Abstract
Three xenarthrans species Chaetophractus villosus, Chaetophractus vellerosus, and Zaedyus pichiy have been used for the analysis of the structure, behavior, and immunochemical features of the XY body during pachytene. In all these species, the sex chromosomes form an XY body easily identifiable in thin sections by the special and regular packing of the chromatin fibers of the internal region of the XY body (“differential” regions) and those of the peripheral region (synaptic region). Spermatocyte spreads show a complete synapsis between the X- and the Y-axis, which lasts up to the end of pachytene. From the early pachytene substages to the late ones, the X-axis develops prominent branches, which in late pachytene span the synaptic region. Synapsis is regular as shown by SYCP1 labeling. Axial development is followed by SYCP3 labeling and in the asynaptic region of the X-axis by BRCA1. Gamma-H2AX labels exclusively the differential (asynaptic) region of the X chromosome. A single focus is labeled by MLH1 in the synaptic region. The location of this MLH1 focus spans from 0.3 to 1.6 μm from the telomere in the analyzed xenarthrans, covering approximately half of the Y-axis length. It is concluded that xenarthrans, as basal placental mammals, harbor the largest pseudoautosomal regions of presently analyzed mammals, and shows the typical features of meiotic sex chromosome inactivation (MSCI).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The differentiation of the mammalian X and Y chromosomes is generally assumed to have evolved since 310 MYA (million years ago) (Waters et al. 2007a). In this evolutionary path, the monotremes have diverged early (210 MYA, Waters et al. 2007a) and have formed an elaborate sex chromosome complex containing five X chromosomes and five Y chromosomes which form a chain multivalent in meiosis (Rens et al. 2004). On the other hand, a later divergence of metatherian from therian mammals (180 MYA, Waters et al. 2007a, b) has conserved in both clades the simple XY/XX sex chromosome system that is the rule (with a few exceptions) in marsupials and eutherian mammals (Fig. 1).
However, a fundamental difference between the genetics of sex between marsupials and eutherians is the presence in eutherians of the gene XIST and the inactivation center in the X chromosome, which is absent or disrupted in marsupials (Deakin et al. 2009; Chaumeil et al. 2011).
Despite this important functional difference between marsupials and eutherians, a particular sex-related mechanism is shared by both clades: the formation of the XY body (Solari 1974, reviewed in Solari 1994 and in Handel 2004) at the pachytene stage of meiosis during spermatogenesis, with the same molecular markers of transcriptional inactivation (Franco et al. 2007; Namekawa et al. 2007).
Thus, formation of the XY body through the transcriptional inactivation and chromatin remodeling at male meiotic prophase seems to be a basic and conserved mechanism both in marsupials and placental mammals (Solari 1994; Franco et al. 2007).
Recently, some reports have raised the question of the general conservation of the features of the XY body, especially in horses (Baumann et al. 2011). Thus, the analysis of possible variations of the XY body in the four eutherian lineages and their evolutionary trend has gained a renewed interest.
Furthermore, recent observations in mice suggest that the amount and permanence of synapsis between the X and Y chromosomes in the XY body may have functional importance in the further development of germ cells (Royo et al. 2010).
In this paper, we analyze the processes of synapsis, meiotic recombination, and chromatin remodeling in several species of armadillos, which belong to the Xenarthra (Fig. 1), which is assumed with Afrotheria to constitute the oldest living radiations of the placental mammals (Hallstrôm and Janke 2010).
We conclude that synapsis between the X and Y chromosomes in armadillos is unusually long and lasts to the late pachytene substage, differing from human and mouse XY pairs (reviewed in Solari 1994).
Furthermore, we prove the existence of a single and obligatory crossover event between the X and Y chromosomes of armadillos, which lies somewhat far from the telomeric region, and thus shows the presence of a sizable pseudoautosomal region (PAR).
Some observed features suggest that in armadillos, the meiotic silencing of sex chromosomes (MSCI, Turner 2007) occurs as in other mammals. Thus, the special packing of chromatin fibers in the non-synapsed regions of the X chromosome is mirrored by the labeling with γ-H2AX. We conclude that armadillos (and probably most Xenarthra) contain a typical, perhaps ancestral, XY body that, with some modifications, is present in the majority of placental mammals.
