Abstract
Genome editing tools (GET), including zinc-finger nucleases (ZFN), transcription activator-like endonucleases (TALENS), and meganucleases possess long recognition sites and are thus capable of cutting DNA in a very specific manner. These genome editing tools mediate targeted genetic alterations by enhancing DNA mutation frequency via induction of double-strand breaks at a predetermined genomic site. Compared to conventional homologous recombination based gene targeting, GETs can increase gene targeting and gene disruption via mutagenic DNA repair more than 10,000-fold. Recently, a novel class of genome editing tools was described that uses RNAs to target a specific genomic site. The CRISPR/Cas9 system is capable of targeting even multiple genomic sites in one shot and thus could be superior to ZFNs or TALEN. Current results indicate that these tools can be successfully employed in a broad range of organisms which renders them useful for improving the understanding of complex physiological systems, producing transgenic animals, including creating large animal models for human diseases, creating specific cell lines, and plants, and even for treating human genetic diseases. This review provides an update on the use of ZFNs to modify the genome of farm animals, summarizes current knowledge on the underlying mechanism, and discusses new opportunities for generating genetically modified farm animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic modification starts with the creation of a double-strand break (DSB) of the DNA. The efficiency of a targeted genetic modification can be significantly enhanced by creating a site-specific DSB (Rouet et al. 1994). Genome editing tools normally consist of a cleavage domain and a DNA binding domain, which can be designed to bind to nearly any DNA sequence. By selecting for different outcomes of DNA repair, either gene knockout or targeted transgene insertion can be obtained.
Homologous recombination is a rare cellular event that has numerous applications, including studies of basic mechanisms in mammalian development and physiology, and the production of transgenic farm animals for xenotransplantation, as human disease models, for gene pharming or simply to increase breeding performance and/or specific agriculturally important traits. In embryonic stem cells, homologous recombination (HR) can be achieved using a positive-negative selection approach based on the presence of an antibiotic selection cassette within the homologous region, which will confer resistance against an antibiotic drug. By using a promoterless approach, the resistance cassette has to be driven by an endogenous active promoter. This approach significantly reduces the amount of false positive cell clones. Combining the promoterless approach with a selection cassette localized outside of the homologous region further reduces the amount of false positive selected cell clones. The overall efficiency obtained by using such an approach normally does not exceed 10−6 HR events. In contrast, several studies have reported 1–18 % homologous recombination events per mammalian cell, when the targeted double-strand break was introduced by natural or artificial endonucleases (Choulika et al. 1995; Donoho et al. 1998; Epinat et al. 2003; Vasquez et al. 2001; Szczepek et al. 2007). Meganucleases were the first genome editing tools that were discovered and used to cut a specific DNA within the host genome. Following the discovery that induction of a double-strand break increases the frequency of homology-directed repair (HDR) by several orders of magnitude, targeted nucleases have emerged as the method of choice for improving the efficiency of HDR-mediated genetic alterations. By co-delivering a site-specific nuclease with a donor plasmid bearing locus-specific homology arms, single or multiple transgenes or mutations can be efficiently integrated into any endogenous locus. In the past few years, new genome editing tools were discovered that cut DNA in a very precise manner, with unprecedented efficiency and in a straightforward manner. These new programmable endonucleases include zinc-finger nucleases, Transcription activator-like effector endonucleases (TALEN) and the most recent addition are RNA-programmed genome editors (CRISPR/Cas9). Here, we provide an update on the recent advances in ZFN application in farm animals.
Zinc-finger nucleases
Structure of zinc-finger nucleases
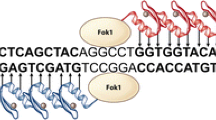
The first zinc-finger (ZF) motif which had specific binding affinity to DNA was discovered as part of the transcription factor IIIa in Xenopus oocytes (Miller et al. 1985). A typical zinc finger (Cys2His2) consists of 30 amino acids which form two anti-parallel β-sheets opposing an α-helix (Pabo et al. 2001). The domain is stabilized by two cysteine and two histidine residues binding a zinc ion, thus forming a compact globular domain. The zinc-finger motif uses residues in the alpha helix to bind to approximately 3 specific base pairs in the major groove of the DNA (Pavletich and Pabo 1991). ZFs can be designed to bind almost any base triplet (Pabo et al. 2001). Multiple ZFs can be combined to form a larger DNA-recognition domain which in turn increases specificity and efficiency of genetic modification. Specific binding of individual zinc fingers is largely independent, with some contacts between adjacent fingers altering base pair recognition. While the zinc-finger motif was discovered in the 1980s (Miller et al. 1985), ZFNs have a shorter history. The first specific zinc-finger nuclease was reported ∼15 years after the discovery of the zinc-finger domain (Kim et al. 1996). A ZFN consists of a site-specific zinc-finger DNA binding domain fused to the nonspecific cleavage domain of the FokI endonuclease. At least, two ZFN molecules are required for genetic modification, since the FokI nuclease must dimerize to cut the DNA. The need of two ZFN molecules doubles the number of specifically targeted base pairs (Smith et al. 2000). The two ZFN molecules bind to the targeted DNA in a tail-to-tail orientation separated by 5–7 bp, with double-stranded DNA cleavage occurring in the spacer region (Fig. 1).
