Abstract
Most species of Citrus and related genera display a similar karyotype with 2n = 18 and a variable number of terminal heterochromatic blocks positively stained with chromomycin A3 (CMA+ bands). Some of these blocks are 45S rDNA sites, whereas others may correspond to the main GC-rich satellite DNA found in several Citrus species. In the present work, the distribution of the 45S rDNA and the main satellite DNA isolated from C. sinensis (CsSat) were investigated by in situ hybridization in seven species of Citrus, two species of closely related genera (Fortunella obovata and Poncirus trifoliata) and four species of the subfamily Aurantioideae, which were less related to Citrus (Atalantia monophylla, Murraya paniculata, Severinia buxifolia, and Triphasia trifolia). In Citrus, Fortunella, and Poncirus, most CMA+ bands colocalized only with CsSat sites, whereas others colocalized only with rDNA sites. However, some of these species displayed a few CMA+ bands that colocalized with sites of both probes and other CMA+ bands that did not colocalized with any of the probes. On the other hand, in the four species less related to Citrus, no CsSat signal was found on chromosomes. On Southern blot, the CsSat probe hybridized with genomic DNA from Citrus, Fortunella, and Poncirus at high stringency only, while under the less stringent conditions, it also hybridized with distantly related species. Therefore, CsSat sequences are the principal component of the heterochromatic blocks of Citrus, Poncirus, and Fortunella, whereas CsSat-like sequences seem to be widespread in the subfamily Aurantioideae. These data further suggest that the variable number of terminal CMA+ bands observed on chromosomes of Citrus and related genera are probably the consequence of amplification or reduction in the number of CsSat-like sequences distributed on chromosome termini, paralleled by mutation and homogenization events, as proposed by the library hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large fraction of plant and animal genomes is represented by repetitive DNA, which may account for as much as 90% of the whole nuclear DNA (Schwarzacher 2003). The repetitive sequences may be dispersed through the genome or organized in tandem forming blocks of hundreds or thousands repeats, which constitute the satellite DNA. Some tandemly repetitive sequences consist of coding genes, as the rDNA sites, but the vast majority of them are noncoding sequences (Hemleben et al. 2007). Satellite DNA alone may correspond to a significant fraction of the genome, reaching 20% or more in plants (Beridze et al. 1992). On the other hand, large clusters of repetitive DNA sequences are cytologically detected as heterochromatin blocks occupying up to 50% of the whole chromosome complement (Guerra 1993a). The difference between biochemical and cytological estimations can be due to an overstaining of the heterochromatic blocks after banding techniques. Further, DNA sequencing of heterochromatic blocks showed that large clusters of repetitive DNA are often intermingled with several other sequences (CSHL/WUGS/PEB Arabidopsis Sequencing Consortium 2000), which, at the cytological level, are visualized as a continuous heterochromatic band.
The distribution of a single-satellite DNA family may be chromosome specific, genome specific, or more widely distributed into a genus or a tribe (Tek et al. 2005; Hemleben et al. 2007; Winterfeld and Röser 2007). On the other hand, a heterochromatic block may be constituted by different members of a single repeat family or by two or more distinct satellite families (Kazama et al. 2006; Lim et al. 2006). The number of copies of a given satellite DNA may vary between species or between individuals of the same species, and this variation in number may be promoted by different mechanisms, such as replication slippage, gene conversion, and unequal crossing-over (Charlesworth et al. 1994).
