Abstract
Sustainable fisheries management into the future will require both understanding of and adaptation to climate change. A risk management approach is appropriate due to uncertainty in climate projections and the responses of target species. Management strategy evaluation (MSE) can underpin and support effective risk management. Climate change impacts are likely to differ by species and spatially. We use a spatial MSE applied to a multi-species data-poor sea cucumber/béche-de-mer fishery to demonstrate the utility of MSE to test the performance of alternative harvest strategies in meeting fishery objectives; this includes the ability to manage through climate variability and change, and meeting management objectives pertaining to resource status and fishery economic performance. The impacts of fishing relative to the impacts of climate change are distinguished by comparing future projection distributions relative to equivalent no-fishing no-climate-change trials. The 8 modelled species exhibit different responses to environmental variability and have different economic value. Status quo management would result in half the species falling below target levels, moderate risks of overall and local depletion, and significant changes in species composition. The three simple strategies with no monitoring (spatial rotation, closed areas, multi-species composition) were all successful in reducing these risks, but with fairly substantial decreases in the average profit. Higher profits (for the same risk levels) could only be achieved with strategies that included monitoring and hence adaptive management. Spatial management approaches based on adaptive feedback performed best overall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Climate change is likely to have a significant impact on both target and non-target marine populations worldwide, with the concomitant need for management strategies capable of sustaining fishing into the future. There is a need to bridge the gap between short-term individual stock forecasts and longer term assessments of community production and resilience (Steele and Gifford 2010). A wide range of methods, ranging from single-species assessment models to ecosystem models, are currently under development to model the effects of climate change on fish and fisheries (Hollowed et al. 2011; Plagányi et al. 2011a, b). Improvements in understanding the functional relationships between climate variability and fish production are increasingly enabling their explicit incorporation in fisheries models (Hollowed et al. 2009; Holt and Punt 2009; Ianelli et al. 2011).
Uncertainty pervades all aspects of fisheries management, with a paucity of data and understanding similarly hindering moves to ecosystem-based fisheries management as well as incorporation of climate impacts. This has motivated the adoption of risk-based assessment methods such as ecological risk assessment (Hobday et al. 2011) and Management Strategy Evaluation (MSE) (Smith et al. 2007). Several risk assessment approaches have been developed to identify, analyse and evaluate the ecological effects of fishing under changing climate and to prioritize management responses (Fletcher 2005; Chin et al. 2010; Hobday et al. 2011). MSE frameworks (also known as an operational management procedure) are key examples of formal risk assessment methods, given their focus on the identification and modelling of uncertainties as well as in balancing different representations of resource dynamics (Sainsbury et al. 2000). Briefly however, it involves modelling each step of the formal adaptive-management approach (Walters 1986) and evaluating the consequences of a range of management strategies, especially in the face of uncertainty. This includes consideration of the implications, for both the resource and its stakeholders, of alternative combinations of monitoring data, analytical procedures, and decision rules. By identifying and evaluating tradeoffs in performance across a range of management objectives, it provides advice on the performance of different management measures and whether they are robust to inherent uncertainties in all inputs and assumptions used (Cooke 1999).
The simulation-testing frameworks used in MSE consist of operating models that simulate alternative plausible scenarios for the “true” dynamics of the resource. They are also used to generate simulated fisheries data that are used when fitting assessment models. The outputs from these assessment models are used by decision or control rules to determine what management actions are taken. In turn, these management actions are implemented inside the operating models with error or filtered by the industry’s social and economic drivers. The active feedback, which is a part of MSE testing, can simulate how well the different management strategies can detect and control changes in fishery production or profitability (Plagányi et al. 2011b). For example, MSE has been used to evaluate the performance of fishery management control rules for walleye Pollock (Theragra chalcogramma) under climate change and the assumption of temperature-induced changes in recruitment pattern (A’mar et al. 2009; Ianelli et al. 2011).
In this paper we provide an example of the use of MSE to assess both the effects of projected climate change impacts and the relative performance of alternative harvest strategies in adapting to those changes and meeting management objectives pertaining to resource status and fishery economic performance. Our MSE risk management framework simultaneously accounts for uncertainty in biological understanding as well as projected climate change impacts.
