Abstract
We previously demonstrated that the detection of circulating cancer cells (CCC) expressing survivin mRNA could provide valuable information for predicting recurrence in patients with breast, lung, gastric and colorectal carcinoma. The purpose of this study is to investigate whether the detection of survivin-expressing CCC in the peripheral blood is also useful for predicting recurrence in patients with esophageal squamous cell carcinoma (ESCC). Blood samples obtained from 108 ESCC patients and 75 healthy volunteers were quantitatively investigated by a technique that detected reverse transcription-polymerase chain reaction products based on a hybridization-enzyme linked immunosorbent essay. Not all of the patients were available for the follow-up study. Only 48 patients who were treated with similar adjuvant therapy regimens were available and followed-up for 33 months after the initial assay test. Survivin-expressing CCC were detected in 51 (47.2%) patients. The presence of survivin-expressing CCC was found to be significantly associated with depth of invasion, vascular invasion, nodal status, and disease stages (P = 0.032, 0.019, 0.018, and 0.001, respectively). During the follow-up period, patients who had positive survivin expressions had a higher relapse rate and a shorter survival time than those who had negative survivin expressions (P = 0.002 and 0.016, respectively). Examination of survivin-expressing CCC could provide valuable information in the prediction of haematogenous recurrence as well as in the prognosis of ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most malignant diseases in the world [1]. Once diagnosed, the prognosis is often poor despite recent advancements made in multidisciplinary treatments. Disease stages and nodal status are the two most important prognostic factors for determining clinical outcomes of ESCC [2]. However, patients with similar disease stages or nodal status tend to have noticeable discrepancies in their survival rates. This occurs because patients with ESCC frequently suffer from recurrent disease even after a curative resection. The reported recurrence rate ranges from 34 to 79% [3]. In light of this, new diagnostic procedures are needed in order to early detect recurrence and metastasis, and to improve the prognosis of patients with ESCC.
Patients who had undergone potentially curative surgeries retain the risk of recurrence mainly because of tumor dissemination via the blood or lymphatic circulation. In recent years, reported studies have used RT-PCR and variations of this technique to detect circulating cancer cells in ESCC patients by evaluating tumor marker expressions in their peripheral blood. Tumor markers such as cytokeratin 19, cytokeratin 20, squamous cell carcinoma antigen (SCC-Ag), deltaNp63, and carcinoembryonic antigen have all been used with varying degrees of sensitivity and specificity [4–10]. However, these studies have also revealed a major problem in that some of these tumor markers are expressed in normal cells of the peripheral blood and frequently in normal epithelial cells as well. This inevitably leads to false-positive results that greatly diminish the correlation between tumor markers and some of the well-known clinical and pathological prognostic factors. In order to avoid false-positive results, careful selection must be made on a particular tumor marker that is uniquely expressed in cancer cells but not in normal cells.
Survivin, a novel inhibitor of the apoptosis protein family, has been found to be expressed in tissues during fetal development and in many common human carcinomas such as ESCC [11]. However, it is rarely found in normal tissues [12]. By detecting survivin expressions in the primary lesion, several studies have been conducted which suggest that survivin might be an ideal tumor marker in the diagnosis and prognosis of ESCC [13–15].
In our previous studies, we demonstrated that: (1) survivin-expressing CCC were detected in about 50% of peripheral blood samples from patients with breast, lung, gastric and colorectal carcinoma, but not in the healthy volunteers that were used as controls; (2) the presence of survivin-expressing CCC was found to be significantly associated with various clinicopathological parameters; and (3) the detection of circulating cancer cells expressing survivin mRNA could provide valuable information for predicting metastasis and recurrence in these patients [16–18].
In our current study, we examined whether or not the detection of survivin-expressing CCC in patients with ESCC could also be used for predicting metastasis and recurrence. To do so, survivin-expressing CCC were analyzed in 108 patients with ESCC. Their correlation with various clinicopathological parameters and their value in predicting metastasis and recurrence were subsequently evaluated.
