Abstract
Aim The aim of our study was to compare the influence of partial hepatectomy, intra-arterial treatment with 3-BrPA and TACE on regional and distant metastases. In order to achieve our objective, we tested the feasibility of both resection, intra-arterial therapy with 3-BrPA and TACE of VX2 liver cancer in New Zealand White rabbits. Methods VX2 tumors were implanted in the left lateral lobe of the liver of 20 rabbits. Tumors were allowed to grow for 14 days. Rabbits were divided in four groups. Group 1 (n = 2) was sacrificed 14 days post implantation. Group 2, 3 and 4 (n = 6) underwent left lateral hepatectomy, a 1 h intra-arterial infusion with 3-BrPA and TACE respectively. Animals in each group were further subdivided into three groups of two animals each corresponding to the time-point of sacrifice after the procedure (7, 14 and 21 days respectively). After sacrifice, organs were harvested, fixed and analyzed. Results Pathologic examination showed lung metastases in all 20 rabbits. Abdominal cavity dissemination was seen in five rabbits in Group 2, two rabbits in Group 3 and all rabbits in Group 4. Kidney metastases were seen in two rabbits treated with TACE. Conclusion The VX2 rabbit model of liver cancer is a suitable model to compare the influence of partial hepatectomy, intra-arterial treatment with 3-BrPA and TACE on tumor recurrence in the form of regional and distant metastases. Our results indicate that intra-arterial delivery of 3-BrPA may result in a favorable metastatic profile when compared to both liver resection and TACE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cause of mortality from cancer worldwide and is responsible for about one million deaths yearly [1, 2]. The number of cases of HCC will rise dramatically over the next 10–15 years in North America because of the high prevalence of chronic hepatitis infections [3]. Liver resection and transplantation are currently considered the only two potentially curative treatments for this cancer [4, 5].
For the treatment of unresectable HCC or intrahepatic recurrences, several effective therapeutic modalities (such as transcatheter arterial chemoembolization (TACE) and radiofrequency ablation) are widely employed [6]. Currently, however, TACE is not utilized in patients with resectable disease confined to the liver. From an oncology point of view, evaluating the therapeutic efficacy of TACE for patients with resectable disease requires a comparison with resection in a randomized controlled study. However, such an approach would raise critical ethical concerns. Therefore, a suitable animal model, to compare these two treatment modalities is of high importance. This animal model should allow direct access to the tumor feeding artery, in order to provide a route for intra-arterial infusion. The VX2 liver tumor model in the rabbit is currently the most suitable animal model that allows access to the tumor feeding hepatic artery, which is necessary to study intra-arterial therapies. Unfortunately, human HCC does not grow in a rabbit liver, due to the rabbit’s intact immune system.
With the recent progress in diagnostic modalities, pre- and postoperative treatment, and operative techniques, the results of resection and TACE for patients with HCC have been improving steadily [7, 8]. However, the long-term prognosis remains poor in most series, with 5-year overall survival rates of 33–69% and 5-year recurrence rates approaching 70% after resection [9–12]. Tumor recurrence may present in the form of metastatic foci in distant organs, such as the lungs, brain, and bone [13]. Therefore it is important to identify new aggressive agents for patients with HCC. One such novel agent, 3-bromopyruvate (3-BrPA), a known inhibitor of glycolysis, has proven to be effective in the treatment of VX2 liver cancer in rabbits, when administered intra-arterially [14].
The aim of our study was to compare the influence of partial hepatectomy, intra-arterial treatment with 3-BrPA and TACE on tumor recurrence in the form of regional and distant metastases. In order to achieve our objective, we tested the feasibility of both resection, intra-arterial therapy with 3-BrPA and TACE of VX2 liver cancer in New Zealand White rabbits.
Materials and methods
All animals received human care and all the experimental procedures were performed according to our Institutional Guidelines.
Tumor implantation
Adult New Zealand White rabbits weighing 8–9 lbs (n = 20; Myrtle’s Rabbitry, Thompson Station, TN) were used for this study. For successful implantation of the VX2 tumor into the liver, the tumor was first grown for 2 weeks in the hind leg of a carrier rabbit. Before tumor implantation all rabbits were anesthetized with a mixture of acepromazine (2.5 mg/kg, Phoenix, St. Joseph, MO) and ketamine hydrochloride (44 mg/kg, Phoenix) administered i.m.; i.v. access was gained via a marginal ear vein and sodium pentobarbital (Abbott Laboratories, Abbott Park, IL) was given to maintain anesthesia. The abdomen was shaved and prepped in a sterile fashion. A midline subxyphoid incision was made. The left lateral lobe of the liver was exposed and the VX2 tumor excised from a carrier rabbit was minced and implanted into the left lobe of the liver using the outer cannula of a 21-gauge angiocatheter. The abdomen was closed in two layers.