Schematic drawing of a zinc-finger nuclease (ZFN) which binds as juxtaposed pairs. Zinc-finger (ZF) molecules bind to their specific DNA sequence. Each ZF molecule is specific to a base triplet. To enhance specificity three to six (or more) ZF molecules are linked to each other to recognize a DNA sequence of 9–18 bp. Dimerization of the FokI endonuclease causes a double-strand break in the DNA
Genetically modification by zinc-finger nucleases
To employ a specific ZFN for genetic engineering, the plasmid DNA or messenger RNA (mRNA) encoding a specific ZFN is introduced into cells or embryos via microinjection or transfection (Hauschild-Quintern et al. 2013a). After translation, the ZFN pair binds to its specific target, the FokI nucleases dimerize, and the DNA is cleaved. ZFN activity can be enhanced by incubating transfected cells at 30 °C for a few days (Doyon et al. 2010). Cultivation at subphysiological temperatures slows down the cell cycle, giving the ZFNs more time to bind and cut at the targeted locus (personal communication Greg Cost, Sangamo Biosciences, CA, USA). A ZFN pair induces a site-specific DSB only at the genomic site for which the molecule had been designed.
After ZFN-mediated DNA cleavage in eukaryotic cells, double-strand break repair is initiated, either by nonhomologous end-joining (NHEJ) or homology-directed repair (HDR). NHEJ is error-prone and often creates short insertions or deletions (indels) of a few base pairs (10–20 bp) at the sealed break (Bibikova et al. 2002). Such mutations can cause frame-shift or disruption of a gene, which in turn leads to the genetic knockout. Since the frequency of genetic modification is generally >1 %, isolation of knockout cells is readily achieved by interrogation of cell clones generated by limiting dilution. Fluorescence-activated cell sorting (FACS) or magnetic bead selection have been successfully employed to enrich the targeted non-immortalized and other poorly clonable cells lines (Yu et al. 2011; Whyte et al. 2011; Hauschild et al. 2011; Li et al. 2012).
Mitotic cells often repair a DSB using homology-based DNA repair. In such a case, the cell normally uses the sister chromosome as a template to repair the DSB, which faithfully restores the original sequence (Fig. 2). The molecule can be used as template when a donor DNA molecule containing homologous arms to both sides of the DSB is co-transfected with the ZFNs. The exogenous DNA sequence placed between the two regions of homology will be copied into the chromosome during the DNA repair process (Moehle et al. 2007). In the absence of a site-specific break, the donor DNA must contain a large region (6–7 kb) homologous to the targeted region for capturing one of the rare spontaneous breaks (Deng and Capecchi 1992). In contrast, ZFN-based targeting strategy is compatible with a significantly shorter stretch of homologous DNA. Typically, 500–1500 bp are used. Even linear <50-bp homologous donor sequences (Orlando et al. 2010) and single-stranded DNA oligonucleotides can be used to induce mutations, deletions, or insertions at the target site (Chen et al. 2011).
Schematic drawing to demonstrate potential outcomes of a double-strand break in DNA generated by a pair of ZFNs, gene disruption, or DNA insertion. If cells are treated with ZFNs alone, the repair process called nonhomologous end-joining results in the rejoining of the broken ends of the DNA. As a consequence, insertions or deletions (indels) result in the generation of a shortened or nonfunctional protein (gene disruption). In contrast, if cells are treated with ZFNs in the presence of donor DNA that encodes for entire new gene or a small mutation of the endogenous gene, the cell can use the donor DNA as a template to repair the broken DNA. By using a coding sequence that is flanked by short arms homologous to the target sequence, a high frequency of targeted integration can be obtained. This process is called homology-directed repair (HDR)
Integration of the ZFN has to be avoided as it would result in permanent transcription of the ZFN and thereby would likely lead to permanent nonspecific DNA cleavage. Usually, ZFN plasmids are rapidly diluted and disappear from the treated cells when a transient transfection protocol is applied. Besides the high efficiency, a major advantage of ZFN-mediated targeting is the lack of random integration, which prevents negative side effects such as insertional mutagenesis. Nuclease-mediated targeted integration normalizes for positional effects that typically confound many types of genetic analysis and enables study of structure-function relations in the complex and native chromosomal environment. ZFNs have been broadly applied in basic research, biotechnology, and medicine, but genome engineering with ZFNs is limited by the random generation of unwanted indels at homology sites (Liu et al. 2013). One potential strategy to overcome this limitation is the targeted introduction into DNA containing a single-strand break (SSB) or nick. A nick can be equivalent to a double-strand break (DSB) and stimulate the HDR pathway (Meselson and Radding 1975; Radding 1982). In contrast to a DSB, a nick is not a bona fide substrate for repair by the NHEJ pathway. Thus, a targeted nick has the potential to restrict repair to the HDR pathway (Wang et al. 2012).