Materials and methods
Patients and sample collection
We investigated 108 cases of ESCC diagnosed and treated between 2002 and 2005 at three different hospitals located in Chengdu, Sichuan, P. R. China. The mean age of the patients at the time of diagnosis was 58.9 years old (range: 36–82 years), and there were 85 males and 23 females. Seventy-five healthy volunteers, who were confirmed to be cancer-free through the use of clinical and imaging examinations, were used as controls in this study. The mean age of the healthy controls was 44.9 years old (range: 36–69 years), and there were a total of 59 males and 16 females. In addition, 15 patients with benign esophageal disease were used as another control group in the study. The mean age of this group was 54.8 years old (range: 32–70 years), and there were 12 males and 3 females.
None of the patients had any pre-operative chemotherapy or radiotherapy. However, after surgery, patients in disease stages I–III received radiotherapy or oral 5-fluorouracil, and patients in disease stage IV received a combination of radiotherapy and 5-fluorouracil-based chemotherapy. The clinicopathological findings were determined according to the classification of malignant tumors as set out by the World Health Organization [19] and the International Union Against Cancer Tumor-Node-Metastasis (TNM) staging system [20]. Out of the 108 ESCC patients, 11 (10.2%) were classified as being in stage I, 32 (29.6%) were considered to be in stage II, 57 (52.8%) were in stage III, and 8 (7.4%) were consigned to stage IV. After the initial assay test, not all patients were available for the follow-up investigation. Only 48 patients who were treated with similar adjuvant therapy regimens were available and followed-up for an average of 19.5 months (range: 1–33 months). The study was approved and monitored by the ethics committee at each of the three hospitals.
A 2 ml sample of peripheral blood from all of the subjects was collected into test tubes containing sodium citrate, which were then sent to the laboratory for immediate processing. All of the samples we analyzed were obtained at the time of diagnosis before any curative resection in patients with stages I–III, or any systemic therapy in patients with stage IV. Furthermore, in order to observe the effects that multiple sampling has on predicting disease recurrence, another set of peripheral blood samples were collected from 23 cancer patients who had undergone a curative resection during the follow-up period. In these patients, six were in stage I/II and 17 were in stage III at the time of the initial assay test.
Detection of circulating cancer cells by RT-PCR ELISA
Detailed procedures for measuring survivin mRNA in the peripheral blood by using the RT-PCR ELISA technique can be found in our previous report concerning breast cancer [16]. The same procedures were utilized here to detect circulating cancer cells in patients with ESCC. Briefly, total RNA of whole blood cells was extracted using a Trizol kit (Invitogen, Carlsbad, CA, USA) and cDNA was synthesized from 5 μg of the total RNA samples. Survivin mRNA fragment in the blood cells was then amplified with fluorescein-labeled SUF and SUB primers to generate fluorescein-labeled PCR products. A pQE-30UA/survivin plasmid diluted in serial concentrations was used as standards and amplified together with the samples in the same batch of assay.
Afterwards, fluorescein-labeled PCR product was added into probe-coated microtiter plates/strips, initially denatured by NaOH/thymol blue solution and hybridized to the coated probe. Finally, the plates/strips were washed and the specifically hybridized survivin-fragment was detected by adding anti-fluorescein antibody-HRP conjugates (Roche, Basel, Switzerland), and 3, 3′, 5, 5′-tetramethylbenzidine (Sigma, St. Louis, MO, USA) was added after subsequent washes. The plates/strips were read at 450/630 nm using a microplate reader (Bio-TEK, Winooski, VT, USA) after stopping the color reaction with HCl. Survivin mRNA concentrations in each of the samples were determined by comparing to the standards with the results being expressed in pg/ml.
Quantitative real-time RT-PCR
Survivin mRNA concentrations from 48 peripheral blood samples were also determined using quantitative real-time RT-PCR on an ABI Prism 7900 HT Sequence Detection System (Perkin-Elmer, Foster City, CA, USA). Initially, 2 μl of cDNA, 5 μl of Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 400 nM of forward primer, 400 nM of reverse primer, 200 nM of probe, and nuclease-free water were all combined to create a total volume of 10 μl in a 384-well plate. The thermal cycling conditions were set to 95°C for 10 min, then 40 cycles of 95°C for 15 s and 53°C for 1 min. In the RT-PCR reaction, a 77-nt PCR product located at nt 130–206 of the survivin mRNA was amplified with a forward primer 5′-TCCACTGCCCCACTGAGAAC-3′ and a reverse primer 5′-TGGCTCCCAGCCTCCA-3′. The survivin probe (5′-CACTGCCCCACTTAGAACGAGCC-3′) was designed using the ABI Primer Express software. A standard curve was calculated using linear regression analysis after the pQE-30UA/survivin plasmid in 2-fold serial dilutions was amplified together with the samples in the same batch of assay. The standard curve showed a linear relationship between the threshold cycle (Ct) values and the logarithm of the initial input plasmid copy numbers. The amount of product in a particular sample was determined by interpolation from the standard curve of Ct values generated from the plasmid dilution series. All RT-PCR assays were done in duplicate and reported as the mean.