Experimental design
All rabbits underwent ultrasound examination to confirm the presence of a liver tumor 14 days after implantation. Rabbits were divided in four groups. Group 1 (n = 2) was sacrificed 14 days post implantation to assess for the presence of metastases. Rabbits in group 2 (n = 6) underwent left lateral hepatectomy 14 days post implantation, and were sacrificed 7, 14 and 21 days respectively, after the procedure. Rabbits in group 3 (n = 6) underwent a 1 h intra-arterial infusion with 3-BrPA 14 days post implantation, and were sacrificed 7, 14 and 21 days respectively, after the procedure. Rabbits in group 4 (n = 6) underwent TACE 14 days post implantation, and were sacrificed 7, 14 and 21 days respectively, after the procedure.
Partial hepatectomy
Fourteen days after tumor implantation a laparotomy was performed in six animals under general anesthesia and with aseptic operative techniques. Heart and respiration rates, were continuously monitored throughout the procedure. The animal was prepped and draped in a sterile fashion. A midline incision from xyphoid to umbilicus was made. The falciform and left triangular ligaments were divided to help free the right and left lateral lobe of the liver. The inferior surface of the liver was completely isolated from the stomach and the gut by means of gauzes. The circulation in the left lateral liver lobe was interrupted by ligation of the hilar vessels with a 2–0 silk tie. Subsequently the ligated lobe was resected about 5 mm distal to the ligature. Blood which drained from the excised liver was sponged from the abdominal cavity and the ligated pedicles inspected for complete hemostasis. The incision was closed in two layers.
Intra-arterial delivery protocol
Fourteen days after tumor implantation a laparotomy was performed under general anesthesia and with aseptic operative techniques. Transcatheter hepatic artery infusion of 3-BrPA was performed under fluoroscopic guidance. The animals were brought to the angiography suite and incubated by using a 3.0-mm endotracheal tube (Mallinkrodt Medical, St Louis, Mo) but not ventilated. Surgical cutdown was performed to gain access into the right common femoral artery, after which a 3-F sheath (Cook, Bloomington, Ind) was inserted. A 2-F JB1 catheter (Cook) was manipulated into the celiac axis, after which a celiac arteriogram was obtained to delineate the blood supply to the liver and confirm the location of the tumor. The tumor could readily be visualized as a region of hypervascular blush located on the left side of the liver near the gastric fundus. The left hepatic artery, which provides most of the blood flow to the tumor, was selectively catheterized via the common hepatic artery by using a Transcend guide wire (Boston Scientific, Natick, Mass.).
Intra-arterial infusion with 3-BrPA
After the catheter was adequately positioned within the left hepatic artery, a syringe containing 25 ml of 1.75 mM 3-BrPA was connected to the end of the JB1 catheter and carefully placed and adjusted on an infusion syringe pump (Harvard Apparatus model 11 infuse/withdraw single syringe pump; Instech Solomon, Plymouth Meeting, Pa). The infusion rate was set to 25 ml per h. During the infusion, maintenance of appropriate positioning of the catheter in the hepatic artery was monitored with fluoroscopy. After completion of the infusion the catheter was removed and the common femoral artery was ligated. All rabbits were monitored during and after the procedure and given analgesics when they showed signs of physical distress.
TACE
After the catheter was adequately positioned within the left hepatic artery, a mixture of doxorubicin (Bedford Laboratories, Bedford, Ohio; 5 mg) and iodized oil (Ethiodol, 0.5 ml; Savage Laboratories, Melville, New York) was injected, followed by embolization with Embosphere particles (Biosphere Medical, Rockland, MA) until flow reduction was observed. After completion of the TACE the catheter was removed and the common femoral artery was ligated. All rabbits were monitored during and after the procedure and given analgesics when they showed signs of physical distress.
Pathology
After partial hepatectomy, the resected lobe was macroscopically inspected and multiple specimens from the resection margin, healthy liver and tumor were fixed in formalin, embedded in paraffin, stained with hematoxylin–eosin and evaluated with a light microscope.