Ingle et al. (1973) observed that Citrus species, among dozens of plant genera investigated, displayed an exceptionally high amount of satellite DNA (25%). Accordingly, many species of Citrus and several other genera of the subfamily Aurantioideae (Rutaceae) exhibit a large number of terminal heterochromatic bands, distributed over karyotypes with 2n = 18 and similar chromosomes size and morphology (Guerra et al. 2000). Such bands stain strongly with the fluorochrome chromomycin A3 (CMA+ bands), which binds preferentially to GC-rich DNA sequences (Hou et al. 2004). Besides, of the terminal heterochromatin, there are a few chromosomes with proximal CMA+ bands, often less brilliant than the terminal ones, corresponding to 45S rDNA sites (Carvalho et al. 2005; Brasileiro-Vidal et al. 2007; Moraes et al. 2007a,b). The chromosomal distribution of CMA+ bands in Citrus species allowed the characterization of seven different chromosome types (Carvalho et al. 2005). Chromosome types A (two terminal and one proximal band), B (one terminal and one proximal), C (two terminals), E (one subterminal/interstitial band), and G (one terminal and one subterminal band) are relatively rare, whereas types D (one terminal), F (without bands), and FL (the largest F chromosome) are the most common. The single pair of FL type is highly conserved and easily identified in all accessions of Citrus and most other genera (Guerra et al. 2000; Guerra 2009). In some accessions, the FL chromosome may exhibit a fine terminal band in the long arm, as in C. sunki and C. maxima, and are referred to as F +L .
Based on chromosome measurements of CMA+ bands, the heterochromatin content of Aurantioideae species was estimate to vary between 3% and 34% (Miranda et al. 1997; Guerra et al. 2000), but the number of satellite DNA families involved in this genome fraction is unknown. Fluorescence in situ hybridization (FISH) using specific satellite DNA families may allow to identify the composition of each chromosomal band and may contribute to understand the evolution of the different banding patterns (Kazama et al. 2006; Lim et al. 2006).
In the last 20 years, some satellite DNA sequences from Citrus species and the closely related Poncirus trifoliata were isolated, cloned, and sequenced. The first satellite DNA was isolated by Beridze et al. (1992) from C. ichangensis, having a 181-bp consensus sequence and an exceptionally high GC content (63%). Similarly, Fann et al. (2001) isolated 48 clones of this DNA family present in C. ichangensis, C. limon, C. sinensis, and P. trifoliata having 179–186 bp, GC content ranging between 58.6% and 68%, and 70%–100% of base pair similarity in relation to the consensus sequence reported by Beridze et al. (1992). The high GC content of this satellite DNA together with its large representation in Citrus and Poncirus species, strongly suggest that it could be the main component of the heterochromatin in this group. Indeed, Kang et al. (2008) hybridized in situ a DNA sequence isolated from C. sunki, using primers designed for this main satellite DNA. They found large blocks of repetitive DNA in chromosomes of several Citrus species, but they did not compare the hybridized sites with the location of heterochromatic bands.
In the present work, the satellite DNA reported for Citrus by Beridze et al. (1992) was amplified by polymerase chain reaction (PCR) from the genomic DNA of C. sinensis and in situ hybridized in seven Citrus species and in representatives of six other related genera, aiming to understand the evolution of heterochromatic bands among Aurantioideae species. All metaphases were previously stained with CMA/DAPI and sequentially used for FISH. A 45S rDNA probe was also used in order to identify the CMA+ bands which colocalize with 45S rDNA sites (Carvalho et al. 2005; Moraes et al. 2007a,b) from those that hybridize with the main satellite.
Material and methods
Materials
Seven species of Citrus, including C. sinensis cv Pêra, the five karyotypically homozygous species of subgenus Citrus (C. deliciosa cv Montenegrine, C. maxima cv Israel, C. medica cv Etrog, C. reshni, and C. sunki), and C. hystrix, a representative of the subgenus Papeda, were investigated together with two cultivars of P. trifoliata (Pomeroy and USDA) and one sample of Fortunella obovata. Both Poncirus and Fortunella are recognized as genera closely related to Citrus, and these species are also referred to as citric species (Swingle and Reece 1967). The five karyotypically homozygous species of Citrus were chosen for this analysis because they may represent the only true species of the subgenus Citrus (Guerra 2009). Further, representatives of four distantly related genera, Atalantia monophylla, Triphasia trifolia, Murraya paniculata, and Severinia buxifolia were also analyzed. All accessions were obtained on the Citrus Active Germplasm Bank of the Embrapa Cassava & Fruits, Cruz das Almas, Bahia, Brazil, except M. paniculata and T. trifolia, cultivated as ornamental plants at the campus of the Federal University of Pernambuco.