Plasma SCC-Ag ELISA
Plasma SCC-Ag concentrations in patients with ESCC were determined by an enzyme immunoassay test kit (Fujirebio Diagnostics AB, Göteborg, Sweden). The assays were performed according to the manufacturer’s protocol. A value of 1.2 ng/ml was viewed as being positive in accordance with the instructions.
Detection of vascular invasion in tumors
Vascular invasion was assessed by measuring microvessel density (MVD) in 86 cases of tumors that were performed with anti-CD34 immunohistochemical stain [21]. The medium value of MVD for all of the cases was 32.3 (range: 18.7–48.2). Thirty-three cases were defined as having high MVD (>32.3), while 53 others were defined with low MVD.
Statistical analysis
Statistical analysis was performed using the SPSS software package (Abacus Concepts, Berkeley, CA, USA). Differences of survivin expression in the peripheral blood between healthy controls, benign disease cases, and patients with early stages or metastatic cancer were compared with using the One-way Analysis of Variance. The One-way Analysis of Variance, the Student’s t-test, and the Pearson Chi-square test were used to determine the association between the detection of survivin mRNA and various clinicopathological parameters. A receiver operating characteristic (ROC) curve analysis was performed to assess the feasibility of using the detection of survivin-expressing CCC as an indicator for predicting metastasis. Binary logistic regression analysis was used to further compare the detection of survivin-expressing CCC as a risk factor against other clinical parameters for metastasis. The Kaplan–Meier method was introduced to estimate recurrence and survival rates as a function of time. Recurrence and survival rates were analyzed using a log-rank test. The Cox proportional hazard model was used to identify prognostic factors for recurrence and survival. Plasma SCC-Ag measurements were also analyzed using the same set of statistical methods. A comparison of the sensitivity and accuracy in predicting metastasis and recurrence between survivin-expressing CCC detection and plasma SCC-Ag measurement was analyzed using Chi-square analysis. P < 0.050 was considered to be statistically significant.
Results
Comparison of RT-PCR ELISA with quantitative real-time RT-PCR
In our previous study on breast cancer, we compared the results obtained from our RT-PCR ELISA with those assayed from a regular RT-PCR in which β-actin cDNA was used as internal control. Survivin expression in each of the samples was first represented as a ratio of survivin to β-actin as determined by a 1.2% agarose gel electrophoresis stained with ethidium bromide, and then analyzed with a Molecular Dynamics laser densitometer. Those results showed that there was a significant correlation between the two methods (r = 0.9378) [16]. In our current study on ESCC, we compared survivin mRNA concentrations from 48 peripheral blood samples assayed by our RT-PCR ELISA with those assayed by a quantitative real-time RT-PCR. These results showed that there was also a significant correlation between the two methods that we used here (y = 0.063 + 0.279x, r = 0.971, P < 0.0001), which further validates the effectiveness of our RT-PCR ELISA technique.
Measurement of survivin-expressing CCC in peripheral blood samples
By using our RT-PCR ELISA technique, survivin mRNA concentration in the peripheral blood samples of healthy controls was determined to range from 0 to 0.96 pg/ml with a mean of 0.442 pg/ml, while the concentration in the benign disease cases ranged from 0 to 0.98 pg/ml with a mean of 0.59 pg/ml. In patients with early stages of ESCC, survivin concentration was detected to range from 0 to 1.39 pg/ml with a mean of 0.694 pg/ml. In patients with metastatic cancer, survivin concentration was found to range from 0 to 4.79 pg/ml with a mean of 1.64 pg/ml. Comparing the cancer results with the control groups, a significant statistical difference was found (One-way analysis of variance: F = 46.7, P < 0.001; Fig. 1). However, no significant difference was found between healthy controls and benign disease cases (P = 0.481).
