Abstract
We have developed a novel hyperthermic treatment modality using magnetic materials for metastatic bone tumors. The purpose of this study is to show the results of novel hyperthermia for metastatic bone tumors. This novel hyperthermic treatment modality was used for 15 patients with 16 metastatic bone lesions. In seven lesions, after curettage of the metastatic lesion followed by reinforcement with a metal intramedullary nail or plate, calcium phosphate cement (CPC) containing powdery Fe3O4 was implanted into the cavity. In one lesion, prosthetic reconstruction was then performed after an intralesional tumor excision. For the remaining eight lesions, metal intramedullary nails were inserted into the affected bone. Hyperthermic therapy was started at 1 week postoperatively. To comparatively evaluate the radiographic results of patients who underwent hyperthermia (HT group), we also assessed eight patients who received a palliative operation without either radiotherapy or hyperthermia (Op group), and 22 patients who received operation in combination with postoperative radiotherapy (Op + RT group). In HT group, all patients had an acceptable limb function with pain relief without any complications. On radiographs, 87, 38, and 91% were, respectively, considered to demonstrate an effective treatment outcome in HT group, Op group, and Op + RT group. The patients in HT group showed a statistically better radiographic outcome than the patients in Op group (P = 0.0042). But when compared between HT group and Op + RT group, there were no significant difference (P = 0.412). This first series of clinical hyperthermia using magnetic materials achieved good local control of metastatic bone lesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with metastatic bone tumors in the extremities sometimes require surgical intervention to prevent reduction of quality of life due to a pathological fracture. The addition of localized radiotherapy after internal fixation reduces the risk of further bone destruction, which leads to increased pain, loss of fixation, and the need for additional orthopedic procedures [1, 2]. However, radiotherapy sometimes induces soft tissue damage, including muscle induration, joint contracture, and skin erosion [2]. Moreover cancer cells are not always radiosensitive.
Hyperthermia has been studied using an experimental animal model to treat various types of advanced cancer from 1940s [3]. Recent progress in cell biology has revealed hyperthermia, variously reported between 40 and 45°C, to trigger tumor cell death by apoptosis although the exact temperature differs in the individual condition [4, 5]. It is now well-known that hyperthermia-induced apoptosis is characterized by the occurrence of intranucleosomal DNA cleavage [6]. Hyperthermia have now reached a stage of clinical application to treat advance cancer, such as recurrent rectal cancer, prostate cancer, uterine cancer, head and neck cancer, lung cancer, and breast cancer, especially in conjunction with radiotherapy or chemotherapy [7–13].
Heating the tumor in hyperthermic applications is usually achieved by means of external sources such as microwave, ultrasound or water bath [14]. However in using these external sources, peritumoral normal tissue may be injured. In contrast, magnetically induced heating of magnetic materials comprises a minimally invasive method providing an internal source. The concept of hyperthermic cancer therapy that utilizes magnetic materials and an alternating magnetic field, have been developed by many researchers [15–17]. Hilger et al. showed the feasibility of thermal ablation of breast cancer with magnetic nanoparticles using animal model [18]. Yan et al. showed the treatment using Fe2O3 nanoparticles combined with magnetic fluid hyperthermia could inhibit not only the proliferation of cultured liver cancer cells, but also induce apoptosis of cultured liver cancer cells. Moreover, they showed that that this hyperthermic strategy has a significant inhibitory effect on the weight and volume of xenograft liver cancer [19]. Johannsen et al. showed the first report on clinical application of hyperthermia of human cancer using magnetic nanoparticles [12]. They injected magnetic nanoparticle suspensions transperineally in to prostate under ultrasound and fluoroscopy guidance, and showed that hyperthermia using magnetic nanoparticles was feasible and well tolerated for a patient with previously irradiated and locally recurrent prostate carcinoma.