Chromosome preparation
For mitotic analyses, root tips from germinating seeds or from adult plants growing in pots were pretreated with 8-hydroxyquinoline (0.002 M) for 5 h at 18°C, fixed in 3:1 ethanol–acetic acid (v/v) for 2–24 h at room temperature and stored at −20°C. The material was washed in distilled water and digested at 37°C for 1 h with a 2% cellulase (Onozuka)–20% pectinase (Sigma, Saint Louis, MO) (w/v) solution. The meristem of each individual root tip was squashed in a drop of 45% acetic acid, and the coverslip was removed in liquid nitrogen. The slides were stained with DAPI (2 μg/ml)–glycerol (1:1, v/v) solution to allow selection of the best preparations. Subsequently, they were destained in ethanol–acetic acid (3:1) for 30 min at room temperature and transferred to absolute ethanol overnight at 4–8°C. Slides were air dried and aged for 3 days at room temperature.
CMA/DAPI staining
After aging, the slides were stained with CMA and DAPI as described previously (Guerra 1993b), with minor modifications. The slides were stained with CMA (0.1 mg/ml) for 1 h, washed in distilled water, air dried, stained with DAPI (1 μg/ml) for 30 min, washed again, dried, and mounted in McIlvaine’s buffer (pH7.0)–glycerol (1:1, v/v). The best metaphases were captured with a Cohu video camera using Leica QFISH software. The slides were destained again and stored at −20°C for posterior in situ hybridization.
Satellite DNA amplification and probe labeling
Genomic DNA of C. sinensis was isolated from young leaves using the protocol of Zellinger et al. (2007). PCR amplification of satellite DNA was carried out using the primer pair F-5′-CGCCCAAAAATACCCGCCCGAAG-3′ and R-5′-CCTATTTTTGGCACACGGAGG-3′ designed to hybridize in the nucleotide positions 16–38 (forward) and 161–181 (reverse) of the 181-bp consensus sequence of C. ichangensis satellite DNA (Accession number in EMBL: X51946) described by Beridze et al. (1992), as indicated in Fig. 1. After an initial denaturation step at 94°C for 5 min, the amplification was carried out in 30 cycles consisting of denaturation at 94°C for 30 s, annealing at 61.7°C for 30 s, and primer extension at 72°C for 30 s, with final extension at 72°C for 7 min. The products of PCR reactions analyzed in agarose gel electrophoresis revealed amplified fragments of about 160, 340, and 520 bp long. The whole PCR product, denominated CsSat, was labeled with rodamine-11–dUTP (Sigma) or digoxigenin-11–dUTP and used as a probe in the FISH or in the Southern blot hybridization, respectively. The probe R2, a 6.5-kb fragment of an 18S-5.8S-25S rDNA repeat unit from Arabidopsis thaliana (Wanzenböck et al. 1997) was labeled with digoxigenin-11–dUTP by nick translation (Invitrogen, Carlsbad, CA, USA).
Consensus sequence of the main satellite DNA of C. ichangensis, according to Beridze et al. (1992). Arrows indicate the nucleotide sequences of the primers used to amplify the satellite DNA by PCR. HaeIII restriction sites (GGCC) are indicated in boldface
Fluorescence in situ hybridization
The FISH procedure followed the protocol used by Brasileiro-Vidal et al. (2007) with minor modifications. The hybridization mixture contained 50% v/v formamide, 5% w/v dextran sulfate, 2 × SSC (Saline-Sodium Citrate), 2–5 ng/μl of the 45S rDNA probe, and the labeled satellite DNA isolated from C. sinensis. The final stringency was ca. 76%. The rDNA probe was detected with sheep anti-digoxigenin–fluorescein isothiocyanate (Boehringer, Ingelheim am Rhein, Germany) and the signal amplified with fluorescein isothiocyanate-conjugated antisheep secondary antibody (Serotec, Raleigh, NC, USA). All preparations were counterstained and mounted with 2 μg/ml DAPI in Vectashield (Vector, Burlingame, CA, USA). Images of cells previously acquired with CMA/DAPI staining were captured again, as described before. The images were optimized for better contrast and brightness with Adobe Photoshop CS3 Extended Version 10.0.