Comparison of survivin mRNA concentrations as determined by RT-PCR ELISA for healthy controls (I), benign disease cases (II), early stages (III), and metastatic ESCC patients (IV). The mean value of survivin concentration in healthy controls was 0.442 pg/ml. A cutoff value of 0.96 pg/ml was determined when the specificity was set to 100% according to ROC curve analysis. Statistical analysis showed that there was a significant difference amongst the four groups (P < 0.001)
Association of survivin-expressing CCC with various clinicopathological parameters
The association between survivin mRNA concentration and various clinicopathological parameters such as age, gender, tumor location, MVD, depth of invasion, nodal status, and disease stages was investigated thoroughly. As evident from the data in Table 1, the association between survivin mRNA concentration and nodal status and disease stages were statistically significant. However, no significant association was found between survivin concentration and the other parameters. To further corroborate the above findings, comparison of positive rates of survivin detection was carried out using Chi-square analysis according to a cutoff value of 0.96 pg/ml as established by ROC curve analysis (see below) for the various subgroups. The association between positive rates of survivin detection and the clinicopathological parameters of MVD, depth of invasion, nodal status, and disease stages were found to be statistically significant. However, this significance did not extend to the parameters of age, gender or tumor location.
Significance of survivin-expressing CCC detection in predicting metastasis
To distinguish between healthy controls and cancer patients, ROC curve analyses were performed. The area under the ROC curve was 0.773 for ESCC patients versus healthy controls, and 0.908 for early stage patients versus patients with metastatic cancer. Using binary logistic regression analysis, survivin-expressing CCC detection was shown to be the only statistically significant risk factor for metastasis (P = 0.019; Table 2). The above results indicate that the detection of survivin-expressing CCC may be an indicator for the existence of metastasis in ESCC patients.
Significance of survivin-expressing CCC detection in predicting recurrence
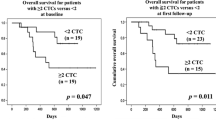
During the entire follow-up period, 18 out of the 48 follow-up patients suffered a relapse. Of the 48 patients, 12 were in disease stage I/II and 36 were in stage III. Survivin-expressing CCC were detected in the peripheral blood samples of 27 out of the 48 patients, 21 of whom had metastasis and six were in the early stages of the disease at the time of the initial assay. Regarding the 27 cases of positive survivin expression, 15 eventually suffered a relapse within the follow-up period. In those recurrent cases, three patients were in the early stages of the disease. By contrast, for the 21 patients who did not show the presence of survivin-expressing CCC in their peripheral blood samples, only three suffered from any form of relapse. Of these, six were in the early stages and 15 had metastatic cancer. In the entire cohort, the cumulative recurrence rate for patients with positive survivin expressions was significantly higher than those with negative expressions (P = 0.002, log-rank test; Fig. 2). Moreover, a multivariate Cox regression analysis showed that the parameters of nodal status and disease stages along with the detection of survivin-expressing CCC had a significant effect on the recurrence rate (Table 3). All these analyses suggest that the detection of survivin-expressing CCC can be used as an independent predictor for cancer recurrence in ESCC patients.
In the investigation that examined the effects that multiple sampling has on predicting recurrence, survivin-expressing CCC were detected in 12 out of the 23 cases initially. After the curative resection, survivin-expressing CCC were detected in 14 patients, of which seven were survivin-positive and seven were survivin-negative at the time of the initial assay test. Regarding the 14 patients who had a positive survivin expression, 10 eventually suffered a relapse. However, for the remaining nine patients who had a negative survivin expression, only two suffered a relapse, of which one was survivin-positive and one was survivin-negative at the time of the initial assay test. The recurrent rate for patients whose blood samples showed positive survivin expressions in the initial assay test or during the follow-up was significantly higher than those whose blood samples showed negative survivin expressions (P = 0.021, Chi-square test). These results indicate that the use of multiple sampling may improve the predictive value of survivin-expressing CCC detection for disease recurrence.