Localized hyperthermic treatment for musculoskeletal tumors with ferromagnetic ceramics was first reported by Ikenaga et al. [20, 21]. In an in vitro study Hilger et al. showed the impact of magnetic thermoablation in muscle tissue using iron oxide particles, and also demonstrated the applicability of local magnetic thermoablation as a therapy of muscle lesions in the long-term [18]. We modified these new hyperthermic strategies as an option to treat metastatic bone tumors, and developed bone cement made of glass ceramic that is partly replaced by magnetite [22–24]. When this magnetite cement was inserted into the metastatic lesion followed by exposure to an alternating electromagnetic field, this magnetite cement generated heat according to the principle of physical energy conversion called “hysteresis loss.” As a result, any metastatic cancer cells thereby suffer apoptotic cell death and necrosis [20, 22, 23, 25]. Since our magnetite cement has not yet been approved by Ministry of Health, Labor, and Welfare of Japan, we decided to use titanium alloy or stainless intramedullary nail or powdery Fe3O4 as magnetic materials. We have continually performed experimental studies using animal models [22, 24, 26], and finally started clinical applications of this treatment modality from March 2003 after obtaining approval from our Institutional Ethics Investigational Review Board.
To our knowledge, this is the first report on the clinical application of hyperthermia for metastatic bone tumors with magnetic materials by generating an alternating electromagnetic field. The purpose of this study is to show the clinical outcome of hyperthermia using magnetic materials on human subjects.
Patients and methods
Patients
The patients who underwent hyperthermia for metastatic bone tumors with magnetic materials (HT group)
Informed consent was obtained from all patients according to the guidelines of the institutional ethics review board. Patients eligible for the study were suffering from previously untreated and histologically confirmed metastatic bone tumors in the extremities. From March 2003 to November 2005, we treated 42 patients with metastatic bone tumors in the extremities. Of 42 patients, 14 patients received only radiotherapy because they had too poor general condition to undergo the operation and two patients underwent only operation because low-priority of radiotherapy for operated limb due to multiple metastases. Of 26 patients, 11 patients with a metastatic lesion at the proximal end of femur or humerus were precluded to undergo our hyperthermia because it was structurally impossible to perform hyperthermia for proximal lesions of the extremities using our electromagnetic field generator. Therefore, our novel hyperthermia modality was performed on 15 patients, including seven males and eight females, with 16 metastatic lesions, and then they were prospectively followed-up (Table 1). The age of the patients at operation ranged from 41 to 80 years of age (median 63 years), and the follow-up period ranged from 3 to 37 months (median 10 months). The primary lesions included three lung cancer, three hepatocellular carcinomas, two renal cell carcinomas, two sarcomas, and others. Regarding the operation sites, eight were the humerus, five the femur, two the tibia, and one the fibula. Seven patients received systemic therapies including chemotherapy, hormonal therapy, and interferon.
The patients of the control group (“Op group” and “Op + RT group”)
We established two control groups; one was the “Op group” who underwent a palliative operation without postoperative radiotherapy from 1995 to 2003, and another is the “Op + RT group” who underwent an operation in combination with postoperative radiotherapy from 2001 to 2003.
“Op group”: from 1995 to 2003, 132 patients with the metastatic bone tumor in their extremities were treated at our institute. Until hyperthermic therapies were started in 2003, we have undergone surgical reinforcement in combination with postoperative radiotherapy for the patients with metastatic bone tumor of the extremities. Therefore there were the limited patients in Op group (Table 2). The selection criteria of the fight patients in the Op group are as follows: (1) a low-priority of radiation therapy for operated limb because of multiple bone metastases in 3, brain metastases in 2, and metastases to other visceral organ in 2, (2) the patient’s rejection of irradiation in one patient.
“Op + RT group”: from 2001 to 2003, 51 patients were treated due to metastatic bone tumors of the extremities. Of 51 patients, 18 patients received only radiotherapy because their general condition was too poor for them to undergo the operation and three patients underwent only an operation because of a low-priority of radiotherapy for the operated limb due to multiple metastases. Of 30 patients, eight patients with a metastatic lesion at the proximal end of femur or humerus underwent a wide tumor resection and a prosthetic reconstruction. Therefore, the remaining 22 patients were selected as the “Op + RT group” (Table 3).