The case study used is the Australian Torres Strait (Fig. 1) bêche-de-mer (sea cucumber) fishery. Though it has a small Gross Value of Production ($321,000 in 2005), it is an important source of income for local Islander communities, especially in east Torres Strait. Historically, the fishery has been characterised by boom and bust cycles as the result of resource depletion and/or price fluctuations (Wilson et al. 2010). The open access rights for all Torres Strait Islanders and the artisanal nature of fishing makes regulatory control very difficult (Wilson et al. 2010). The underlying Operating Model (OM) (Plagányi et al. 2011c) is both spatial (27 reefs) and multi-species (8 bêche–de-mer species) to enable assessment of the performance of different management strategies from a spatial and multi-species perspective. Furthermore, there are substantial differences in the value of the different species and hence we compare the overall profit arising from alternative fishing strategies.
2 Methods
2.1 The Torres Strait Sea cucumber fishery
The Torres Strait bêche-de-mer fishery began in the late 1800s up until about 1939, and restarted in about 1990. Sandfish (Holothuria scabra) on Warrior Reef (Fig. 1) provided the bulk of the early catches in the fishery, which peaked at over 1200 t (wet gutted weight) in 1995. A survey in 1998 (Skewes et al. 1998) found that the population was severely depleted and the sandfish fishery was closed. Subsequent surveys found a small recovery in the population, especially of the breeding cohort, but it is still considered heavily depleted (Murphy et al. 2011).
After the closure of sandfish in 1998, the fishery mostly targeted black teatfish (H. whitmaei), deepwater redfish (Actinopyga echinites), surf redfish (A. mauritiana), blackfish (mostly A. miliaris) and white teatfish (Holothuria fuscogilva). However, a survey in March 2002 found that black teatfish and surf redfish were probably overexploited (Skewes et al. 2003), and a prohibition on the harvest of these species was introduced in January 2003. A survey in 2009 found that the density of black teatfish had recovered to near natural (unfished) densities (Skewes et al. 2010) and it was recommended that this species be reopened to fishing but with a modest TAC of 25 t and community-based harvest strategies to manage the spatial effort of this species (Skewes et al. 2010).
2.2 Climate change in the Torres Straits
We used existing reviews and analyses of climate change in Torres Strait to summarise the related changes to several environmental variables: sea surface temperature, sea level rise, changes to current systems, storms and cyclones, rainfall, and ocean acidification; two critical habitats: seagrass and coral reefs; and an important survival parameter for larval holothurians: phytoplankton productivity (Online Resource 1). The projections were considered only to 2030 as this has higher management relevance than longer term projections, for which there would be increasing uncertainty. Projections of global warming were considered only for the mid-high range greenhouse gas emissions scenario, A1B (IPCC 2007)—in any case there is little deviation by 2030 among different emission scenarios.
2.3 Assessing and classifying risks
The potential impacts of the projected changes to physical variables and critical habitats were assessed for a range of life history variables (growth, mortality, movement and distribution and reproduction) separately for three sea cucumber life history stages (larvae, juvenile and adult) in Torres Strait. Each potential impact was described and quantified to the fullest extent possible using information from literature reviews, unpublished experimental studies and expert consultation, based on the approach of Norman-Lόpez et al. (2012). Considerable uncertainty exists for most combinations of physical and biological variables. We took the view, in this case, of using all available information to outline likely potential impacts for use in our model.
Following the Australian national risk assessment approach, risk rankings for potential impacts on sea cucumber biological variables from climate-change-related changes in environmental variables were formulated from the likelihood of the climate-related change occurring and the consequences, or severity of impact, of such changes to sea cucumber biology (Table 1). Likelihood scores of the physical variable changing were assigned based on confidence ratings by experts in the field (Poloczanska et al. 2009); where >70 % likelihood was considered as high, <30 % low and in between these values medium (Table 1). The relative consequence of the potential impacts was a subjective assessment based on expert opinion—generally impacts that resulted in greater than a 5 % change in a biological property were considered as a high consequence, and greater than 2 % as a medium consequence. Less than 2 % was considered as low consequence. The ratings criteria used were arbitrary, but acknowledged that relatively small changes in biological rates could potentially have a large effect on overall population productivity (Norman-Lόpez et al. 2012).
Risk for potential impacts for all combinations of climate change variable and life history parameter were then formulated (see Online Resource 1, Table 2). Interactions between multiple impacts (e.g. temperature and acidification on mortality) were considered by taking an iterative approach to formulating potential impacts: all potential impacts were assessed in a cyclical fashion several times and potential interactions between them were accounted for in the final assessment.