Correlation of survivin-expressing CCC with patient survival
Within the follow-up period of 33 months, there were 21 cancer-related deaths in the total follow-up population of 48. Six deaths came from the 21 patients who had a negative detection of survivin-expressing CCC, while 15 deaths were from the 27 patients who had a positive detection. In the entire cohort, the overall survival rate for patients who had tested negative was significantly higher (P = 0.016, log-rank test; Fig. 3). In addition, a multivariate Cox regression analysis again showed that the parameters of nodal status and disease stages along with the detection of survivin-expressing CCC had a significant effect on the survival rate (Table 3). The analyses suggest that the detection of survivin-expressing CCC can also be used as an independent prognostic factor for patients with ESCC.
Comparison with plasma SCC-Ag
To compare the efficiency of survivin-expressing CCC detection, measurements of plasma SCC-Ag, considered to be a tumor marker for ESCC [22], were included in our study. Concentrations of plasma SCC-Ag were 0.19 ± 0.04 ng/ml in healthy controls, 3.51 ± 1.31 and 0.42 ± 0.12 ng/ml in patients with and without metastatic cancer, respectively. The differences between healthy controls and cancer patients and between early stage patients and patients with metastatic cancer were all statistically significant (P = 0.008 for healthy controls versus ESCC patients, and P = 0.020 for early stage patients versus patients with metastatic cancer). The area under the ROC curve was 0.717 for early stages versus later stages of ESCC. Comparisons of the sensitivity and accuracy between survivin-expressing CCC detection and plasma SCC-Ag measurement in terms of predicting metastasis and recurrence are shown in Table 4. There was no statistical significance in the accuracy between the two methods. However, the sensitivity of survivin-expressing CCC detection was significantly higher than that of the plasma SCC-Ag measurement (P = 0.017 and 0.003, respectively). Furthermore, plasma SCC-Ag measurement was not a significant indicator for metastasis in the binary logistic regression analysis (Table 2), or an independent predictive factor for recurrence and prognosis in the multivariate Cox regression analysis (Table 3). All in all, these results showed that the detection of survivin-expressing CCC was more sensitive than the measurement of plasma SCC-Ag in predicting metastasis and recurrence in ESCC.
Discussion
Previously, we had demonstrated our RT-PCR ELISA technique to be a specific, sensitive, reproducible and yet simple procedure for quantitatively measuring survivin mRNA in the peripheral blood cells of breast cancer patients [16]. In this study, we further validated our RT-PCR ELISA technique by comparing the results against those obtained from a quantitative real-time RT-PCR. Compared to the real-time RT-PCR, our technique used less expensive commercial reagents and equipment such as an ELISA reader. As such, our RT-PCR ELISA technique may prove to be a reliable alternative for detecting gene expression.
Esophageal cancer is notorious for its aggressive biological behavior, local infiltration, involvement in adjacent lymph nodes, and a wide metastasis through haematogenous spread. It has been reported that the frequency of haematogenous recurrence was remarkably high despite radical surgery with lymph node dissection [23]. In this regard, the detection of cancer cells in the blood could be important in identifying patients with high risks of relapse [24]. However, most circulating cancer cells are apoptotic [25], and recurrence is a complex process in which disseminated cancer should be a phenotype tending to be more successful in establishing metastastic tumors.
Recently, the concept of a “migrating cancer stem cell” has been proposed and developed in [26]. The main characteristics of this model are the mobilized cancer stem cells that arise from nonmobilized cancer stem cells through persistence of activation of stemness and initiation of epithelial-mesenchymal transition (EMT). Aberrant activation of Wnt pathway is involved in both stem cell formation and the induction of EMT. One of the features of Wnt pathway activation is the nuclear accumulation of its main effector β-catenin. Stimulation of target gene expression such as survivin by β-catenin might impose a stem cell-like phenotype in tumor cells [27]. Moreover, several recent studies have examined survivin expression in ESCC and found that it was expressed in the majority of cases, and its presence led to unfavorable clinical outcomes [11, 13, 14]. The results, together with the finding that survivin was not expressed in normal blood cells [28], allowed us to hypothesize that the detection of survivin-expressing CCC in peripheral blood samples might be an ideal tumor marker for identifying migrating cancer stem cells, for predicting the development of metastasis and recurrence, and for estimating prognosis.