Surgical procedure
All surgical procedures were performed under general anesthesia. The surgical procedures for the patients who underwent hyperthermic treatment can be categorized into two groups. For eight patients not expected to have a long survival, only reinforcement with an intramedullary nail was performed without any excision of the metastatic lesion. In contrast, for eight patients who were expected to have a long survival, after curettage of the lesion followed by either a prosthetic replacement (n = 1) or reinforcement with a metal intramedullary nail or plate (n = 7), calcium phosphate cement (CPC) containing a powdery Fe3O4 was implanted into the cavity. As a CPC, we used BIOPEX®-R (PENTAX Corp., Tokyo, Japan [27, 28]). A powdery Fe3O4, which consists of 13-(m-diameter particle was made by Nippon Electric Glass Corp. Ltd., Ohtsu, Japan. The tumor was exposed via an appropriately sized bone window, and careful curettage was then performed using a curette. After curettage of the tumor, internal reinforcement was performed using intramedullary nail or plate fixation. Next, the powdery Fe3O4 was prepared to become a 40% weight ratio of total CPC weight. Thereafter, the liquid components, the powder of the CPC and Fe3O4 were mixed manually together until a smooth consistency was obtained (Fig. 1a, b). The bone defect was filled with the CPC containing powdery Fe3O4 either by hand or by injection through a blunt needle, depending on the shape and size of the bone defect (Fig. 1c). After sufficiently washing the wound using physiological saline, the muscle, subcutaneous tissue, and skin were then carefully closed in layers.
The surgical method to fill calcium phosphate cement (CPC) containing Fe3O4 after reinforcement using an intramedullary nail. (a) CPC and the powdery Fe3O4 were prepared. (b) The liquid components, the powder of the CPC and Fe3O4 were mixed manually together to become a smooth consistency. (c) The bone defect was filled with the CPC containing powdery Fe3O4 by injection through a blunt needle and by hand
Exposure to alternating electromagnetic field
Alternating electromagnetic field generator was developed by Yamamoto Vinita Co. Ltd., Osaka, Japan [22, 23, 26]. The affected limb was inserted into a cylinder-form coil of the generator (Fig. 2). The power of the electromagnetic field was 4 kW in output, and 1.5 MHz in fixed frequency. The intensity was adjusted to obtain a temperature of around 43°C on the surface of cortical bone. Hyperthermic treatment was performed postoperatively on days 8, 10, 12, 15, 17, 19, 22, 24, 26, and 29. The exposure time was 15 min per day. The treatment method including the treatment schedule and exposure time were decided according to the experimental studies using rabbit and human cadavers [23, 26]. The temperature of the bone surface was monitored with a fluoroptic thermometer (model 3000; Laxtron, Mountain View, CA, USA) under local anesthesia.
Hyperthermia was performed by inserting the affected limb into a cylinder-form coil of the electromagnetic field generator. The power of the electromagnetic field was 4 kW in output, and 1.5 MHz in fixed frequency. Hyperthermic treatment was performed postoperatively on days 8, 10, 12, 15, 17, 19, 22, 24, 26, and 29. The exposure time was 15 min per day
Assessment
The assessments were based on clinical examinations and radiographic findings. The functional assessments were performed according to the scoring system of Musculoskeletal Tumor Society [29]. The radiographic outcome was evaluated by radiographs at 3 months after surgery. The radiographic outcome was assessed according to following criteria. “Excellent” means a reduction of the lesion with visible bone formation. “Good “means no progression of the lesion for more than 3 months. “Poor” means a progression of the lesion. To evaluate the effectiveness of hyperthermia on radiographic findings, a univariate analysis was performed using the Mann–Whitney U-test for non-parametric data and StatView statistical software (Version 5.0; SAS Institute Inc., Cary, NC, USA).
Results
There were no adverse effects after the operation (e.g., excessive postoperative drainage, erythema) in the early postoperative period. No patients developed a postoperative infection. Regarding the adverse effects related to hyperthermia, three patients felt a heat sensation during exposure to electromagnetic field. However, these feelings of heat disappeared immediately when the generator switch was turned off, and hyperthermia was carried out for 15 min of total exposure time. All patients underwent ten sessions of hyperthermic treatment as planned. None of patients suffered any burns. In addition, no soft tissue injury was observed due to elevated temperatures, including muscle necrosis, vascular damage, and nerve injury. No allergic reaction was observed during the follow-up period. None of the patients had any pain at the operation site. In addition, no toxicity was detected in routine blood examinations.
In all patients who underwent hyperthermic treatment, pain relief was obtained after operation.
The functional assessment after hyperthermia ranged from 57 to 100% (average 80%) in the upper extremities, and from 57 to 97% (average 72%) in the lower extremities.