After euthanization, the abdomen of all rabbits was opened and inspected for metastases. The primary tumor (if present), lungs, heart, liver, spleen and kidneys were removed and weighed. The number of macroscopic metastases was determined per organ. Multiple biopsy specimens from the primary tumor (if present) lungs, heart, liver, spleen and kidneys, were fixed in formalin, embedded in paraffin, stained with hematoxylin–eosin (H&E) and evaluated with a light microscope.
Results
In all 20 rabbits a solid liver tumor was seen on ultrasound examination 14 days after implantation (Fig. 1).
Partial hepatectomy
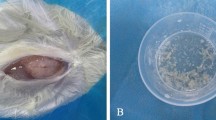
All six partial hepatectomies were successful in removing the tumor completely. No complications occurred during the procedure or during the follow-up period (Fig. 2). Blood loss during the procedure was minimal. Mean procedure time was 20 min. At macroscopic examination a resection margin of at least 1 cm was observed in all animals.
(a) Shows the midline incision from xyphoid to umbilicus. (b) Shows the isolation of the inferior surface of the liver from the stomach and the gut by means of gauzes. (c) Shows the ligation of the hilar vessels with a 2–0 silk tie in order to interrupt the circulation in the left lateral liver lobe. (d) Shows the resected left liver lobe
Intra-arterial infusion with 3-BrPA
In all six rabbits catheterization of the left hepatic artery was successful. The 1 h infusion with 25 ml of 1.75 mM 3-BrPA was tolerated well. All animals recovered without complications.
TACE
In all six rabbits catheterization of the left hepatic artery was successful. The TACE procedure was tolerated well. All animals recovered without complications.
Pathology
Results of pathologic examination of the two rabbits that were sacrificed 14 days after tumor implantation are shown in Table 1. Pathologic analysis showed the presence of lung metastases in one rabbit. None of the rabbits had abdominal cavity, kidney or brain metastases.
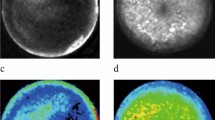
Results of pathologic examination of the rabbits that underwent partial hepatectomy are shown in Table 2. Pathologic analysis showed the presence of lung metastases in six of the six rabbits (Fig. 3). Abdominal cavity dissemination was seen in one of the rabbits that were sacrificed 7 days after partial hepatectomy and in all rabbits that were sacrificed 14 and 21 days after partial hepatectomy. Local recurrence near the resection margin was seen in one rabbit that was sacrificed 7 days after partial hepatectomy. None of the rabbits had kidney or brain metastases.
Results of pathologic examination of the rabbits that underwent intra-arterial infusion with 3-BrPA are shown in Table 3. Pathologic analysis showed the presence of lung metastases in six of the six rabbits (Fig. 4). Abdominal cavity dissemination was seen in none of the rabbits that were sacrificed 7 days after the infusion and in one rabbit that was sacrificed 14 days after infusion and in one rabbit that was sacrificed 21 days after infusion. None of the rabbits had kidney or brain metastases. Pathologic analysis of the liver tumor showed that their primary liver tumors had not grown from baseline, were mostly necrotic and surrounded by a capsule (Fig. 5).
Results of pathologic examination of the rabbits that underwent TACE are shown in Table 4. Pathologic analysis showed the presence of lung metastases in six of the six rabbits (Fig. 6). Abdominal cavity dissemination was seen in all rabbits. Kidney metastases were present in two of the six rabbits. None of the rabbits had brain metastases. Pathologic analysis of the primary liver tumor showed only partial necrosis, with an increase in tumor size corresponding to the time-point of sacrifice (Fig. 7).
Discussion
The present study aimed to compare the impact of partial hepatectomy, intra-arterial treatment with 3-BrPA and TACE on tumor recurrence in the form of regional and distant metastases. The VX2 rabbit model of liver cancer has proven to be suitable for the study of intra-arterial approaches. In order to achieve our objective of comparing partial hepatectomy to two forms of intra-arterial therapies, we tested the feasibility of partial hepatectomy in rabbits bearing VX2 liver cancer.
The success of scientific research, as measured by the ability to correctly obtain the answers to important questions, is predominantly influenced by the quality and availability of appropriate models. In the case of medical research, this frequently involves selecting the best available animal model. Model selection is perhaps the most essential step in determining the success of a research venture. An ideal model should satisfy several criteria: it should be truly representative or a close approximation of the condition being studied; it should permit necessary tests or procedures to be performed.