Certainly, the results of this study showed that the detection of survivin-expressing CCC was significantly associated with nodal status and disease stages, which are considered to be the most important prognostic indicators for determining clinical outcomes of esophageal cancer. In the follow-up study, the majority of recurrent cases for ESCC came from patients who had a positive detection of survivin-expressing CCC at the time of the initial assay test regardless of disease stages. Results from the binary logistic regression analysis also demonstrated that the detection status of survivin-expressing CCC was a strong indicator for metastasis in ESCC, and the multivariate analyses further indicated that the detection status of survivin-expressing CCC together with nodal status and disease stages could form strong and independent predictors for recurrence as well as prognosis.
All of these results lend credence to the idea that the detection of survivin-expressing CCC in ESCC has an important clinical application in that it can be used to early identify patients with high risks of relapse. Nevertheless, to fully substantiate the concept, further investigations with a larger population and a longer follow-up period after the initial assay test are needed.
Measurement of plasma SCC-Ag concentrations has been suggested to be a useful prognostic indicator for ESCC [22]. However, plasma SCC-Ag did not appear to be a significant indicator for either metastasis or recurrence in our study. Interestingly, the detection of survivin-expressing CCC was shown to be more sensitive in predicting metastasis and recurrence than the measurement of plasma SCC-Ag. This may be due to the fact that plasma SCC-Ag is also expressed in a variety of normal tissues, benign tumors, and non-neoplastic diseases [29], which limits its efficiency in clinical applications.
In summary, similar to our previous studies on breast, lung, gastric and colorectal carcinoma, we have demonstrated that: (1) survivin-expressing CCC was detected in the peripheral blood of patients with ESCC; (2) the presence of survivin-expressing CCC was significantly associated with the clinicopathological parameters of depth of invasion, nodal status and disease stages; (3) the detection of survivin-expressing CCC could provide valuable information in the prediction of metastasis and recurrence as well as in the prognosis of ESCC; and (4) the method of detecting survivin-expressing CCC in ESCC patients was more sensitive than traditional methods such as the measurement of plasma SCC-Ag for the early diagnosis of metastasis and recurrence. Nevertheless, as mentioned before, a more definitive answer on these findings will require further investigations into assessing cancer recurrence on a larger number of cohort patients. Likewise, additional in vitro experiments and studies on animal models are needed to validate the hypothesis that the detection of circulating cancer cells expressing survivin mRNA is more successful in establishing metastatic tumors.
References
Patel M, Ferry K, Franceschi D et al (2004) Esophageal carcinoma: current controversial topics. Cancer Invest 22:897–912. doi:10.1081/CNV-200039672
Roder JD, Stein HJ, Siewert JR (1995) Oesophageal carcinoma. In: Hermaneck P, Gospodarowicz MK, Henson DE (eds) Prognostic factors in cancer, UICC. Springer-Verlag, Berlin, p 37
Lee SJ, Lee KS, Yim YJ et al (2005) Recurrence of squamous cell carcinoma of the oesophagus after curative surgery: rates and patterns on imaging studies correlated with tumour location and pathological stage. Clin Radiol 60:547–554. doi:10.1016/j.crad.2004.09.002
Huang P, Wang J, Guo Y et al (2003) Molecular detection of disseminated tumor cells in the peripheral blood in patients with gastrointestinal cancer. J Cancer Res Clin Oncol 129:192–198
Ikoma D, Ichikawa D, Ueda Y et al (2007) Circulating tumor cells and aberrant methylation as tumor markers in patients with esophageal cancer. Anticancer Res 27:535–539
Ito H, Kanda T, Nishimaki T et al (2004) Detection and quantification of circulating tumor cells in patients with esophageal cancer by real-time polymerase chain reaction. J Exp Clin Cancer Res 23:455–464
Kaganoi J, Shimada Y, Kano M et al (2004) Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg 91:1055–1060. doi:10.1002/bjs.4593
