promotes migration on MDA-MB-231 breast cancer cells through an arachidonic acid-dependent pathway. Int J Biochem Cell Biol 42:306-317
Soto-Guzman A, Navarro-Tito N, Castro-Sanchez L, Martinez-Orozco R, Salazar EP (2010) Oleic acid promotes MMP-9 secretion and invasion in breast cancer cells. Clin Exp Metastasis 27:505–515
Acknowledgments
The authors would like to thank Cary Mariash for helpful discussions and critique of the manuscript. We thank Pfizer, Inc., for providing the ACAT inhibitor CP-113818. This study was supported by a Clarian Values Fund for Research grant (to CJA) and the Methodist Research Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antalis, C.J., Uchida, A., Buhman, K.K. et al. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin Exp Metastasis 28, 733–741 (2011). https://doi.org/10.1007/s10585-011-9405-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9405-9