The rabbit VX2 tumor model, an orthotopic model of liver cancer, was developed in 1940 from a papilloma virus-transformed keratinocyte [15]. VX2 tumors have been shown to exhibit fast tumor growth and extensive metastases in a short time [16, 17]. The main advantages of the VX2 liver tumor model are a straightforward tumor-inoculation technique and a higher incidence of tumor metastases [18]. Therefore, we further developed the VX2 model to be used in studies focusing on tumor therapy, especially on therapy of recurrent HCC after partial liver resection.
For in vivo experiments, rabbits readily allow surgical procedures and/or repeated, blood sampling and can carry a much greater tumor burden (particularly with orthotopic tumors) than small rodents, thereby increasing both the time available to study the tumor and the amount of tumor tissue obtainable. More importantly, rabbits allow direct access for intra-arterial delivery of anti-cancer treatments. Our study showed that partial hepatectomy was a straightforward and feasible procedure on rabbits. The techniques required for our model are simple and reproducible. All procedures can be carried out quickly. Our method was associated with no procedure related animal mortality and is favorable for a large scale application.
Although liver resection and liver transplant are the first choice of treatment for patients with HCC, recurrence is still a major set back of these approaches [11]. TACE has proven to significantly improve survival in patients with unresectable HCC [19]. However, it is unclear whether patients with surgically resectable HCC should always be treated with hepatectomy as opposed to TACE. Our results show that TACE caused only partial necrosis in the primary liver tumor. Furthermore, when compared to resection, more rabbits receiving TACE presented with abdominal and kidney metastases. One possible explanation for this finding is that in our study rabbits only received one TACE treatment, whereas in the clinical setting, TACE treatments typically are repeated until tumor response is achieved [19].
In previous studies we showed that 3-BrPA acts as an irreversible inhibitor of glycolytic enzymes [20]. Many cancer types, including HCC, are associated with a high glycolytic rate and therefore are ideal targets for 3-BrPA [21]. Moreover, we established intra-arterial delivery of 25 ml, 1.75 mM 3-BrPA over 1 h as the optimal method of delivery [14]. In this study we compared this treatment regimen to resection and TACE. Our results indicate that rabbits treated with 3-BrPA exhibit less abdominal metastases than rabbits treated with either resection or TACE. Kidney and brain metastases after 3-BrPA treatment were absent which was comparable to resection and superior to TACE. Interestingly all animals showed lung metastases. This finding suggests that metastatic spread in the VX2 liver tumor model is a result of hematogenic rather than local seeding. Clinically, lung metastases are known to be an important metastatic pathway of HCC.
After successful surgical resection of the primary liver tumor, HCC may recur in various distant sites such as lungs, brain and abdominal cavity. Whenever metastases are present, patients are essentially incurable and current treatment strategies are unsatisfactory [22, 23]. The described method of left lateral hepatectomy could be useful as an animal model to test new anti-cancer agents for this patient group. Our results indicate that this animal model reflects the recurrent metastatic features of HCC after liver resection. Furthermore, this model uses animals with tumor material implanted in orthotopic sites which offers better tumorigenicity and metastatic potential [24, 25].
This study has several limitations. First, our sample size was relatively small, so further studies with a larger sample size are needed to confirm our conclusions. However, our results are in line with previous studies on intra-arterial therapy with 3-BrPA [14, 26]. Second, the VX2 tumor used in our study is of non-hepatic origin. However, it has proven to be convenient to study liver cancer in the animal because of the similarities in blood supply, genotype and metabolism to advanced human HCC.
Conclusion
The VX2 rabbit model of liver cancer is a suitable model to compare the influence of partial hepatectomy, intra-arterial treatment with 3-BrPA and TACE on tumor recurrence in the form of regional and distant metastases. Our results indicate that intra-arterial delivery of 3-BrPA may result in a favorable metastatic profile when compared to both liver resection and TACE.