Materials and methods
Testicular tissue from adult males of three different species of armadillos Chaetophractus villosus (n = 3), Chaetophractus vellerosus (n = 2), and Zaedius pichiy (n = 2), coming from different Argentinian regions, were analyzed by optical microscopy, electron microscopy (EM), and immunolocalization of meiotic proteins. The animals were collected as part of a project on the phylogeny and evolution of xenarthrans in South America (see Acknowledgments).
The testicular tissue was subdivided in smaller pieces to perform different techniques. One piece of tissue was processed using routine methods for histological analysis. Another piece of tissue was fixed in 2% glutaraldehyde, post-fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.2) and embedded in Araldite. Semi-thin sections (0.5 μm thick) were stained with toluidine blue to analyze the seminiferous epithelium in detail with the light microscope and with Reynold’s solution to analyze the spermatocytes by EM.
Spermatocyte microspreads for synaptonemal complexes (SCs) were performed as previously described by Sciurano et al. (2009). Some slides were stained either with 4% phosphotungstic acid in ethanol or silver nitrate (Howell and Black 1980) and, others were kept at −70°C until used for immunofluorescence microscopy. For immunolocalization of meiotic proteins (with the exception of γ-H2AX), microwave and pressure cooker epitope retrieval were performed in 0.01 M sodium citrate buffer (pH 6) before the blocking step (see Sciurano et al. 2009). Slides were blocked with PBT [5% bovine serum albumin and 0.1% Tween 20 in phosphate-buffered saline (PBS)] for 20 min at room temperature before incubation. The following primary antibodies were incubated at 4°C: a mouse anti-SC protein SYCP1 at 1:100 (P.J. Moens and B. Spyropoulus, York University, Toronto, Ontario, Canada); a rabbit anti-SYCP3 at 1:100 (P.J. Moens and B. Spyropoulus); a rabbit anti-BRCA1 (Santa Cruz Biotech, CA, USA) at 1:10; a mouse anti-MLH1 at 1:10 (BD Pharmingen, USA); a rabbit anti-SMC3 at 1:500 (Merck Millipore, MA, USA); and human CREST serum at 1:10 (Laboratorios IFI, Buenos Aires, Argentina). A mouse anti-γ-H2AX antibody (Abcam Ltd, Cambridge, UK) was used at 1:500 in PBS and incubated at 37°C. All incubations were performed overnight in a humid chamber. After washing, the following secondary antibodies were used at the specified dilutions in PBS for 2 h: a fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit at 1:50; a tetramethyl-rhodamine isothiocyanate (TRITC)-labeled goat anti-mouse at 1:25; a TRITC-labeled goat anti-rabbit at 1:25; a FITC-labeled goat anti-mouse at 1:25; and a FITC-labeled anti-human gamma-globulin IgG at 1:400. Slides were counterstained with 4,6-diamidino-2-phenylindole (0.2 mg/ml) and mounted in glycerol with 1,4-diazobicyclo-(2,2,2)-octane antifade. Slides were examined using a LEICA DM microscope (Leica Microsystems, Wetzlar, Germany) and photographed with a Leica DFC 300 FX digital camera (Cambridge, UK). The separate images were superimposed using the program Adobe Photoshop CS (Adobe Systems Inc., USA).
The distance of MLH1 foci from the telomere in the XY pair, the length of the entire chromosome set, and the percentage of each chromosome of the entire set were measured using the freeware computer application Micromeasure version 3.3 (Reeves 2001).
The length of PAR in mega base pairs was calculated as the subtraction of the maximum and the minimum distance of the MLH1 foci from the telomere (in micrometer) × total DNA content (in mega base pairs, see Redi et al. 2005)/total length of the entire chromosomal set (in micrometer).
Results
Synaptic behavior of XY pair along the pachytene stage
The analysis of 60 primary spermatocytes by EM and 300 spermatocytes by immunolocalization shows that one of the peculiarities of the XY pair in armadillos is the long synaptonemal complex, which is formed between the X and Y chromosomes and covers practically all the Y-axis (Fig. 2). This synaptic peculiarity remains all along the pachytene stage, from early (Fig. 2a–c) to late (Fig. 2g–i) pachytene.