2.4 MSE simulations
We used a modified form of the spatial MSE developed by Plagányi et al. (2011c) for 8 béche-de-mer species inhabiting 27 reef groupings (hereafter called subzones) in the Torres Straits. The model was conditioned over the historic period 1995–2010 using all available survey data (Skewes et al. 2010). A Reference Set (see Rademeyer et al. 2007) of alternative model parameterisations was used to collectively capture some of the key biological uncertainties (e.g. alternative natural mortality estimates and steepness of the stock-recruitment relationship), as well as uncertainty as to the likelihood (using high risk scenarios only versus assuming both high and medium risk scenarios occur) and consequence (accounting for a doubling of the severity of each postulated effect) of climate-change effects. By using a Reference Set (rather than a single base-case operating model) (see Rademeyer et al. 2007) we were able to integrate across this range of biological and climate-impact uncertainties. Finally we tested a range of alternative harvest strategies to evaluate their performance under changing climate.
2.5 Spatial Operating Model (OM)
The OM used is that developed by Plagányi et al. (2011c) for the Torres Strait region, with a full description of the spatial age-structured population model described therein. The model includes 8 bêche-de-mer species, with populations distributed across 27 subzones, in turn grouped into 8 zones (Fig. 1) (see Online Resource 2). Species modelled are sandfish, black teatfish, surf redfish, white teatfish, deepwater redfish, hairy blackfish (Actinopyga miliaris), prickly redfish (Thelenota ananas), and leopardfish (Bohadschia argus).
The time period covered in the model was 1995 to 2010, with a 20 year future projection time period. Selected key equations are reproduced below.
The resource dynamics are modelled by the following set of population dynamics equations:
where:
- N syar :
-
is the number of holothurians of species s at the start of year y (which refers to a calendar year), at age a in subzone r;
- \( R_{sy}^Z \) :
-
is the total recruitment (number of 0-year-old holothurians) in zone Z of species s at the start of year y;
- ρ s,r :
-
is the proportion of the total recruitment of species s that settles in subzone r;
- M s :
-
denotes the (age-independent) natural mortality rate of species s; fishing was approximated as a pulse at the end of the 3rd quarter to simplify the computations and because this corresponds to the peak observed in monthly catch statistics;
- C syar :
-
is the predicted number of holothurians of species s, in year y, of age a, caught in subzone r; and,
- m :
-
is the maximum age considered (taken to be a plus-group and set equal to 5 for all species).
The number of recruits of species s at the start of year y is assumed to be related to the spawning stock size (i.e. the biomass of mature holothurians) by a modified Beverton-Holt stock-recruitment relationship (Haddon 2011). The spawning biomass is summed over all subzones r located within zone Z, and the predicted recruitment is then assigned to each subzone r as per Equation (1) and after allowing for annual fluctuations about the deterministic relationship, with such fluctuations varying by species but not spatially:
where:
- α s , β s :
-
are spawning biomass-recruitment relationship parameters for species s (re-parameterised in terms of the pre-exploitation equilibrium spawning biomass \( K_s^{sp } \) for each species s (and for the entire model area), and the “steepness”, h s , of the stock-recruitment relationship—see Haddon 2011);
- ζ sy :
-
reflects variation about the expected recruitment for species s in year y, and is assumed to be normally distributed with a mean of zero and standard deviation σ sR (which is input, based on the historic level of variability deduced from survey data). The –(σ sR )2/2 is a log-normal bias correction term;
- \( B_{syr}^{sp } \) :
-
is the spawning biomass for species s, in year y, in subzone r, computed as:
except for black teatfish, which spawns in the Austral winter and hence is modified as follows:
where:
- \( w_{sa}^{strt } \) :
-
is the begin-year mass of a holothurian of species s, age a, and strt is start-of-year;
- \( w_{sa}^{mid } \) :
-
is the mid-year mass of species s, age a, and mid is the middle of the year; and
- f sa :
-
is the proportion of holothurians of species s and of age a that are mature.
2.6 Reference set of OMs
Two key uncertainties in modelling resource status and productivity were: a) the natural mortality M s of each species, and b) the steepness parameter h s of the stock-recruitment functions. A Reference Set (RS) of operating models was thus constructed to include a sufficiently representative range of potential estimates of these parameters. In addition, to account for c) uncertainty as to the risks of posited climate-change effects, both high risk only and high-plus-medium risk combined scenarios were included in the RS. The fourth key uncertainty included in the RS was d) the consequence of the predicted impact of climate change on population variables, with an alternative scenario d) assuming a doubling of the impacts.
The RS cases were thus as follows:
-
M. Natural mortality:
-
M1: The average mortality estimates for each species were used, together with the growth parameters (length-weight-age relationships; Online Resource 2);
-
M2: The lower bound of the mortality estimates were used for each species, and because this is also a slow growth scenario, were combined with slow growth assumptions for the two teatfish species for which slow growth has been proposed as likely (Skewes et al. 2003; Uthicke et al. 2004).