Southern blot hybridization
Different amounts of genomic DNA of the same species analyzed by FISH were isolated, as indicated before, digested with HaeIII, fractionated on a 1% agarose gel and blotted onto a nylon membrane (Hybond N+; Amersham Biosciences, Buckinghamshire, UK) by capillary transfer. The satellite DNA used as a probe was amplified by PCR and labeled with digoxigenin (DIG-11-dUTP; Roche, Basel, Switzerland) as described above. The membranes were hybridized overnight with the satellite DNA in hybridization buffer [1% blocking reagent (Roche), 0.1% sodium dodecyl sulfate (SDS), 5% dextran sulfate, in 5 × SSC, pH 7.0] at 37°C, according to Sambrook and Russell (2001). After hybridization, the membranes were washed twice in 2 × SSC, 0.1% SDS for 5 min and in 0.5 × SSC, 0.1% SDS for 15 min, at 45°C and 68°C for low and high stringency, respectively. The satellite DNA probe was detected using anti-DIG alkaline phosphatase conjugate (Roche) and CDP-Star (Roche). Hybridization signals were captured on x-ray ECL film (Amersham Biosciences).
Results
Distribution of rDNA and CsSat sites in relation to CMA+ bands
All species investigated presented 2n = 18 and karyotype formulae identical to those previously described, except by a variation observed in C. deliciosa (Guerra et al. 2000; Pedrosa et al. 2000; Carvalho et al. 2005; Brasileiro-Vidal et al. 2007; Moraes et al. 2007a,b). In order to allow a detailed comparison between the CMA+ bands of chromosomes and the hybridization sites of CsSat and 45S rDNA probes, the slides were firstly stained with CMA/DAPI, photographed, and reused for FISH.
Both CsSat and rDNA probes hybridized only on CMA bands, with the exception of a single very small terminal band in C. sinensis. In this species, with karyotype formula 2B + 2C + 7D + 7F, the 45S rDNA probe hybridized on the terminal band of a D chromosome and the proximal bands of two B, whereas the CsSat probe hybridized on all remaining CMA+ bands (Figs. 2a, b, 5a). Surprisingly, a very small site of CsSat was observed on the distal euchromatic region of a D chromosome type (insert in Fig. 2a, b). In C. medica (2B + 8D + 8F) and C. maxima (4A + 2C + 4D + 8F), all terminal CMA+ bands hybridized with the CsSat probe, except the small band of the F +L chromosome pair of C. maxima (arrowheads in Figs. 2e, f, 5b, c). This band is not always observed in both homologues of cells of the same root tip, probably due to its very small size. The proximal bands of type A chromosomes of C. maxima and type B of C. medica hybridized with the 45S rDNA probe (Fig. 2c–f ).
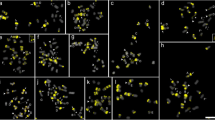
Distribution of CMA bands (yellow), CsSat sites (red), and 45 S rDNA sites (green) in C. sinensis (a, b), C. medica (c, d), C. maxima (e, f), C. sunki (g, h), C. reshni (i, j), and C. deliciosa (k, l). Letters in a, c, e, g, i, and k indicate most characteristic chromosome types. Small white dots indicate distended proximal secondary constriction of B chromosomes. Yellow curved arrow in e points to an A chromosome superimposed with another A chromosome. Arrowheads in e, f, g, h, i, j, k, and l indicate CMA+ bands not detected by FISH in F +L and D chromosomes, respectively. Inserts present magnified view of different chromosome features: a, b a small terminal CsSat site (b) not detected by CMA (a); e, f the small terminal CMA band of the short arms of A chromosomes (e) also detected with CsSat probe (f); e, f, i, j the long arm of F +L chromosomes with a small terminal CMA band (e, i) and the same arms after FISH (f, j); i–l CMA bands (i, k) colocalized with CsSat and rDNA adjacent sites (j, l). Bar in l corresponds to 5 µm
Citrus sunki as well as C. reshni (both with 14D + 4F) had 10 out of their 14 terminal CMA+ bands of D chromosomes hybridized only with CsSat, two other bands hybridized with the rDNA probe, and the remaining two bands did not hybridize with any of these probes (Figs. 2g–j, 5d, e). In C. reshni, the only chromosome pair bearing an rDNA site also displayed a terminal CsSat site located more terminally on the same CMA+ band (Fig. 2j). The small terminal CMA+ band on the F +L chromosome pair, sometimes visible only on one of the homologues, did not hybridize with any of the probes in both species (Fig. 2g–j).