Genetically modifications of farm animals using zinc-finger nucleases
Transgenic farm animals, specifically the domestic pig, increasingly serve as model in human medicine, including xenotransplantation, basic research, and human disease models. The latter is an important complementation to the laboratory mouse where it has frequently been shown that the typical disease manifestation does not fully mimic the human disease symptoms. Pigs share many genetic, anatomical, and physiological features with humans, and have rapidly emerged as a suitable model for specific diseases, including cystic fibrosis, diabetes, cancer, and several neurological disorders (Flisikowska et al. 2014). Pigs are also considered as suitable organ donors for xenotransplantation to reduce or even eliminate the shortage of suitable human organs (Cooper and Ayares 2011; Petersen et al. 2009). This requires genetic modification of the donor pigs to overcome the severe immunological rejection responses occurring after pig-to-primate xenotransplantation. Conventional targeting is extremely inefficient and usually does not lead to a biallelic KO. Moreover, true germline competent pluripotent cells are not yet available from pigs and other domestic animals, which prevents selection for rare HDR events as it is feasible in laboratory mice (Nowak-Imialek and Niemann 2012). The production of transgenic farm animals is significantly facilitated by effective somatic cell nuclear transfer (SCNT) protocols (Petersen et al. 2008). This cell-mediated transgenesis is compatible with screening for genetic modifications and analysis of the transgenic genotype in vitro rather than in animals ‘on the farm.’ These cells are then used to produce animals with the desired phenotype. While cell-mediated transgenesis is more labor-intensive than direct transgenesis, in vitro genetic manipulation of cells followed by detailed genome analysis bears significant advantages. First, it reduces the total number of animals required to generate a useful transgenic offspring. Second, it increases dramatically the number of independent transgene integration events that can be screened and investigated. Third, it facilitates the engineering of precisely controlled genetic alterations (gene targeting) by allowing selection and isolation of rare integration events resulting from homologous recombination. Fourth, the use of a selected cell clone as cell donor for SCNT leads to a syngenic clone cohort, which facilitates detailed analysis of the phenotype. Finally, biallelic knockout via ZFN provides a significant time advantage compared with traditional knockout via homologous recombination, which significantly streamlines the production of relevant large animal models. Genome editing technology has been successfully applied to zebrafish (Bedell et al. 2012), rabbits (Flisikowska et al. 2011) and rodents (Geurts et al. 2009) by direct injection of mRNA or DNA of genome editors into embryos. Injection of ZFN mRNA or DNA into zygotes can also be used to generate null phenotype offspring in large animals with high efficiencies (Lillico et al. 2013). This high versatility of genome editing tools allowed many laboratories worldwide the use of this technology.
Pig
The first experiments with ZFNs to modify the pig genome were conducted to target the transgenic eGFP (pCX-eGFP) locus in the domestic pig, with ∼10 genomic integration sites. After targeting, the rate of nonfluorescent cells increased from 6 % (control) to 21 % (ZFN-targeted cells), showing that in ∼15 % of the cells, nearly all copies of the eGFP gene had been disrupted. Sequencing of several nonfluorescent cell clones revealed that wild-type DNA (non-mutated eGFP) variants remained, implying that at least one intact eGFP copy was silenced (Watanabe et al. 2010).