-
-
H. Steepness parameter:
-
H1: h is fixed at 0.7 (Myers et al. 1995);
-
H2: h is fixed at a more conservative value of 0.5;
-
-
R. Risks:
-
R1: High risks only included;
-
R2: High plus medium risks included.
-
-
I. Impact:
-
I1: The predicted impacts on biological parameters as shown in Online Resource 1;
-
I2: All impacts (positive and negative) doubled.
-
2.7 Hand collectibles location choice model
Location choice within the hand collectibles fishery is modelled as a simple function describing utility by zone (see Online Resource 2). Weightings are placed on “habituation”, w h , profitability, w p , proximity of each zone to communities, w r , and habitat area within each zone, w a . The TAC or effort is then distributed in accordance with the relative utility of each zone.
2.8 Harvest strategy testing
The RS was projected 20-year forwards under a range of future fishing scenarios and management strategies (Table 3), which are hypothetical and have been selected to illustrate the range. The first scenario assumed that future catches continue roughly in the same manner as current (i.e. zero adaptation to climate change). The second set of six scenarios explore future strategies that are not reliant on monitoring, and are used to test the efficacy of some data-limited strategies under changing climate. The final set of five scenarios include a range of alternatives that would be possible with monitoring and spatial management (i.e. adaptive feedback in response to climate change):
-
A.
Current catch (average of past 5 years, w h = 1), plus small catch for species with current zero TAC
-
B.
No monitoring or adaptation:
-
B1. Double all catches (although unlikely, this is included to provide a contrast, as well as test a scenario in which there is increased pressure on some species due to declines in other resources)
-
B2. Profit maximisation (w p =1 to simulate future fisher location choice governed by profit considerations, with constant overall TAC)
-
B3. Location choice based on habitat area and distance from community (w r , w a = 0.5 to simulate future fisher location choice governed by habitat area and travel distance, with constant overall TAC)
-
B4. Spatial rotation (3-year spatial rotation strategy alternating between zones)
-
B5. Spatial closure (as no detailed data are assumed to become available to inform the choice of closure, this option tests an example that stops all fishing on sandfish, a sensitive species, and closes Warrior Reef, a known sensitive area)
-
B6. Multi-species catch composition (this option assumes that even without detailed monitoring, fishers will have some notion of changes in the overall species composition (simulated as the proportional abundance estimated with an error term added) and hence the TAC will be modified upwards (20 %) or temporary species-specific spatial closures implemented if the relative abundance of a species is thought to have changed by more than 20 % i.e. this is sampled with error in the model)
-
-
C.
Adaptive feedback/monitoring:
-
C1. Broken stick based on overall species-specific depletion (i.e. based on future monitoring (simulated with an error term corresponding to a survey sampling CV of 25 %), if the overall abundance of a species drops below a trigger reference point (Btrig) of 30%K, the fishing mortality for that species is reduced proportionately, and set to zero for depletion levels below the limit reference point (Blim) of 20%K. Broken stick control rules imply constant rates until some trigger biomass threshold followed by linear declines in fishing mortality down to a limit threshold after which no fishing occurs. Thus the control rule resembles a bent or broken stick)
-
C2. Broken stick with spatial adaptive management (i.e. if the local abundance (at the finer reef scale) of a species drops below Btrig, the fishing mortality for that species in that zone is reduced proportionately, and set to zero for depletion levels below Blim).
-
C3. Spatial closure whereby fishing is prohibited (spatially) on a species in a zone where it is estimated to be depleted below a more conservative limit reference point of 30%K, based on monitoring.
-
C4. Spatial closure (Entire Zone) whereby (for practical reasons), an entire spatial zone is closed if any of the eight species is estimated to be depleted to 30 % or less of K, based on monitoring.
-
C5. Spatial closures (Entire Zone) (as above but with 20 % trigger depletion level)
-
2.9 Performance Statistics
The following performance statistics were computed for each harvest strategy (HS) tested over a 20-year projection period:
-
1.
\( B_{2030}^{sp }/B_{1995}^{sp } \), the expected spawning biomass at the end of the projection period, relative to the starting (1995) level (used as a proxy for K, the unfished biomass), for each species averaged across the entire area and separately for each zone.
-
2.
\( B_{2030}^{sp }/B_{2030}^{sp } \) (no fishing, no climate change), the expected spawning biomass at the end of the projection period, relative to the comparable simulation no-fishing trial with no climate-change effects, for each species averaged across entire area. The same set of random numbers was used to generate sets of 480 no-fishing projections for each species and zone, as a baseline for comparisons with the range of projections with fishing and climate change.