Although C. deliciosa has previously been described as having 2C + 10D + 6F (Cornélio et al. 2003; Moraes et al. 2007b), we observed a minor terminal CMA+ band in one F chromosome pair, not described previously, which did not hybridize with any of the probes. In this case, it seems quite possible that the plant investigated here was originated by a cross with other Citrus species. In this sample, CsSat hybridized on the terminal band of 10 out of 11 D chromosomes as well as on both terminal bands of the C chromosome pair. The CMA+ band of one of the D chromosome pairs hybridized with the 45S rDNA probe, but one of its homologues also had a small terminal site of CsSat (Figs. 2k, l, 5f). Therefore, the only chromosome pair bearing 45S rDNA was heterozygous for the presence of CsSat.
Citrus hystrix (3B + 10D + 2E + 1G + 2F) exhibited five sites of rDNA, three of them colocalized with the proximal CMA band of the B chromosomes and two with the terminal band of D chromosomes. The CsSat probe hybridized on all remaining CMA bands except on the terminal band of the single G chromosome and on a D chromosome (Figs. 3a, b, 5g). We had previously assumed that G chromosomes could have been originated by an inversion or a duplication involving the terminal CMA+ band of a F +L chromosome (Carvalho et al. 2005). However, it is clear now that the terminal and the subterminal bands of the G type are formed by different repetitive sequences and, therefore, are not related to each other.
Distribution of CMA bands (yellow), CsSat (red), and 45S rDNA sites (green) in Citrus hystrix (a, b), P. trifoliata (c, d), and F. obovata (e, f). Arrowheads indicate CMA bands not labeled by the probes. Inserts in (d) show magnified chromosomes with CsSat site adjacent to rDNA sites. Bar in f corresponds to 5 µm
Among the representatives of the six other genera investigated here, only P. trifoliata and F. obovata showed in situ hybridization sites for CsSat. The two accesses of P. trifoliata investigated exhibited identical karyotype formulae (4B + 8D + 6F), with CsSat signals on terminal CMA+ bands of three B and seven D chromosomes (Figs. 3c, d, 5h). The fourth B chromosome displayed a very small terminal CMA+ band colocalized with an rDNA site instead of a CsSat. This unique terminal rDNA site in a B chromosome type had already been reported for some P. trifoliata cultivars (Brasileiro-Vidal et al. 2007). rDNA sites were also found on the proximal band of all B chromosomes and on the short arms of a D chromosome pair of P. trifoliata. In one of these D chromosomes, both probes hybridized on the terminal band, having the CsSat repeats on a more terminal position (Fig. 3d). In F. obovata (1A +1B + 2C + 11D + 1E + 2F +L ), the CsSat probe hybridized on the terminal CMA+ bands of A and B chromosomes as well as on eight D chromosomes and on the interstitial band of the E chromosome (Fig 3e, f). This chromosome was classified as E, in spite of having a very fine terminal band, because its interstitial band was relatively large and the chromosome size was small, whereas typical G chromosomes are large and the subterminal band is very small (Carvalho et al. 2005; Moraes et al. 2007a). The rDNA probe hybridized on the proximal CMA+ bands of A and B chromosomes and on one of the terminal bands of the C chromosome pair. Therefore, there was no hybridization on the bands of three D chromosomes, the small band of the FL+ pair and one band of each C type of F. obovata (Fig. 5i).
Finally, in A. monophylla (8D + 10F), M. paniculata (2C + 14D + 2F), T. trifolia (15D + 4F), and S. buxifolia (2C + 4D + 12F), no in situ hybridization signal was found with the CsSat probe. The 45S rDNA probe hybridized only on the CMA bands of one or two D chromosome pairs of the three former species and on one of the terminal bands of a C chromosome pair of S. buxifolia (Fig. 4).