References
Motola-Kuba D, Zamora-Valdes D, Uribe M, Mendez-Sanchez N (2006) Hepatocellular carcinoma: an overview. Ann Hepatol 5:16–24
Jemal A, Siegel R, Ward E et al (2008) Cancer statistics, CA: a cancer. J Clin 58:71–96
Leong TY, Leong AS (2005) Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB (Oxford) 7:5–15
Blum HE, Spangenberg HC (2007) Hepatocellular carcinoma: an update. Arch Iran Med 10:361–371
Forner A, Hessheimer AJ, Isabel Real M, Bruix J (2006) Treatment of hepatocellular carcinoma. Crit Rev Oncol/Hematol 60:89–98. doi:10.1016/j.critrevonc.2006.06.001
Poon RT, Fan ST, Lo CM, Liu CL, Wong J (1999) Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 229:216–222. doi:10.1097/00000658-199902000-00009
Zimmerman MA, Ghobrial RM, Tong MJ et al (2008) Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg 143:182–188. Discussion 188. doi:10.1001/archsurg.2007.39
Lo CM, Ngan H, Tso WK et al (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology (Baltimore, Md.) 35:1164–1171. doi:10.1053/jhep.2002.33156
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917. doi:10.1016/S0140-6736(03)14964-1
Befeler AS, Di Bisceglie AM (2002) Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 122:1609–1619. doi:10.1053/gast.2002.33411
Poon RT, Fan ST, O’Suilleabhain CB, Wong J (2002) Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg 195:311–318. doi:10.1016/S1072-7515(02)01226-7
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference: European Association for the Study of the Liver. J Hepatol 35:421–430. doi:10.1016/S0168-8278(01)00130-1
Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL (2000) Extrahepatic metastases of hepatocellular carcinoma. Radiology 216:698–703
Vali M, Liapi E, Kowalski J et al (2007) Intraarterial therapy with a new potent inhibitor of tumor metabolism (3-bromopyruvate): identification of therapeutic dose and method of injection in an animal model of liver cancer. J Vasc Interv Radiol 18:95–101. doi:10.1016/j.jvir.2006.10.019
Rous P, Kidd JG, Smith WE (1952) Experiments on the cause of the rabbit carcinomas derived from virus-induced papillomas, II: loss by the Vx2 carcinoma of the power to immunize hosts against the papilloma virus. J Exp Med 96:159–174. doi:10.1084/jem.96.2.159
Ling R, Li Y, Yao Q et al (2005) Lymphatic chemotherapy induces apoptosis in lymph node metastases in a rabbit breast carcinoma model. J Drug Target 13:137–142. doi:10.1080/10611860400027725
Phillips PC, Than TT, Cork LC et al (1992) Intrathecal 4-hydroperoxycyclophosphamide: neurotoxicity, cerebrospinal fluid pharmacokinetics, and antitumor activity in a rabbit model of VX2 leptomeningeal carcinomatosis. Cancer Res 52:6168–6174
Chen JH, Lin YC, Huang YS, Chen TJ, Lin WY, Han KW (2004) Induction of VX2 carcinoma in rabbit liver: comparison of two inoculation methods. Lab Anim 38:79–84. doi:10.1258/00236770460734434
Georgiades CS, Hong K, Geschwind JF (2008) Radiofrequency ablation and chemoembolization for hepatocellular carcinoma. Cancer J (Sudbury, Mass.) 14:117–122. doi:10.1097/PPO.0b013e31816a0fac
Ko YH, Pedersen PL, Geschwind JF (2001) Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett 173:83–91. doi:10.1016/S0304-3835(01)00667-X
Peng SY, Lai PL, Pan HW, Hsiao LP, Hsu HC (2008) Aberrant expression of the glycolytic enzymes aldolase B and type II hexokinase in hepatocellular carcinoma are predictive markers for advanced stage, early recurrence and poor prognosis. Oncol Rep 19:1045–1053
Yang Y, Nagano H, Ota H et al (2007) Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery 141:196–202. doi:10.1016/j.surg.2006.06.033
Zhu AX (2008) Development of sorafenib and other molecularly targeted agents in hepatocellular carcinoma. Cancer 112:250–259. doi:10.1002/cncr.23175
Fidler IJ (1995) Critical factors in the biology of human cancer metastasis. Am Surg 61:1065–1066
Khanna C, Hunter K (2005) Modeling metastasis in vivo. Carcinogenesis 26:513–523. doi:10.1093/carcin/bgh261
Vali M, Vossen J, Buijs M et al (2008) Targeting of VX2 rabbit liver tumor by selective delivery of 3-bromopyruvate: a biodistribution and survival study. J Pharmacol Exp Ther
Acknowledgments
The work was supported by NCI RO1 CA100883-01, the Abdulrahman Abdulmalik Research Fund and the Charles Wallace Pratt Research Fund
Author information
Authors and Affiliations
Corresponding author
Additional information
Josephina A. Vossen and Manon Buijs have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Vossen, J.A., Buijs, M., Syed, L. et al. Development of a new orthotopic animal model of metastatic liver cancer in the rabbit VX2 model: effect on metastases after partial hepatectomy, intra-arterial treatment with 3-bromopyruvate and chemoembolization. Clin Exp Metastasis 25, 811–817 (2008). https://doi.org/10.1007/s10585-008-9195-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-008-9195-x