The first live ZFN-mediated KO pig carried a hemizygous transgenic eGFP allele. Porcine fibroblasts were co-transfected with a pair of ZFN plasmids and a red-fluorescent CAG-tomato plasmid (transient selectable fluorophore). Two percent of the cells showed red fluorescence and could be sorted by FACS. A second round of selection for green cells by FACS led to 5 % eGFP negative cells. Selected cells used in SCNT led to the delivery of six out of seven piglets with knocked out eGFP fluorescence. Sequencing revealed several deletions and insertions at the targeted locus. A third litter with six piglets was entirely eGFP negative. One piglet carried an unusual large deletion of 700 bp deleting nearly the entire eGFP coding sequence (Whyte et al. 2011). Yang et al. showed that ZFNs can be used to target endogenous genes and that these cells are capable to generate live offspring (Yang et al. 2011a). They targeted the endogenous peroxisome proliferator-activated receptor-γ (PPAR-γ) locus by using ZFNs. PPAR-γ−/− animals could be a useful model for studies on cardiovascular diseases. Male fibroblasts were co-transfected with a PPAR-γ-specific ZFN pair and a neomycin-resistance gene. After selection with G418, 4 % of screened cell clones carried a mutated PPAR-γ gene and served as donors in SCNT. Two live-born piglets carried a mutation in one of the PPAR-γ alleles. Western blotting analysis confirmed the successful production of heterozygous PPAR-γ KO animals (Yang et al. 2011b).
The first pigs with a biallelic KO of an endogenous gene via ZFN targeting were produced by our laboratory (Hauschild et al. 2011). Transfection of fetal fibroblasts with a pair of ZFN plasmids directed against exon 9 (catalytic domain) of the α1,3-galactosyltransferase (GGTA1, Gal) gene induced biallelic mutations in 1 % of the cells. The α1,3-galactosyltransferase synthesizes galactose-epitopes on the surface of porcine cells, which are the major antigen after xenotransplantation and are recognized by 1 % of all antibodies circulating in human blood flow. Magnetic beads were used to counter-select for Gal-negative cells, reaching a purity of >99 % Gal-negative cells. The Gal-negative cells served as donor cells in somatic cell nuclear transfer (SCNT) and led to the birth of nine live GGTA1-knockout piglets. Sequencing revealed five different haplotypes with two homozygous and three heterozygous (individual mutations on each allele) mutations. The GGTA1 gene showed deletions from 1 to 7 bp in size and one unusual large deletion of 96 bp. The GGTA1-KO fibroblasts derived from the ZFN approach were protected against lysis in a complement in vitro assay. Disruption of both alleles by conventional HR generally involves production of mono-allelic knockout clones followed by breeding with other heterozygous knockouts to obtain a homozygous knockout in 25 % of the offspring (Whyte and Prather 2012). Compared to conventional gene targeting, the use of ZFNs to generate a functional gene knockout led to a 10,000-fold efficiency increase.
In a follow-up study, we showed that the efficiency of the ZFNs is not influenced by the gender of the cells (Hauschild-Quintern et al. 2013b), thus allowing production of knockout pigs of both sexes with similar efficiency. Our results to disrupt the porcine GGTA1-locus by using ZFNs have been confirmed by other groups (Li et al. 2012; Bao et al. 2014), showing the robustness and reproducibility of this technology.
After knockout of GGTA1, the Hanganutziu-Deicher antigen remains a major antigen that is implicated in subsequent xenograft rejection (Ezzelarab et al. 2005). The responsible porcine gene for the generation of the HD-antigen on porcine cells is the CMP-N-acetylneuraminic acid hydroxylase (CMAH). ZFNs designed to target exon 8 of the CMAH locus led to 9.1 % targeted alleles when donor DNA coding for a neomycin resistance cassette was not added to the transfection mix. A dramatic increase of targeting frequency (41.7 %) was observed when donor DNA with a 789-bp homologous 5′ arm and a 763-bp homologous 3′ arm was added. Biallelic knockouts were in all cases associated with integration of the exogenous DNA (Kwon et al. 2013). A possible explanation for this difference is the difficulty of separating non-transfected from total cells used for the transfection without a selection marker. This study demonstrated for the first time gene targeting using ZFN-assisted HR of donor DNA in porcine somatic cells (Kwon et al. 2013).
In these studies, the ZFN-encoding DNA was introduced into nuclear donor cells for SCNT to produce genetically modified pigs. However, plasmid DNA can also be integrated into the genome of cells, which may result in disruption of endogenous genes and constitutive expression of ZFNs. This drawback of plasmid DNA can be eliminated by the use of ZFN-encoding mRNA, which cannot be inserted into the host genome. Watanabe et al. (2013) applied ZFN-encoding mRNA to knockout the interleukin-2 receptor gamma (IL2RG) gene on the X-chromosome of male porcine fibroblasts; these cells supported development to live offspring after SCNT (Watanabe et al. 2013). The IL2RG KO pigs obtained in this study lacked T and NK cells but showed normal B cell populations which mimic adequately the phenotype of human XSCID patients. Due to the limited capacity of their immune system, IL2RG KO pigs are susceptible to any kind of pathogen. To keep such pigs for longer studies, expensive gnotobiotic housing conditions are necessary. Nevertheless, IL2RGKO pigs represent the first step toward developing a porcine SCID model and can contribute not only to cancer and stem cell research but also to preclinical evaluation of the transplantation of pluripotent stem cells such as iPS cells (Watanabe et al. 2013).