a–i Parallelism between electron microscopy and immunolocalization of BRCA1 and synaptonemal complex proteins of the XY pair along pachytene stage. a–c Early pachytene. Electron micrographs of silver-stained XY pair show a fully synapsed Y chromosome (arrows) and a slightly thick X-axis (a) labeled by BRCA1 (b–c). d–f Mid-pachytene. The differential region of the X-axis splits into several strands and it usually shows loops and irregularities (e, arrowhead) in the upper part of the SC. BRCA1 is located on each strand of the differential X-axis. g–i Late pachytene. The X and Y chromosomes remain completely synapsed at very late pachytene showing a fully split X-axis. The synapsed region of the XY pair is labeled by the SYCP1 protein and the split branches are labeled by BRCA1, including the PAR (h–i). Bars a 1 μm, b–c 5 μm, d 1 μm, e 0.5 μm, f 5 μm, g 1 μm, h–i 5 μm
At early pachytene, the differential segment of the X-axis forms a thick axis (compared to the synapsed region, PAR) which is labeled by the DNA damage repair protein, BRCA1 (Fig. 2b–c). As pachytene progresses, this differential axis becomes split into a number of strands that stem from the single original axis. Moreover, some of these strands form bridges which close the loop formed by the split X-axis (Fig. 2d–f). Towards the end of the pachytene stage, the X-axis is completely split including the pseudoautosomal region which has the synapsed main axes of the X and Y chromosomes labeled by SYCP1 and the split region labeled by BRCA1 (Fig. 2g–i).
Frequently, the X-axis twists near the end of the SC formed in the paired segment (Fig. 2e). These peculiarities correspond to the segment of the SC which is more distant from the nuclear envelope. Conversely, the large recombination region has a regular shape.
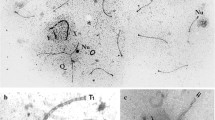
The large recombination region in armadillos
Even though the synapsed region between the X and Y chromosomes is particularly large, there is only one recombination nodule (RN) localized in the half nearest to the nuclear envelope (Fig. 3). The localization of this RN (Fig. 3a) is consistent with the presence of a single MLH1 focus in the analyzed armadillos (Fig. 3b–d). The average position of the MLH1 foci and its range are calculated in Table 1 showing that a crossover event could span from the third part of Y chromosome, nearest to the nuclear envelope, to the middle of this chromosome (Table 1). According to the content of the genomic DNA of the analyzed armadillos, summarized by Redi et al. (2005), the recombination region (PAR) comprises from 8.4 to 13.6 Mbp in the three species (Table 1).
a–d Recombination in the XY pair of three different species of armadillos. a PTA staining allows the detection of a single recombination nodule (RN, arrowhead), which is located near the attachment site of the SC with the nuclear envelope. b–d The position of the RN is consistent with the presence of a single MLH1 focus (white arrows). The drawings at scale summarize the average location of the MLH1 focus (black lines) and its large range (light gray areas) regarding the extremely acrocentric Y chromosome in the three analyzed species. KX (Y): kinetochores of X (Y) chromosome. Bars a 1 μm, b–d 5 μm
Chromatin remodeling in the XY body
The analysis of thin sections of spermatocyte nuclei shows that the XY body is attached to the nuclear envelope. This XY body shows two remarkably different regions: the internal, tightly packed chromatin of the differential region of the X-axis, and the peripheral, more sparsely packed chromatin of the long synapsed segment between the X and Y chromosomes (Fig. 4a). Moreover, this mentioned difference between both types of chromatin is consistent with the labeling with γ-H2AX of the asynaptic segment of the X chromosome, thus showing the remodeling of that chromatin piece. As shown in Fig. 4b–f, the large synapsed region of the XY body, which corresponds to almost all the length of Y chromosome, lacks γ-H2AX.
a–f Differential remodeling of the chromatin in the XY body of armadillos and the presence of variant histone γ-H2AX. a Thin sections of spermatocytes show the ultrastructural differences between the chromatin of the differential X-axis (asterisk) and the chromatin that is associated with the extensive SC of the XY pair. b–f The remodeling of the asynaptic chromatin is indicated by γ-H2AX in the three species (excluding the fully synapsed Y chromosome, white arrows). Bars a 1 μm, b–f 5 μm
Discussion
Armadillos as basal eutheria
Even though the antiquity of the Xenarthra radiation compared to that of the Afrotheria is not settled (Hallstrôm and Janke 2010; Waters et al. 2007b), there is a consensus that both clades are the older sister radiations of the eutherian root (Hallstrôm and Janke 2010). It is interesting to remark that the existent recent data on Afrotheria have shown that they share several features with the rest of eutherian species (reviewed in Solari 1994), including one xenarthran species (Grinberg et al. 1966). Thus, in Afrotheria the females have specific X chromosome inactivation in somatic cells resulting in stable Barr body formation that is a common phenomenon to all placentalia (Waters et al. 2007b), and males show the formation of an XY body during male meiotic prophase, similar to other placentals, which has a pseudoautosomal region (PAR) and a single recombination nodule shown as a MLH1 focus (Waters et al. 2007b).