-
3.
Risk of falling below limit biomass level of 20 % unfished biomass during the projection period for each species across all simulations and replicates, and for all spatial areas combined as well as for individual zones.
-
4.
Average catch: \( \frac{1}{20}\sum {C_y } \), over 2011 to 2030 (for each zone as well as the entire area, and for four groups of species: very high (sandfish), high (black teatfish and white teatfish), medium (surf redfish, prickly redfish, deepwater redfish and hairy blackfish) and low value (leopardfish).
-
5.
Species composition computed as the relative abundance (in 2030) of each species compared with the species composition from a no-fishing no-climate-change scenario. This takes into account the sometimes large historic catches (Plagányi et al. 2011c) as well as allowing for a 20-year recovery period.
2.9.1 Summary risk metrics
To allow easier visualisation and cross-comparison of results, the following summary risk statistics were defined and computed for each HS (Table 3):
-
1.
Risk of sub-optimal management: the percentage of species for which the median 2030 spawning biomass level was less than Btarg (0.48 K)
-
2.
Risk of depletion below Blim: percentage of species for which the lower 90 % confidence limit of the 2030 RS projections was less than Blim
-
3.
Risk of depletion below 30 % unfished: as above, but a more conservative risk measure
-
4.
Risk of local depletion: percentage of all individual runs that fell below Blim at any stage during the projection period
-
5.
Average annual profit (US$ million) computed as the landed weight of each species multiplied by current average market prices. This does not account for costs of monitoring and adaptive management.
3 Results
3.1 Climate change and its impacts
Growth in all life history stages (larval, juvenile and adults) was assessed as being at high risk related mostly to a likely increase in sea temperatures (Tables 1 and 2). This effect was assessed as being mostly positive for production and yields given the expected faster growth leading to larger sizes and increased fecundity. Medium risks contained both positive and negative effects. Positive effects were associated with an increase in larval growth due to projected increases in primary production (Brown et al. 2010), and faster adult growth and bigger sea cucumbers resulting in an increase in adult reproduction. Negative effects were associated with increased larval and juvenile mortality related to higher sea surface temperatures and detrimental effects on the juvenile sandfish seagrass habitats.
3.2 Reference set of operating models
The posited climate change impacts resulted in both negative and positive effects, and when these were modelled as affecting the various life history variables in combination, the net effect was slightly more negative for most species. The largest differences between the 16 OMs in the reference set resulted from the more severe climate impact cases, with the more severe impact case (I2) leading to more negative spawning biomass trajectories (Fig. 2, see also Online Resource 3). Sensitivity varied across species and spatial areas—for example, some of the largest differences were evident for the redfish species. The next largest effect overall was due to whether the high risks only (R1) or high-plus-medium risk (R2) case was used, with the latter typically more negative. However for the shallow-water specialist species (black teatfish, sandfish, surf redfish) for which sea-level rise is predicted to increase habitat availability, there was less of a difference between the high-risk and high-plus-medium risk cases, and projections were more sensitive to the mortality and growth assumptions. The mortality and growth scenario M1 produced slightly more positive population trajectories than the low mortality and slow growth scenario M2 (Fig. 2). Recruitment variability is naturally high (sigma = 0.5) and this largely swamped variability due to changing the steepness of the stock-recruit curve (H1 and H2). There were some differences between species, for example, those Leopardfish cases with a combination of high mortality rates and low steepness resulted in negative spawning biomass trajectories. As expected, teatfish population trajectories were more negative under the high mortality and slow growth cases (Fig. 2). Median spawning biomass trajectories are shown in Online Resource 3.
Reference Set model spawning biomass (t) projections for two species in each of the zones in which the species occurs, under no-fishing scenarios but with climate change effects. The alternative model versions differ in terms of choice of natural mortality (M), the steepness parameter hs of the stock-recruitment functions (H), whether both high risk only and high-plus-medium risk combined climate change scenarios are used (R) and the consequence of the predicted impact of climate change on population variables (I). Full results for all species are provided in Online Resource 3
3.3 Harvest strategy testing
None of the populations in any of the spatial zones decreased below B lim when the RS was projected 20-year forwards under the assumption of zero future fishing. However, if future patterns of catches were similar to current (in terms of total TACs, spatial distribution of fishing effort and choice of species), there were substantial declines predicted in some species and local crashes of populations (Fig. 2, Online Resource 3). Similar results were obtained when assuming future fishing location choices were based on drivers such as profit maximisation and distance from communities, without adaptively modifying harvest strategies to account for climate change. The probability envelopes encompassing the range of biological and climate uncertainties in the projections were fairly broad, but it was nonetheless possible to discriminate between alternative harvest strategies.