Lillico et al. (2013) tested a pair of ZFNs with a targeted location of 1496 to 1532 bp relative to the translational start site in porcine RELA cDNA sequence (Lillico et al. 2013). The RELA locus might play an important role in generating pigs resistant against African swine fever. Transfer of embryos injected with 10 ng/μl RELA ZFN mRNA failed to generate a pregnancy, while two transfers of embryos injected with 2 ng/μl RELA ZFN mRNA resulted in two pregnancies and the birth of nine piglets. One of the nine piglets (11 %) carried a biallelic editing event at the ZFN targeting site, and the remaining eight piglets did not show any modification at the RELA locus.
Cattle
In cattle, ZFN-mediated gene targeting was conducted to produce beta-lactoglobulin (BLG)-KO animals. BLG is the major whey protein in bovine milk and is the critical milk allergen. Bovine fetal fibroblasts were transfected with mRNA coding for ZFNs designed against the BLG gene. Sequencing revealed that ∼15 % of the cells carried a mutated variant and 3 % of the single cell colonies showed a biallelic BLG gene knockout. Homozygous KO-cells were used in SCNT and 8 cloned animals were born; one survived the postnatal period. The mutated BLG gene was shorter (9- and 15-bp deletion, no frame shift) than the wild-type version. Off-target site mutations induced by the ZFN pair were also analyzed for BLG. While a 1-bp mismatch with the targeting sequence led to 7 % gene targeting (single nucleotide polymorphism in cattle), 3- and 7-bp mismatches did not result in a mutated phenotype in sheep and pigs. Results suggest that ZFN-mediated targeting is promising for specific gene editing in large domestic animals with little risk of off-target site cleavage (Yu et al. 2011).
Mastitis costs the dairy industry billions of dollars annually and is the most consequential disease of dairy cattle. Therefore, the use of genome editing tools to integrate mastitis resistance via transgenes such as human lysozyme (Liu et al. 2014) or lysostaphin (Liu et al. 2013) in the β-casein locus may open a unique avenue for the creation of transgenic cows with enhanced mastitis resistance and improved health and welfare of livestock. ZFNickase-stimulated gene addition at the endogenous bovine locus is feasible and compatible with the production of cloned bacterial lysostaphin-transgenic cows (Liu et al. 2013). In this particular study, the FokI catalytic domain was mutated at amino acid D450 in one of the two ZFNs necessary for dimerization and subsequent DNA cleavage, leading to nickase activity of the ZFN pair. A lysostaphin coding vector was transfected into bovine fetal fibroblasts along with expression plasmids encoding ZFN/ZFNickase to introduce a nick in intron 2 of the CSN2locus. Finally, 69 cell clones with a correct integration of the lysostaphin vector at the CSN2 locus were obtained and using these cells as donors in somatic cell nuclear transfer resulted in 16 transgenic calves. When calves were induced to lactate, the milk contained lysostaphin-mediated antibacterial activity.
The relatively high percentage of integration of a long DNA fragment into a predetermined locus in the bovine genome demonstrates that ZFNickases are active in bovine cells and can be used to further minimize the risk of potential off-target events. Thereby, the use of ZFNickases ensures that only a single copy of the transgene is integrated into the host genome. This can further facilitate a range of new transgenic technologies beneficial for both agriculture and biomedicine.
Concluding remarks and future directions
Genome editing tools such as ZFNs, TALENs, and RNA-guided DNA endonucleases (CRISPR/Cas) have emerged as valuable molecular tools that have already been shown to revolutionize biological research with great benefits for personalized medicine. These emerging technologies significantly expand the ability to create and study model organisms, including large animals, and will be instrumental for correcting many genetic diseases in livestock species and humans. With the aid of these tools, researchers are able to develop biomedical models in species that are physiologically closer related to humans than mice. The domestic pig is particularly promising in this regard. The growing number of human disease models in pigs supports this assumption (Flisikowska et al. 2014).
Due to the high degree of physiological similarity with humans, porcine organs are considered as promising solution to satisfy the growing demand of human organs for allotransplantation. To achieve this goal and to avoid immune rejection responses, the porcine genome has to be modified to ensure long-term survival of porcine organs in patients after xenografting. ZFNs, TALENs, and CRISPR/Cas can now be used to elegantly knockout candidate pig genes or to precisely knockin transgenes at specific genomic sites in the porcine genome to produce pigs specifically tailored as organ donors.