Even though in most placental mammals presently examined there is the so-called meiotic sex chromosome inactivation (MSCI) during meiotic prophase in the XY body, Afrotheria is a basal branch in which the present data on γ-H2AX and transcriptional inhibition are lacking.
When comparing the available data on the Afrotherian XY and those of the present work, it is remarkable the differences in the location of the single MLH1 focus. In Afrotheria, the MLH1 focus is described as “always at the terminal ends of the XY pair” (Waters et al. 2007b). On the other hand, the single focus in the presently analyzed xenarthrans shows a range of locations as regards to the Y terminus (see “The pseudoautosomal region and recombination in the xenarthrans” section).
Furthermore, synapsis between the sex chromosomes in Afrotheria is notably shorter and brief compared to Xenarthra (see “Results”). Thus, besides the common features there are significant differences between these two clades as regards the XY body.
Synapsis and chromatin remodeling in the XY of Xenarthra
Partial synapsis between the X and Y chromosomes is the rule among placental mammals (Solari 1970; Moses et al. 1975; reviewed in Solari 1994). A few exceptional species have a non-synaptic relationship without any trace of a SC between the sex chromosomes, as is the case of Psammomys obesus (Solari and Ashley 1977; Franco et al., unpublished observations) and other gerbils (Franco et al., unpublished). Among the vast majority of placental mammals having partial synapsis, the SC, which is formed at the earliest substage of pachytene, is the longest one. This SC shortens, in subsequent stages, to reach a minimal length at late pachytene that is variable among species (reviewed in Solari 1994).
On the other hand, there are very few instances of an SC between the X and Y chromosomes that does not shorten during pachytene as in the rodent Galea musteloides (Solari et al. 1993). However, even in these exceptional cases, the SC does not cover the full length of the Y-axis, or undergoes morphological changes (Solari et al. 1993).
The present observations in three species of Xenarthra show a special behavior of the SC in the XY body. The length of the SC remains intact up to late pachytene, although splitting of the X-axis is evident in the late substages (see “Results”). The present observations confirm and enlarge the previous morphological descriptions of Scavone et al. (2000) and Sciurano et al. (2006).
Remodeling of the differential regions of the sex chromosomes in the XY body is shown by a specific packing of the chromatin fibers surrounding the X-axis and by the molecular marker γ-H2AX (see “Results”). As regards to this remodeling, Xenarthra behave like the other placental mammals, in which this remodeling has been associated with transcriptional inactivation (Turner et al. 2005; reviewed in Burgoyne et al. 2009). Thus, MSCI seems to be a general feature in both placental mammals and in metatherians (marsupials) (Franco et al. 2007; Namekawa et al. 2007).
A single and curious exception has been reported up to date, the XY body of the domestic horse (Equus caballus). In this single case, it has been reported that γ-H2AX does not cover the chromatin but only the axial elements in the XY body (Baumann et al. 2011). Furthermore, the same authors claim that RNAPol II can label the chromatin of this XY body. However, it will be necessary to confirm these claims by the use of the conventional techniques that preserve protein deterioration in these types of spreads by the use of protease inhibitors (Anderson et al. 1999).
At this point, the mass of the experimental evidence supports the concept that the XY body, which is an ancestral feature derived before the marsupial branching from the placental mammals, is associated with MSCI (Franco et al. 2007).