Our simulation testing suggested that the following harvest strategies would perform better under changing climate:
-
HS1: In data-limited situations with no future monitoring assumed, spatial rotation harvest strategies, such as those based on a 3-year rotation (Table 3), substantially reduced the risk to the resource under changing climate, and simultaneously resulted in moderately high overall profits being achieved.
-
HS2. In data-rich scenarios, with regular updates of resource status possible based on surveys (with assumed error added), a broken stick control rule was shown to substantially reduce the risk to the resource under changing climate, and simultaneously resulted in high overall profits being achieved. Spatial closures based on monitoring information were also successful in reducing the risks of overall as well as localised resource depletion.
Sandfish and leopardfish (respectively very high and low value species) are predicted to increase in relative abundance under a fishing and changing climate scenario (Fig. 3). The largest decrease in relative abundance compared with a reference species composition was predicted for black teatfish, a high value species.
Comparison of no-fishing no-climate-change scenario compared with a fishing and climate-change scenario on a model projected species composition (proportion of total biomass in year 2030) and b proportion of the total biomass under each scenario that is comprised of very high (VH), high (H), medium (M) and low (L) value species
4 Discussion
4.1 Risk assessment
Climate-change related changes in environmental variables such as temperature (Table 2) were predicted to have a positive effect on the growth of bêche-de-mer, with this counteracted by negative effects due to increased larval and juvenile mortality, as well as declines in seagrass habitats necessary for sandfish juveniles.
Although there is considerable uncertainty associated with this analysis, the effects of this uncertainty have been explored in the assessment, which can be updated in the future as more information becomes available. It is thus a practical first step towards linking the range of climatic effects over a range of life history components and critical habitats for fisheries and quantifying the resultant impact on fisheries productivity (Norman-Lόpez et al. 2012).
4.2 Risk management
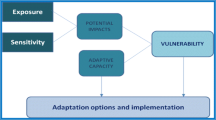
Traditional fisheries management specifies a number of objectives that seek to minimise risks (usually quantified as a high probability of achieving a favourable outcome) such as a) the risk of sub-optimal management; b) risk of overall and local depletion; c) risk of ecosystem effects; and d) risk of a non-viable fishery (profits, catch rates non-profitable). Climate change poses an additional risk, namely that of not responding appropriately or in a timely manner to changes in resource and ecosystem productivity, abundance, and distribution in response to climate change. Uncertainty pervades all aspects of fisheries management, from understanding of climate impacts, biology, monitoring, predicting future fisher behaviour and market changes. Our approach provides a biological complement to climate ensemble modeling approaches, and accounts for important sources of uncertainty that are an integral part of effective risk management decision making (Fig. 4).
Use of a Reference Set of models, rather than a single model, enabled collective capturing of some of the key biological uncertainties (model parameterisation—e.g. natural mortality estimates and steepness of the stock-recruitment relationship), as well as uncertainty as to the likelihood (using high risk scenarios only versus assuming both high and medium risk scenarios occur) and consequence (accounting for a doubling of the severity of each postulated effect) of climate-change effects. The posited climate effects generally had a greater impact on model projections than the biological uncertainties (Fig. 2). We did not incorporate in our analyses uncertainty pertaining to climate modelling, especially as there is a lack of deviations among the various models until about 2030, but future work could include alternative emission scenarios or use of multiple climate models, although such modelling should focus on periods after 2050.
Our paper provides a demonstration that MSE adheres to the guiding principles, summarized in the Australian national risk framework (Anon. 2009), that are needed to underpin and support effective risk management: Creates and protects value (contributes to societal objectives); informs decision making; explicitly addresses uncertainty; is systematic, structured and timely; is based on best available information; is tailored; considers and takes account of human and cultural factors; is transparent and inclusive; is dynamic, iterative and responsive to change; and facilitates continual improvement. The adaptive risk management framework we summarise in Fig. 4 has broad applicability to many natural resource management problems where it is important to consider both biological and climate variability and uncertainty in a balanced way.
4.3 Comparison of harvest strategies
Bêche-de-mer fisheries are difficult to manage globally, and there is some evidence from observations that management approaches based on reduced quotas, license restrictions, spatial rotation and adaptive management may lead to some success (Anderson et al. 2011). In evaluating alternative harvest strategies based on the setting of Total Allowable Catches, we simultaneously accounted for uncertainty in biological understanding as well as projected climate change impacts. In addition to more traditional performance measures to compare strategies (i.e. risk of depletion, overall profit), we demonstrated the utility of a novel measure based on multi-species composition.