However, to exploit the full potential of these new technologies, important questions and challenges must be addressed. A high degree of specificity is a main challenge and would be a critical prerequisite for employing these technologies in human patients or for the generation of livestock animals. Comprehensive profiling of off-target cleavage sites will provide insight into the stringency of target recognition in each system, which in turn will help to increasing the specificity of the systems and to develop algorithms that calculate the most promising sequences to be targeted within a specific locus. The feasibility to use ZFNickases for genetic alterations of farm animals is a great step forward to lower the risk of off-target events, making the technology more predictable.
Although CRISPR/Cas seems to show the greatest promise and flexibility for genetic engineering, sequence requirements within the PAM sequence may constrain some applications. Therefore, evolution of the Cas9 protein should pave the way toward PAM independence and may also provide means to generate an even more efficient Cas9 endonuclease. Additional studies will also be required to evaluate the specificity and toxicity of RNA-guided DNA endonucleases in vitro and in vivo. Recent developments, in which an inactivated Cas element was conjugated to the FokI endonuclease, which requires dimer formation is promising as thereby a higher specificity can be achieved (Tsai et al. 2014; Guilinger et al. 2014). Biophysical and biochemical studies on CRISPRs could help to improve the design of next-generation genome editing tools.
The different genome editing tools have their individual advantages and disadvantages and the selection of a specific system seems more to depend on the expertise of the individual researcher rather than on the weaknesses of one of these technologies. In summary, genome editors are valuable tools, scientists 10 years ago could only dream of. These technologies expand and revolutionize our ability to explore and alter any genome and constitute a new and promising paradigm to understand and treat diseases.
Abbreviations
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- DSB:
-
Double-strand break
- EGFP:
-
Enhanced green fluorescent protein
- FACS:
-
Fluorescence-activated cell sorting
- GET:
-
Genome editing tools
- HR:
-
Homologous recombination
- HDR:
-
Homology-directed repair
- IPS:
-
Induced pluripotent stem cells
- NHEJ:
-
Nonhomologous end joining
- SCNT:
-
Somatic cell nuclear transfer
- SSB:
-
Single-strand break
- TALEN:
-
Transcription activator-like effector endonuclease
- ZF:
-
Zinc-finger
- ZFN:
-
Zinc-finger nuclease
References
Bao L, Chen H, Jong U, Rim C, Li W, Lin X, Zhang D, Luo Q, Cui C, Huang H, Zhang Y, Xiao L, Fu Z (2014) Generation of GGTA1 biallelic knockout pigs via zinc-finger nucleases and somatic cell nuclear transfer. Sci Chin Life sci 57(2):263–268. doi:10.1007/s11427-013-4601-2
Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, Fahrenkrug SC, Carlson DF, Voytas DF, Clark KJ, Essner JJ, Ekker SC (2012) In vivo genome editing using a high-efficiency TALEN system. Nature 491(7422):114–118. doi:10.1038/nature11537
Bibikova M, Golic M, Golic KG, Carroll D (2002) Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 161(3):1169–1175
Chen F, Pruett-Miller SM, Huang Y, Gjoka M, Duda K, Taunton J, Collingwood TN, Frodin M, Davis GD (2011) High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat Methods 8(9):753–755. doi:10.1038/nmeth.1653
Choulika A, Perrin A, Dujon B, Nicolas JF (1995) Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol 15(4):1968–1973
Cooper DK, Ayares D (2011) The immense potential of xenotransplantation in surgery. Int J Surg 9(2):122–129. doi:10.1016/j.ijsu.2010.11.002
Deng C, Capecchi MR (1992) Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus. Mol Cell Biol 12(8):3365–3371
Donoho G, Jasin M, Berg P (1998) Analysis of gene targeting and intrachromosomal homologous recombination stimulated by genomic double-strand breaks in mouse embryonic stem cells. Mol Cell Biol 18(7):4070–4078
Doyon Y, Choi VM, Xia DF, Vo TD, Gregory PD, Holmes MC (2010) Transient cold shock enhances zinc-finger nuclease-mediated gene disruption. Nat Methods 7(6):459–460