The pseudoautosomal region and recombination in the xenarthrans
The region which undergoes crossovers between the X and Y chromosomes is characteristically variable in size among eutherians (Raudsepp et al. 2011). The human PAR is the best known and its size is 2.6 Mb (Mangs and Morris 2007) comprising at least 24 genes (Rappold 1993; Mangs and Morris 2007). The PAR of mice is substantially shorter, about 700 kb (Perry et al. 2001; Rohozinski et al. 2002) and with only three protein-coding genes (reviewed in Raudsepp et al. 2011). However, there are new evidences that neither the human PAR nor that of mice is typical for most placental mammals (reviewed in Raudsepp et al. 2011). In fact, one of the best known PARs of domestic animals, the dog’s one (Canis lupus familiaris), is considerably larger than the human, it is about 6.6 Mb, and it contains at least 9 of the 24 protein-coding genes of the human PAR, in the same order, plus some other genes absent in the human one (see NCBI map viewer, C. lupus familiaris, Build 2.1).
In a comparative study of the PAR from several mammals, Raudsepp et al. (2011) have shown that compared with the human PAR, much larger PARs exist in the domestic dog, cat, and cattle (up to 6.6 Mb in the canine PAR and possibly a larger one in cats). In the same paper, it is concluded that the small and gene-poor PAR of mice is an outlier as regards to the general size and structure of mammalian PARs. Furthermore, it has been assumed that AMEL loci (coding for amelogenin) reveal the possible boundary of an ancestral PAR in eutherian mammals (Iwase et al. 2003). If this is so, an ancestral PAR could cover more than the 12 Mb that is the distance of the AMELY locus from the Y chromosome terminus in the human species.
There is a consensus that the X and Y chromosomes present in living placental mammals have originated from “conserved” regions of the X (XCR) and the Y that are represented also in marsupials that lack any PAR, plus an additional chromosomal piece that is autosomal in marsupials, and that has been labeled “X-added-region” (XAR) (Graves 1995; Delgado et al. 2009). This added region is considered to be originated prior to Boroeutheria (all mammals except Afro–Xenarthra) radiation, about 91 MYA, and this XAR contains both a newly conserved region and the variable PAR (Delgado et al. 2009). The recent observation on a representative of Afrotheria, the elephant Loxodonta africana, which contains 17 genes in the same order of the human X chromosome (including the gene XG) shows that gene conservation in mammalian sex chromosomes is stricter than what was imagined by the comparisons between mice and humans. The gene XG spans the PAR boundary and is X-specific in the human, but in the elephant gives similar labels in both the X and Y, suggesting that it is pseudoautosomal (Delgado et al. 2009).
Thus, the presently available data suggest that PARs exceedingly larger than the human exist in placental mammals, containing a substantial quantity of similar genes, despite large variations in total size of the PAR in diverse species, and inclusion (or exclusion) of some of the typical pseudoautosomal genes.
The results of the present work show that the analyzed xenarthrans have a recombination region which spans approximately half of the Y-axis (see Table 1). According to the genomic DNA content of this species and considering the relative length of the Y-axis in the SC karyotypes of the species examined here (see “Results”), it may be concluded that the Y chromosome of the examined armadillos contain an average of 32–35 Mbp of total DNA. Considering the range of location of MLH1 foci as a measure of the PAR, we conclude that the PAR in armadillos comprises 8.4–13.6 Mbp, and thus it could harbor the AMEL loci as well as the XG locus. Further experiments with FISH may be crucial to confirm this hypothesis.
As regards to the nature and evolution of the PAR among mammals, it is assumed that during a very early stage of eutherian evolution, the sex chromosomes recruited an autosomal piece (XAR), which would be shared by both the X and Y chromosomes through regular meiotic recombination. In this way, eutherians deeply departed from marsupials, as the latter have a non-chiasmatic mechanism to ensure regular segregation: the “dense plate” (reviewed in Solari et al. 1993 and in Franco et al. 2007). Once a somatic region was chosen for the obligatory recombination in the XY pair (PAR), the remaining regions without crossovers in the Y chromosome may have undergone gene deterioration (Charlesworth 1991).
The PAR would be subjected to a specific function: to ensure a crossover, but diverse shortenings and rearrangements would be allowed, giving rise to the present variation of the PAR among examined species (Raudsepp et al. 2011). In fact, it is surprising that in spite of size variations, the gene content of the PAR is similar in many species. Xenarthrans may give decisive clues to fill an important evolutionary gap about the origin of the pseudoautosomal region in eutherians.