When compared with a reference species composition for the entire Torres Straits area, sandfish and leopardfish (respectively very high and low value species) were predicted to increase in relative abundance under a fishing and changing climate scenario (Fig. 3). The largest decrease in relative abundance was predicted for black teatfish, stressing that climate change can impact not only the composition but also the value of a fishery. The multi-species catch composition harvest strategy performed well in terms of reducing the risk of resource depletion, without overly reducing profits, and hence merits further exploration as a strategy for data-poor multi-species fisheries.
Overall our results suggested that status quo management would result in half the species falling below target levels (suboptimal management occurs 50 % of the time), moderate risks of overall and local depletion, and significant changes in species composition (Table 3). The three simple strategies with no monitoring (spatial rotation, closed areas, multi-species composition) were all successful in reducing these risks, but with fairly substantial decreases in the associated average annual profit (Table 3). Higher profits (for the same risk levels) could only be achieved with strategies that included monitoring and hence adaptive management. Spatial management approaches based on adaptive feedback performed best overall. Dowling et al. (2008) similarly concluded that harvest control rules that include triggers and the use of spatial management may be most appropriate for small data-poor fisheries, and stressed the need for identifying data gathering protocols and simple analyses for these hard-to-manage fisheries.
References
A’mar ZT, Punt AE, Dorn MW (2009) The evaluation of two management strategies for the Gulf of Alaska walleye pollock fishery under climate change. ICES J Mar Sci 66:1614–1632
Anderson SC, Flemming JM, Watson R, Lotze HK (2011) Serial exploitation of global sea cucumber fisheries. Fish and Fisheries 12:317–339
Anon. (2009) National emergency risk assessment guidelines. Australian Emergency Management Committee Tasmanian State Emergency Service, Hobart
Brown CJ, Fulton EA, Hobday AJ, Matear R, Possingham HP et al (2010). Effects of climate-driven primary production change on marine food webs: implications for fisheries and conservation. Glob Chang Biol 16:1194–1212
Chin A, Kyne PM, Walker TI, McCauley RB (2010) An integrated risk assessment for climate change: analysing the vulnerability of sharks and rays on Australia's Great Barrier Reef. Glob Chang Biol 16:1936–1953
Cooke JG (1999) Improvement of fishery-management advice through simulation testing of harvest algorithms. ICES J Mar Sci 56:797
Dowling NA, Smith DC, Knuckey I, Smith ADM, Domaschenz P, Patterson HM, Whitelaw W (2008) Developing harvest strategies for low-value and data-poor fisheries: case studies from three Australian fisheries. Fish Res 94:380–390
Fletcher WJ (2005) The application of qualitative risk assessment methodology to prioritize issues for fisheries management. ICES J Mar Sci 62:1576–1587
Haddon M (2011) Modelling and quantitative methods in fisheries. Chapman & Hall, 2nd edn. 449p, p282–284
Hobday AJ, Smith ADM, Stobutzki IC, Bulman C, Daley R et al (2011) Ecological risk assessment for the effects of fishing. Fish Res 108:372–384
Hollowed AB, Bond NA, Wilderbuer TK, Stockhausen WT, A’mar ZT, Beamish RJ, Overland JE, Schirripa MJ (2009) A framework for modelling fish and shellfish responses to future climate change. ICES J Mar Sci 66:1584–1594
Hollowed AB, Barange M, Ito S-I, Kim S, Loeng H, Peck M (2011) Effects of climate change on fish and fisheries: forecasting impacts, assessing ecosystem responses, and evaluating management strategies. ICES J Mar Sci 68:984–985
Holt CA, Punt AE (2009) Incorporating climate information into rebuilding plans for overfished groundfish species of the U.S. west coast. Fish Res 100:57–67
Ianelli JN, Hollowed AB, Haynie AC, Mueter FJ, Bond NA (2011) Evaluating management strategies for eastern Bering Sea walleye Pollock (Theragra chalcogramma) in a changing environment. ICES J Mar Sci. doi:10.1093/icesjms/fsr010