Epinat JC, Arnould S, Chames P, Rochaix P, Desfontaines D, Puzin C, Patin A, Zanghellini A, Paques F, Lacroix E (2003) A novel engineered meganuclease induces homologous recombination in yeast and mammalian cells. Nucleic Acids Res 31(11):2952–2962
Ezzelarab M, Ayares D, Cooper DK (2005) Carbohydrates in xenotransplantation. Immunol Cell Biol 83(4):396–404. doi:10.1111/j.1440-1711.2005.01344.x
Flisikowska T, Thorey IS, Offner S, Ros F, Lifke V, Zeitler B, Rottmann O, Vincent A, Zhang L, Jenkins S, Niersbach H, Kind AJ, Gregory PD, Schnieke AE, Platzer J (2011) Efficient immunoglobulin gene disruption and targeted replacement in rabbit using zinc finger nucleases. PLoS One 6(6):e21045. doi:10.1371/journal.pone.0021045
Flisikowska T, Kind A, Schnieke A (2014) Genetically modified pigs to model human diseases. J Appl Genet 55(1):53–64. doi:10.1007/s13353-013-0182-9
Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Menoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R (2009) Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325(5939):433. doi:10.1126/science.1172447
Guilinger JP, Thompson DB, Liu DR (2014) Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol. doi:10.1038/nbt.2909
Hauschild J, Petersen B, Santiago Y, Queisser AL, Carnwath JW, Lucas-Hahn A, Zhang L, Meng X, Gregory PD, Schwinzer R, Cost GJ, Niemann H (2011) Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A 108(29):12013–12017. doi:10.1073/pnas.1106422108
Hauschild-Quintern J, Petersen B, Cost GJ, Niemann H (2013a) Gene knockout and knockin by zinc-finger nucleases: current status and perspectives. Cellular and molecular life sciences : CMLS 70(16):2969–2983. doi:10.1007/s00018-012-1204-1
Hauschild-Quintern J, Petersen B, Queisser AL, Lucas-Hahn A, Schwinzer R, Niemann H (2013b) Gender non-specific efficacy of ZFN mediated gene targeting in pigs. Transgenic Res 22(1):1–3. doi:10.1007/s11248-012-9647-6
Kim YG, Cha J, Chandrasegaran S (1996) Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A 93(3):1156–1160
Kwon DN, Lee K, Kang MJ, Choi YJ, Park C, Whyte JJ, Brown AN, Kim JH, Samuel M, Mao J, Park KW, Murphy CN, Prather RS, Kim JH (2013) Production of biallelic CMP-Neu5Ac hydroxylase knock-out pigs. Sci rep 3:1981. doi:10.1038/srep01981
Li P, Estrada JL, Burlak C, Tector AJ (2012) Biallelic knockout of the alpha-1,3 galactosyltransferase gene in porcine liver-derived cells using zinc finger nucleases. Journal of surgical research, The. doi:10.1016/j.jss.2012.06.035
Lillico SG, Proudfoot C, Carlson DF, Stverakova D, Neil C, Blain C, King TJ, Ritchie WA, Tan W, Mileham AJ, McLaren DG, Fahrenkrug SC, Whitelaw CB (2013) Live pigs produced from genome edited zygotes. Sci rep 3:2847. doi:10.1038/srep02847
Liu X, Wang Y, Guo W, Chang B, Liu J, Guo Z, Quan F, Zhang Y (2013) Zinc-finger nickase-mediated insertion of the lysostaphin gene into the beta-casein locus in cloned cows. Nat Commun 4:2565. doi:10.1038/ncomms3565
Liu X, Wang Y, Tian Y, Yu Y, Gao M, Hu G, Su F, Pan S, Luo Y, Guo Z, Quan F, Zhang Y (2014) Generation of mastitis resistance in cows by targeting human lysozyme gene to beta-casein locus using zinc-finger nucleases. Proc Biol sci / The Royal Society 281(1780):20133368. doi:10.1098/rspb.2013.3368
Meselson MS, Radding CM (1975) A general model for genetic recombination. Proc Natl Acad Sci U S A 72(1):358–361
Miller J, McLachlan AD, Klug A (1985) Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J 4(6):1609–1614
Moehle EA, Rock JM, Lee YL, Jouvenot Y, DeKelver RC, Gregory PD, Urnov FD, Holmes MC (2007) Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc Natl Acad Sci U S A 104(9):3055–3060. doi:10.1073/pnas.0611478104
Nowak-Imialek M, Niemann H (2012) Pluripotent cells in farm animals: state of the art and future perspectives. Reprod Fertil Dev 25(1):103–128. doi:10.1071/RD12265
Orlando SJ, Santiago Y, DeKelver RC, Freyvert Y, Boydston EA, Moehle EA, Choi VM, Gopalan SM, Lou JF, Li J, Miller JC, Holmes MC, Gregory PD, Urnov FD, Cost GJ (2010) Zinc-finger nuclease-driven targeted integration into mammalian genomes using donors with limited chromosomal homology. Nucleic Acids Res 38(15):e152. doi:10.1093/nar/gkq512