Abbreviations
- AMEL:
-
AMELogenin
- BRCA1:
-
BReast CAncer 1
- Chr.:
-
Chromosome
- CREST:
-
Calcinosis Raynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasia
- DABCO:
-
1,4-DiAzoBiCyclo-(2,2,2)-Octane
- DAPI:
-
4,6-DiAmino-2-PhenylIndol
- DNA:
-
Deoxyribonucleic acid
- EM:
-
Electron Microscopy
- FISH:
-
Fluorescence In Situ Hybridization
- FITC:
-
Fluorescein IsoThioCyanate
- MLH1:
-
MutL Homolog 1
- MSCI:
-
Meiotic Sex Chromosomes Inactivation
- MYA:
-
Million Years Ago
- PAB:
-
PAR Boundary
- PAR:
-
PseudoAutosomal Region
- PBS:
-
Phosphate Buffer Saline
- RN:
-
Recombination Nodule
- RNAPol II:
-
RNA polymerase type II
- SC:
-
Synaptonemal Complex
- SMC3:
-
Structural Maintenance of Chromosomes 3
- SYCP1:
-
SYnaptonemal Complex Protein 1
- SYCP3:
-
SYnaptonemal Complex Protein 3
- TRITC:
-
Tetramethyl Rhodamine IsoThioCyanate
- XAR:
-
X-Added Region
- XCI:
-
X-Chromosome Inactivation
- XCR:
-
X Conserved Region
- XG:
-
Xg blood group antigen
- XIST:
-
X-Inactive Specific Transcript
- γ-H2A.X:
-
phosphorylated (Ser139) Histone 2 A.X
References
Anderson LK, Reeves A, Webb LM, Ashley T (1999) Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 151:1569–1579
Baumann C, Daly CM, McDonnell SM, Viveiros SM, De la Fuente R (2011) Chromatin configuration and epigenetic landscape at the sex chromosome bivalent during equine spermatogenesis. Chromosoma 120:227–244
Burgoyne PS, Mahadevaiah SK, Turner JMA (2009) The consequences of asynapsis for mammalian meiosis. Nature Rev Genet 10:207–216
Charlesworth B (1991) The evolution of sex chromosomes. Science 251:1030–1033
Chaumeil J, Waters PD, Koina E, Gilbert C, Robinson TJ, Graves JA (2011) Evolution from XIST-independent to XIST-controlled X-chromosome inactivation: epigenetic modifications in distantly related mammals. PLoS One 6:e19040
Deakin JE, Chaumeil J, Hore TA, Graves JAM (2009) Unraveling the evolutionary origins of X chromosome inactivation in mammals: insight from marsupials and monotremes. Chrom Res 17:671–685
Delgado CL, Waters PD, Gilbert C, Robinson TJ, Graves JA (2009) Physical mapping of the elephant X chromosome: conservation of gene order over 105 million years. Chromosome Res 17:917–926
Franco MJ, Sciurano RB, Solari AJ (2007) Protein immunolocalization supports the presence of identical mechanisms of XY body formation in eutherians and marsupials. Chromosome Res 15:815–824
Graves JAM (1995) The origin and function of the mammalian Y chromosome and Y-borne genes—an evolving understanding. Bioessays 17:311–320
Grinberg MA, Sullivan MM, Benirschke K (1966) Investigation with tritiated thymidine of the relationship between the sex c hromosomes, sex chromatin and the drumstick in the cells of the female nine-banded armadillo, Dasypus novemcinctus. Cytogenetics 5:64–74
Hallstrôm BM, Janke A (2010) Mammalian evolution may not be strictly bifurcating. Mol Biol Evol 27:2804–2816
Handel MA (2004) The XY body: a specialized meiotic chromatin domain. Exp Cell Res 296:57–63
Howell WM, Black DA (1980) Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: a 1 step method. Experientia 36:1014–1015
Iwase M, Satta Y, Hirai Y, Hirai H, Imai H, Takahata N (2003) The amelogenin loci span an ancient pseudoautosomal boundary in diverse mammalian species. Proc Natl Acad Sci USA 100:5258–5263
Mangs AH, Morris BJ (2007) The human pseudoautosomal region (PAR): origin, function and future. Curr Genomics 8:129–136
Moses MJ, Counce SJ, Paulson DF (1975) Synaptonemal complex complement of man in spreads of spermatocytes, with details of the sex chromosome pair. Science 187:363–365
Murphy WJ, Pringle TH, Crider TA, Springer MS, Miller W. (2007) Using genomic data to unravel the root of the placental mammal phylogeny. Genome Res 17:413–421