IPCC (2007) Climate Change 2007: the physical science basis. In: Solomon S et al (eds) Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ. Press, Cambridge, p 996
Myers RA, Bridson J, Barrowman NJ (1995) Summary of worldwide stock and recruitment data. Canadian Technical Report of Fisheries and Aquatic Sciences. 2024, pp 327
Murphy NE, Skewes TD, Filewood F, David C, Seden P, Jones A (2011) The recovery of the Holothuria scabra (sandfish) population on Warrior Reef, Torres Strait. CSIRO Wealth from Oceans Flagship Final Report, CSIRO, Cleveland, p 44
Norman-Lόpez A, Plagányi ÉE, Skewes T, Poloczanska E, Dennis D, Gibbs M, Bayliss P (2012) Linking physiological, population and socio-economic assessments of climate change impacts on fisheries. Fish Res. doi:10.1016/j.fishres.2012.02.026
Plagányi ÉE, Bell J, Bustamante R, Dambacher J, Dennis D, Dichmont C, Dutra L, Fulton E, Hobday A, van Putten I, Smith F, Smith T, Zhou S (2011a) Modelling climate change effects on Australian and Pacific aquatic ecosystems: a review of analytical tools and management implications. Mar Freshw Res 62(9):1132–1147
Plagányi ÉE, Weeks S, Skewes T, Gibbs M, Poloczanska E, Norman-Lόpez A, Blamey L, Soares M, Robinson W (2011b) Assessing the adequacy of current fisheries management under changing climate: a southern synopsis. ICES J Mar Sci 68:1305–1317. doi:10.1093/icesjms/fsr049
Plagányi ÉE, Skewes TD, Dowling N, Haddon M (2011c) Evaluating management strategies for data-poor bêche de mer species in Torres Strait. CSIRO/DAFF Report, Brisbane, Australia, 84 pp
Poloczanska ES, Hobday AJ, Richardson AJ (eds) (2009) Report card of marine climate change for Australia, NCCARF Publication 05/09, ISBN 978-1-921609-03-9
Rademeyer RA, Plagányi ÉE, Butterworth DS (2007) Tips and tricks in designing management procedures. ICES J Mar Sci 64:618–625
Sainsbury KJ, Punt AE, Smith ADM (2000) Design of operational management strategies for achieving fishery ecosystem objectives. ICES J Mar Sci 57:731–741
Skewes TD, Burridge CM, Hill BJ (1998) Survey of Holothuria scabra on Warrior Reef, Torres Strait. CSIRO Division of Marine Research Report to the Queensland Fisheries Management Authority, CSIRO, Cleveland, p 12
Skewes TD, Dennis DM, Koutsoukos A, Haywood M, Wassenberg T, Austin M (2003) Stock survey and sustainable harvest strategies for Torres Strait bêche-de-mer. CSIRO Division of Marine Research Final Report, Cleveland Australia. AFMA Project Number: R01/1343. ISBN 1 876996 61 7, 50 pp
Skewes TD, Murphy NE, McLeod I, Dovers E, Burridge C, Rochester W (2010) Torres Strait Hand Collectables, 2009 survey: Sea cucumber. CSIRO, Cleveland, 70 pp
Smith ADM, Fulton EJ, Hobday AJ, Smith DC, Shoulder P (2007) Scientific tools to support the practical implementation of ecosystem-based fisheries management. ICES J Mar Sci 64:633–639
Steele JH, Gifford DJ (2010) Reconciling end-to-end and population concepts for marine ecosystems. J Mar Syst 83:99–103
Uthicke S, Welch D, Benzie J (2004) Slow growth and lack of recovery in overfished holothurians on the Great Barrier Reef: evidence from DNA fingerprints and repeated large-scale surveys. Conserv Biol 18:1395–1404
Walters CJ (1986) Adaptive management of renewable resources. MacMillan Pub. Co, New York, USA, p 374
Wilson DT, Curtotti R, Begg GA (eds) (2010) Fishery status reports 2009: status of fish stocks and fisheries managed by the Australian Government, Australian Bureau of Agricultural and Resource Economics – Bureau of Rural Sciences, Canberra
Acknowledgements
EP gratefully acknowledges funding from the organisers to attend the International Workshop on Climate and Ocean Fisheries, Rarotonga, 3–5 October 2011. This research was funded by CSIRO, Australia. We thank Rik Buckworth, Nicole Murphy and three anonymous reviewers for comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Special Issue on "Climate and Oceanic Fisheries" with Guest Editor James Salinger.
Rights and permissions
About this article
Cite this article
Plagányi, É.E., Skewes, T.D., Dowling, N.A. et al. Risk management tools for sustainable fisheries management under changing climate: a sea cucumber example. Climatic Change 119, 181–197 (2013). https://doi.org/10.1007/s10584-012-0596-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10584-012-0596-0