Pabo CO, Peisach E, Grant RA (2001) Design and selection of novel Cys2His2 zinc finger proteins. Annu Rev Biochem 70:313–340. doi:10.1146/annurev.biochem.70.1.313
Pavletich NP, Pabo CO (1991) Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science 252(5007):809–817
Petersen B, Lucas-Hahn A, Oropeza M, Hornen N, Lemme E, Hassel P, Queisser AL, Niemann H (2008) Development and validation of a highly efficient protocol of porcine somatic cloning using preovulatory embryo transfer in peripubertal gilts. Cloning Stem Cells 10(3):355–362. doi:10.1089/clo.2008.0026
Petersen B, Carnwath JW, Niemann H (2009) The perspectives for porcine-to-human xenografts. Comp Immunol Microbiol Infect Dis 32(2):91–105. doi:10.1016/j.cimid.2007.11.014
Radding CM (1982) Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet 16:405–437. doi:10.1146/annurev.ge.16.120182.002201
Rouet P, Smih F, Jasin M (1994) Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci U S A 91(13):6064–6068
Smith J, Bibikova M, Whitby FG, Reddy AR, Chandrasegaran S, Carroll D (2000) Requirements for double-strand cleavage by chimeric restriction enzymes with zinc finger DNA-recognition domains. Nucleic Acids Res 28(17):3361–3369
Szczepek M, Brondani V, Buchel J, Serrano L, Segal DJ, Cathomen T (2007) Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat Biotechnol 25(7):786–793. doi:10.1038/nbt1317
Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ, Joung JK (2014) Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol 32(6):569–576. doi:10.1038/nbt.2908
Vasquez KM, Marburger K, Intody Z, Wilson JH (2001) Manipulating the mammalian genome by homologous recombination. Proc Natl Acad Sci U S A 98(15):8403–8410. doi:10.1073/pnas.111009698
Wang J, Friedman G, Doyon Y, Wang NS, Li CJ, Miller JC, Hua KL, Yan JJ, Babiarz JE, Gregory PD, Holmes MC (2012) Targeted gene addition to a predetermined site in the human genome using a ZFN-based nicking enzyme. Genome Res 22(7):1316–1326. doi:10.1101/gr.122879.111
Watanabe M, Umeyama K, Matsunari H, Takayanagi S, Haruyama E, Nakano K, Fujiwara T, Ikezawa Y, Nakauchi H, Nagashima H (2010) Knockout of exogenous EGFP gene in porcine somatic cells using zinc-finger nucleases. Biochem Biophys Res Commun 402(1):14–18
Watanabe M, Nakano K, Matsunari H, Matsuda T, Maehara M, Kanai T, Kobayashi M, Matsumura Y, Sakai R, Kuramoto M, Hayashida G, Asano Y, Takayanagi S, Arai Y, Umeyama K, Nagaya M, Hanazono Y, Nagashima H (2013) Generation of interleukin-2 receptor gamma gene knockout pigs from somatic cells genetically modified by zinc finger nuclease-encoding mRNA. PLoS One 8(10):e76478. doi:10.1371/journal.pone.0076478
Whyte JJ, Prather RS (2012) CELL BIOLOGY SYMPOSIUM: Zinc finger nucleases to create custom-designed modifications in the swine (Sus scrofa) genome. J Anim Sci 90(4):1111–U1159. doi:10.2527/jas. 2011-4546
Whyte JJ, Zhao J, Wells KD, Samuel MS, Whitworth KM, Walters EM, Laughlin MH, Prather RS (2011) Gene targeting with zinc finger nucleases to produce cloned eGFP knockout pigs. Mol Reprod Dev 78(1):2. doi:10.1002/mrd.21271
Yang D, Yang H, Li W, Zhao B, Ouyang Z, Liu Z, Zhao Y, Fan N, Song J, Tian J, Li F, Zhang J, Chang L, Pei D, Chen YE, Lai L (2011a) Generation of PPARgamma mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Res 21(6):979–982. doi:10.1038/cr.2011.70
Yang D, Yang H, Li W, Zhao B, Ouyang Z, Liu Z, Zhao Y, Fan N, Song J, Tian J, Li F, Zhang J, Chang L, Pei D, Chen YE, Lai L (2011b) Generation of PPARgamma mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Res. doi:10.1038/cr.2011.70
Yu S, Luo J, Song Z, Ding F, Dai Y, Li N (2011) Highly efficient modification of beta-lactoglobulin (BLG) gene via zinc-finger nucleases in cattle. Cell Res 21(11):1638–1640. doi:10.1038/cr.2011.153
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editors: Natalay Kouprina and Vladimir Larionov
Rights and permissions
About this article
Cite this article
Petersen, B., Niemann, H. Advances in genetic modification of farm animals using zinc-finger nucleases (ZFN). Chromosome Res 23, 7–15 (2015). https://doi.org/10.1007/s10577-014-9451-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-014-9451-7