Namekawa SH, VandeBerg JL, McCarrey JR, Lee JT (2007) Sex chromosome silencing in the marsupial male germ line. Proc Natl Acad Sci USA 104:9730–9735
Perry J, Palmer S, Gabriel A, Ashworth A (2001) A short pseudoautosomal region in laboratory mice. Genome Res 11:1826–1832
Rappold GA (1993) The pseudoautosomal regions of the human sex chromosomes. Hum Genetics 92:315–324
Raudsepp T, Das PJ, Avila F, Chowdhary BP (2011) The pseudoautosomal region and sex chromosome aneuploidies in domestic species. Sex Dev. doi:10.1159/000330627
Redi CA, Zacharias H, Merani S, Oliveira-Miranda M, Aguilera M, Zuccotti M, Garagna S, Capanna E (2005) Genome sizes in Afrotheria, Xenarthra, Euarchontoglires, and Laurasiatheria. J Hered 96:485–493
Reeves A (2001) MicroMeasure: a new computer program for the collection and analysis of cytogenetic data. Genome 44:439–443
Rens W, Grützner F, O’brien PC, Fairclough H, Graves JAM, Ferguson-Smith MA (2004) Resolution and evolution of the duck-billed platypus karyotype with an X1Y1X2Y2X3Y3X4Y4X5Y5 male sex chromosome constitution. Proc Natl Acad Sci USA 101:16257–16261
Rohozinski J, Agoulnik AL, Boettger-Tong HL, Bishop CE (2002) Successful targeting of mouse Y chromosome genes using a site-directed insertion vector. Genesis 32:1–7
Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, Bradley A, de Rooij DG, Burgoyne PS, Turner JM (2010) Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol 20:2117–2123
Scavone MDP, Oliveira C, Bagagli E, Foresti F (2000) Analysis of the synaptonemal complex of the nine-banded armadillo, Dasypus novemcinctus. Genet Mol Biol 23:613–616
Sciurano RB, Merani MS, Bustos J, Solari AJ (2006) Synaptonemal complexes and XY behavior in two species of argentinian armadillos: Chaetophractus villosus and Dasypus hybridus. Biocell 30:57–66
Sciurano RB, Luna Hisano CV, Rahn MI, Brugo Olmedo S, Rey Valzacchi G, Coco R, Solari AJ (2009) Focal spermatogenesis originates in euploid germ cells in classical Klinefelter patients. Hum Reprod 24:2353–2360
Solari AJ (1970) The spatial relationship of the X and Y chromosomes during meiotic prophase in mouse spermatocytes. Chromosoma 29:217–236
Solari AJ (1974) The behavior of the XY pair in mammals. Internat Rev Cytol 38:273–317
Solari AJ (1994) Sex chromosomes and sex determination in vertebrates. CRC, Florida
Solari AJ, Ashley T (1977) Ultrastructure and behavior of the achiasmatic, telosynaptic XY pair of the sand rat (Psammomys obesus). Chromosoma 62(4):319–336
Solari AJ, Merani MS, Burgos MH (1993) Dissociation of the synaptonemal complex in the XY body of Galea musteloides (Rodentia, Caviidae). Biocell 17:25–37
Turner JMA (2007) Meiotic sex chromosome inactivation. Development 134:1823–1831
Turner JMA, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng C, Burgoyne PS (2005) Silencing of unsynapsed meiotic chromosomes in the mouse. Nature Genet 37:41–47
Waters PD, Wallis MC, Graves JAM (2007a) Mammalian sex—origin and evolution of the Y chromosome and SRY. Semin Cell Dev Biol 18:389–400
Waters PD, Ruiz-Herrera A, Dobigny G, Garcia Caldés M, Robinson TJ (2007b) Sex chromosomes of basal placental mammals. Chromosoma 116:511–518
Acknowledgments
The able technical help of C. Deparci is gratefully acknowledged. This work was supported by UBACYT 20020100100030 (AJS), PICT-2010-2718 (RBS), and PIP 11220090100204 (“Phylogeny and Evolution of South American Xenarthrans, MSM”).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Walther Traut.
Rights and permissions
About this article
Cite this article
Sciurano, R.B., Rahn, M.I., Rossi, L. et al. Synapsis, recombination, and chromatin remodeling in the XY body of armadillos. Chromosome Res 20, 293–302 (2012). https://doi.org/10.1007/s10577-012-9273-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-012-9273-4