On radiographs, in HT group, eight lesions (50%) showed an excellent outcome (Figs. 3, 4, 5), while six lesions (37%) showed a good outcome. Two lesions (13%) showed a poor outcome and thus required additional surgery because of local recurrence. When compared the radiographic results between the patients who underwent hyperthermia with CPC containing a powdery Fe3O4 and the patients without that, there were no significant difference (P > 0.999). In contrast, in Op group, three lesions (38%) showed a “good,” while five lesions (62%) showed a “poor.” In Op + RT group, seven lesions (32%) showed a “excellent,” and 13 lesions (59%) showed a “good,” while two lesions (9%) showed a “poor.” When the lesions with an “excellent” or “good” outcome were classified to the “effective” treatment, 87, 38, and 91% were, respectively, considered to demonstrate an effective treatment outcome in HT group, Op group and Op + RT group. The patients in HT group showed a statistically better radiographic outcome than the patients in Op group (P = 0.0042). But when compared between HT group and Op + RT group, there were no significant difference (P = 0.412).
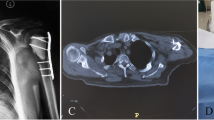
(a) A 63-year-old patient with metastatic lung cancer to the humerus (open arrow). (b) After curettage of the lesion followed by reinforcement with intramedullary Kurschner wire, CPC containing Fe3O4 was implanted into the cavity. (c) At 6 months after undergoing hyperthermia, massive new bone formation has become visible (open triangle)
(a) A 63-year-old patient with metastatic hepatocellular carcinoma to the humerus (open arrow). (b) Reinforcement with intramedullary nailing was performed. The lytic area of the humerus was found to have gradually disappeared at (c) 3, (d) 5, and (e) 7 weeks postoperatively (open triangle). (f) In this patient, the temperature of the surface of cortical bone reached more than 40°C within 2 min, and then it reached a plateau within 4 min, thus showing a temperature of higher than 42°C
In three patients (patients 1, 6, and 7 in Table 1), we monitored the temperature of the bone surface using thermometer. The heat was monitored at the surface of the cortical bone set up at the center of the coil of the electromagnetic generator. We showed the temperature change of the surface of the cortical bone during exposure to the electromagnetic field in the patient who was treated using intramedullary nail as magnetic material (patient no. 7 in Table 1) (Fig. 5f). In this patient, the temperature of the bone surface reached more than 40°C within 2 min, and then it reached a plateau within 4 min, thus showing a temperature of higher than 42°C.
Discussion
The bone has several special properties, which discourage the oncologist from developing hyperthermic therapeutic strategy. The bone is deeply located in the body, too hard to approach penetrating the cortex, and low-thermal conductive with highly vascularized medulla. Hyperthermia for bone tumors using the polymerization heat of polymethylmethacrylate bone cement is now widely performed [30, 31]. However, the generated heat tends to be unreliable and insufficient to reduce bone tumor growth [22]. Microwave-induced hyperthermia [8], laser-induced thermotherapy [32, 33], and radiofrequency ablation [33] are recently used especially for spinal and pelvic metastasis. But these therapeutic modalities are unsatisfactory for the lesions located in the long tubular bone of the limb, because pathological fractures cannot be prevented without a surgical reinforcement of the bone lesion. Therefore, we developed a new hyperthermic therapeutic strategy with magnetic materials for metastatic bone tumors based on experimental studies [22–24, 26], and have also started clinical investigations. This first series of a new clinical hyperthermia treatment modality showed a good clinical outcome.
No serious adverse effects were observed during the follow-up period. Only three patients experienced a heat sensation during exposure to an electromagnetic field. These feelings of heat disappeared immediately when the generator switch was turned off, and hyperthermia could thus be carried out as planned in all patients. In an experimental study using human cadavers, the temperature at the cortical surface increase to 43–45°C when the implanted materials were heated above 60°C [26]. In the experimental study using VX-2 tumor-transplanted rabbit model, there were no histological changes in the surrounding soft tissue after hyperthermia [26]. We speculate that the absence of any heat-related soft tissue injury may be due to a “radiator effect” which is produced by the microvessels, which absorb the heat of microenvironment. We concluded that our hyperthermia with magnetic materials is therefore a safe therapeutic procedure.
The functional assessment after hyperthermia ranged from 57 to 100% (average 80%) in the upper extremities, and from 57 to 97% (average 72%) in the lower extremities. The limb function of the patients who underwent an operation for metastatic bone tumors is sometimes worse than that for primary bone tumors, because the former often have other lesions, which affect the patient’s motility and activity of daily life. For example, no. 11 patient (Table 1) who was operated for metastatic renal cell cancer got a poor functional result, because a metastatic spinal lesion in sixth and seventh cervical spine caused paresis and intractable pain of the affected limb. We believe that the patients treated by our hyperthermia modality thus had an acceptable limb function.