Koike M, Hibi K, Kasai Y et al (2002) Molecular detection of circulating esophageal squamous cell cancer cells in the peripheral blood. Clin Cancer Res 8:2879–2882
Liu Z, Jiang M, Zhao J et al (2007) Circulating tumor cells in preoperative esophageal cancer patients: quantitative assay system and potential clinical utility. Clin Cancer Res 13:2992–2997. doi:10.1158/1078-0432.CCR-06-2072
Nakashima S, Natsugoe S, Matsumoto M et al (2003) Clinical significance of circulating tumor cells in blood by molecular detection and tumor markers in esophageal cancer. Surgery 133:162–169. doi:10.1067/msy.2003.9
Chiou SK, Jones MK, Tarnawski AS (2003) Survivin–an anti-apoptosis protein: its biological roles and implications for cancer and beyond. Med Sci Monit 9:125–129
Ambrosini G, Adida C, Altieri D (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921. doi:10.1038/nm0897-917
Grabowski P, Kühnel T, Mühr-Wilkenshoff F et al (2003) Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br J Cancer 88:115–119. doi:10.1038/sj.bjc.6600696
Mega S, Miyamoto M, Li L et al (2006) Immunohistochemical analysis of nuclear survivin expression in esophageal squamous cell carcinoma. Dis Esophagus 19:355–359. doi:10.1111/j.1442-2050.2006.00604.x
Rosato A, Pivetta M, Parenti A et al (2006) Survivin in esophageal cancer: an accurate prognostic marker for squamous cell carcinoma but not adenocarcinoma. Int J Cancer 119:1717–1722. doi:10.1002/ijc.21923
Yie SM, Luo B, Ye NY et al (2006) Detection of Survivin-expressing circulating cancer cells in the peripheral blood of breast cancer patients by a RT-PCR ELISA. Clin Exp Metastasis 23:279–289. doi:10.1007/s10585-006-9037-7
Yie SM, Lou B, Ye SR et al (2008) Detection of survivin-expressing circulating cancer cells (CCCs) in peripheral blood of patients with gastric and colorectal cancer reveals high risks of relapse. Ann Surg Oncol 15:3037–3082. doi:10.1245/s10434-008-0069-x
Yie SM, Lou B, Ye SR et al (2009) Clinical significance of detecting survivin-expressing circulating cancer cells in patients with non-small cell lung cancer. Lung Cancer 63:284–290. doi:10.1016/j.lungcan.2008.05.024
Watanable H, Jass JR, Sobin LH (eds) (1990) Histological typing of esophageal and gastric tumors. Springer-Verlag, Berlin
Sobin H, Wittekind C (eds) (2002) UICC: TNM classification of malignant tumors, 6th edn. Wiley, New York
Yonenaga Y, Mori A, Onodera H et al (2005) Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology 69:159–166
Mino-Miyagawa N, Kimura Y, Hamamoto K (1990) Tumor-antigen 4: its immunohistochemical distribution and tissue and serum concentrations in squamous cell carcinoma of the lung and esophagus. Cancer 66:1505–1512. doi:10.1002/1097-0142(19901001)66:7<1505::AID-CNCR2820660712>3.0.CO;2-V
Katayama A, Mafune K, Tanaka Y et al (2003) Autopsy findings in patients after curative esophagectomy for esophageal carcinoma. J Am Coll Surg 196:866–873. doi:10.1016/S1072-7515(03)00116-9
Jiao X, Krasna MJ (2002) Clinical significance of micrometastasis in lung and esophageal cancer: a new paradigm in thoracic oncology. Ann Thorac Surg 74:278–284. doi:10.1016/S0003-4975(01)03376-8
Mehes G, Witt A, Kubista E et al (2001) Circulating breast cancer cells are frequently apoptotic. Am J Pathol 159:17–20
Brablets T, Jung A, Spaderna S et al (2005) Migrating cancer stem cells-an integrated concept of malignant tumour progression. Nat Rev Cancer 5:744–749. doi:10.1038/nrc1694
Zhang T, Otevrel T, Gao ZQ et al (2001) Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res 61:8664–8667
Moriai R, Asanuma K, Kobayashi D et al (2001) Quantitative analysis of the anti-apoptotic gene survivin expression in malignant haematopoietic cells. Anticancer Res 21:595–600
Torre GC (1998) SCC antigen in malignant and nonmalignant squamous lesions. Tumour Biol 19:517–526. doi:10.1159/000030045
Acknowledgements
This study was partially supported by grants from the Chengdu Municipal Department of Science and Technology (Grant No. 07GGYB240SF-143), and the Sichuan Provincial Department of Science and Technology (Grant No. 07JY029-091).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, M., Yie, SM., Wu, SM. et al. Detection of survivin-expressing circulating cancer cells in the peripheral blood of patients with esophageal squamous cell carcinoma and its clinical significance. Clin Exp Metastasis 26, 751–758 (2009). https://doi.org/10.1007/s10585-009-9274-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-009-9274-7