On radiographs, when the lesions with an “excellent” or “good” outcome were classified to the “effective” treatment, 87, 38, and 91% were, respectively, considered to demonstrate an effective treatment outcome in HT group, Op group and Op + RT group. The patients in HT group showed a statistically better radiographic outcome than the patients in Op group (P = 0.0042). But when compared between HT group and Op + RT group, there were no significant difference (P = 0.412). These results suggest that the hyperthermia using magnetic material may be so effective as an operation being combined with postoperative radiotherapy in term of radiographic outcome (Table 4).
Our hyperthermic strategy has the following advantages for the treatment of metastatic tumor of long tubular bone, compared to radiotherapy. First of all, our hyperthermic treatment is minimal invasive for surrounding soft tissue although irradiation sometimes induces soft tissue damage, including muscle induration, and joint contracture and skin erosion [2]. Second, our hyperthermic strategy can prevent pathological fractures because of the surgical reinforcement of the bone lesion. Moreover, this surgical reinforcement never requires any special techniques, which may further burden the orthopedic surgeon. Finally, our hyperthermic treatment can be repeatedly applied for recurrent metastatic lesions, even though radiotherapy cannot surpass the normal dose limitations.
We did not always monitor the thermometric parameters of hyperthermic treatment, because the heat monitoring system is invasive for the patients. In three patients (patient no. 1, 6, and 7 in Table 1), we monitored the temperature of the surface of the cortical bone using thermometer. The heat was monitored at bone surface set up at the center of the coil of the electromagnetic generator. In these patient, temperature of the surface of cortical bone reached more than 40°C within 2 min, and reach plateau within 4 min thus showing a temperature of more than 42°C. Because the radiographic assessment showed good response in these three patients, we could not demonstrate any relationship between tumor temperature and tumor response in this study. Further clinical study is needed.
In conclusion, our novel clinical hyperthermia modality using magnetic materials was thus found to achieve a good local control of metastatic bone lesions. We have recently started a randomized study to compare the clinical results between “HT group” and “Op + RT group.” Further investigations are needed before this technique can be employed as a standard therapy for metastatic bone tumors.
References
Frassica DA, Frassica FJ (1998) Nonoperative management. In: Simon MA, Springfield D (eds) Surgery for bone and soft-tissue tumors. Lippincott-Raven, Philadelphia, pp 633–637
Yazawa Y, Frassica FJ, Chao EY et al (1990) Metastatic bone disease. A study of the surgical treatment of 166 pathologic humeral and femoral fractures. Clin Orthop Relat Res 251:213–219
van der Zee J (2002) Heating the patient: a promising approach? Ann Oncol 13:1173–1184
Harmon BV, Corder AM, Collins RJ et al (1990) Cell death induced in a murine mastocytoma by 42–47 degrees C heating in vitro: evidence that the form of death changes from apoptosis to necrosis above a critical heat load. Int J Radiat Biol 58:845–858
Robins HI, D’Oleire F, Grosen E, Spriggs D (1997) Rationale and clinical status of 41.8 degrees C systemic hyperthermia tumor necrosis factor, and melphalan for neoplastic disease. Anticancer Res 17:2891–2894
Sellins KS, Cohen JJ (1991) Hyperthermia induces apoptosis in thymocytes. Radiat Res 126:88–95
Rau B, Wust P, Hohenberger P et al (1998) Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: a phase II clinical trial. Ann Surg 227:380–389
Fan QY, Ma BA, Qlu XC et al (1996) Preliminary report on treatment of bone tumors with microwave-induced hyperthermia. Bioelectromagnetics 17:218–222
van Ginkel RJ, Schraffordt Koops H, de Vries EG et al (1996) Hyperthermic isolated limb perfusion with cisplatin in four patients with sarcomas of soft tissue and bone. Eur J Surg Oncol 22:528–531
Kapp DS (1989) Indications for the clinical use of deep local and regional hyperthermia in conjunction with radiation therapy. Strahlenther Onkol 165:724–728
Sakurai H, Hayakawa K, Mitsuhashi N et al (2002) Effect of hyperthermia combined with external radiation therapy in primary non-small cell lung cancer with direct bony invasion. Int J Hyperthermia 18:472–483
Johannsen M, Gneveckow U, Eckelt L et al (2005) Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperthermia 21:637–647
Grunhagen DJ, de Wilt JH, Graveland WJ et al. (2006) Outcome and prognostic factor analysis of 217 consecutive isolated limb perfusions with tumor necrosis factor-alpha and melphalan for limb-threatening soft tissue sarcoma. Cancer 106:1776–1784
van der Zee J (2002) Heating the patient: a promising approach? Ann Oncol 13:1173–1184
Ivkov R, DeNardo SJ, Daum W et al (2005) Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin Cancer Res 11:7093s–7103s
Ito A, Shinkai M, Honda H, Kobayashi T (2005) Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng 100:1–11
Kawashita M, Tanaka M, Kokubo T et al (2005) Preparation of ferrimagnetic magnetite microspheres for in situ hyperthermic treatment of cancer. Biomaterials 26:2231–2238
Hilger I, Hergt R, Kaiser WA (2000) Effects of magnetic thermoablation in muscle tissue using iron oxide particles: an in vitro study. Invest Radiol 35:170–179
Yan S, Zhang D, Gu N et al (2005) Therapeutic effect of Fe2O3 nanoparticles combined with magnetic fluid hyperthermia on cultured liver cancer cells and xenograft liver cancers. J Nanosci Nanotechnol 5:1185–1192
Ikenaga M, Ohura K, Yamamuro T et al (1993) Localized hyperthermic treatment of experimental bone tumors with ferromagnetic ceramics. J Orthop Res 1:849–855
Ikenaga M, Ohura K, Kotoura Y et al (1994) Hyperthermic treatment of canine tibia through RF inductive heating of an intramedullary nail: a new experimental approach to hyperthermia for metastatic bone tumours. Int J Hyperthermia 10:507–516
Kusaka M, Takegami K, Sudo A et al (2002) Effect of hyperthermia by magnetite cement on tumor-induced bone destruction. J Orthop Sci 7:354–357
Takegami K, Sano T, Wakabayashi H et al (1998) New ferromagnetic bone cement for local hyperthermia. J Biomed Mater Res 43:210–214
Uchida A, Wakabayashi H, Okuyama N et al (2004) Metastatic bone disease: pathogenesis and new strategies for treatment. J Orthop Sci 9:415–420
Akagi M, Tsuboyama T, Ikenaga M et al (1997) Anti-tumour effects of localized hyperthermia on an experimental bone tumour using an intramedullary nail. Int J Hyperthermia 13:387–400
Morita K, Morita S, Tsujiguchi M et al (2002) A method of local hyperthermia with ferromagnetic bone cement. Improvement for clinical medicine. Orthop Ceram Implants 19–20:97–100
Matsumine A, Kusuzaki K, Matsubara T et al (2006) Calcium phosphate cement in musculoskeletal tumor surgery. J Surg Oncol 93:212–220
Asaoka N, Misago M, Hirano M, Takeuchi H (1999) Mechanical and chemical properties of the injectable calcium phosphate cement. Bioceramics 12:525–528
Enneking WF, Dunham W, Gebhardt MC et al (1993) A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 286:241–246
Malawer MM, Marks MR, McChesney D et al (1988) The effect of cryosurgery and polymethylmethacrylate in dogs with experimental bone defects comparable to tumor defects. Clin Orthop Relat Res 226:299–310
Sturup J, Nimb L, Kramhoft M, Jensen JS (1994) Effects of polymerization heat and monomers from acrylic cement on canine bone. Acta Orthop Scand 65:20–23
Vogl TJ, Mack MG, Straub R et al (2001) MR-guided laser-induced thermotherapy of the infratemporal fossa and orbit in malignant chondrosarcoma via a modified technique. Cardiovasc Intervent Radiol 24:432–435
Groenemeyer DH, Schirp S, Gevargez A (2002) Image-guided percutaneous thermal ablation of bone tumors. Acad Radiol 9:467–77
Acknowledgments
We thank the secretarial staff of the Department of Orthopedic Surgery, Mie University School of Medicine, for their generous cooperation. This work is supported in part by the grant from the Ministry of Health, Labor, and Welfare (Grants-in Aid for Clinical Cancer Research).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumine, A., Kusuzaki, K., Matsubara, T. et al. Novel hyperthermia for metastatic bone tumors with magnetic materials by generating an alternating electromagnetic field. Clin Exp Metastasis 24, 191–200 (2007). https://doi.org/10.1007/s10585-007-9068-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-007-9068-8