Southern blot hybridization
In order to further investigate the occurrence of CsSat repeats not detected by FISH, all the species were also analyzed by Southern blot hybridization. The enzyme HaeIII was chosen to digest the genomic DNAs because the consensus sequence of CsSat (Beridze et al. 1992) presents four sites for this enzyme (Fig. 1). The cleavage of internal sites results in short fragments of similar size, which may facilitate the detection of even small amount of this sequence in the membrane. Initially, samples containing 1 μg of digested genomic DNA of all Citrus species as well as the representatives of the other six genera investigated were run on 1% agarose gel and blotted onto nitrocellulose membrane. In the stringency used here (∼82%), only the DNA of Citrus, Poncirus, and Fortunella produced a strong band at about 40 bp (data not shown).
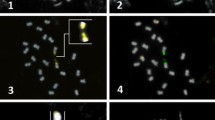
A second hybridization was performed using two samples of C. sinensis genomic DNA (15 ng and 400 ng) and a much higher amount of genomic DNA of S. buxifolia, T. trifolia, and M. paniculata (6.5–22 μg, depending on the availability of ours stocks). Once again, there was a strong hybridization with both samples of C. sinensis and only faint signals for S. buxifolia, T. trifolia, and M. paniculata (Fig. 6a). However, when the stringency was lowered (∼58%), the probe hybridized with the genomic DNA of these species in a similar position in the gel (Fig. 6b), indicating that CsSat-like sequences were present.
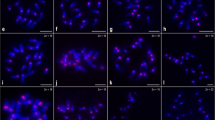
Karyograms of all species investigated based on metaphases of Figs. 2 and 3. a C. sinensis, b C. medica, c C. maxima, d C. sunki, e C. reshni, f C. deliciosa, g C. hystrix, h P. trifoliata, and i F. obovata. Chromosomes of the same type are indicated by a white line. CMA bands are observed in yellow, CsSat sites in red, and 45S rDNA sites in green. Bar corresponds to 5 µm
Discussion
The relationship among CMA+ bands and CsSat sites
The karyotype formulae observed here, based on chromosomal distribution of CMA+ bands and rDNA sites, coincided with the formulae previously published (Guerra et al. 2000; Pedrosa et al. 2000; Carvalho et al. 2005; Brasileiro-Vidal et al. 2007; Moraes et al. 2007a, b). In the present work, the sequential staining with the fluorochromes CMA and DAPI, followed by FISH with 45S rDNA and CsSat probes, revealed four different types of CMA+ heterochromatin in Citrus species: (1) large blocks of CsSat repeats, which represent most of the bands; (2) 45S rDNA sites, colocalized with only two to six bands per diploid chromosome complement; (3) bands composed by blocks of both sequences investigated; and (4) a few small bands constituted by repetitive sequences not detected by rDNA or CsSat probes, which were found only in some Citrus species. These results further support the molecular data from Beridze et al. (1992) and Fann et al. (2001), which indicate that this repeat family is the major component of the satellite DNA fraction of Citrus and Poncirus. Kang et al. (2008) had already demonstrated that chromosomes of Citrus species hybridized in many sites with a similar satellite DNA probe, but they could not demonstrate the correlation with the CMA+ bands.
Because the CsSat probe is constituted by all fragments amplified from C. sinensis by PCR, it should be constituted by a family of different repeats rather than by a single type of repeat. Fann et al. (2001) analyzed the sequence of 11 clones of the main C. sinensis satellite and found that the genetic similarity between the C. ichangensis consensus sequence and those of C. sinensis varied between 78% and 93%. Similar variation is expected here among repeats of the probe and on CMA+ bands. Data from other plant groups have showed that different members of a repeat family are usually represented in most or in all heterochromatic bands bearing the main satellite DNA family (Kazama et al. 2006; Hemleben et al. 2007).
In C. sinensis, besides the sites colocalized with CMA bands, an extra small cluster of CsSat was detected on the short arm of a D chromosome, which was not detected by CMA staining neither here nor in previous works (Guerra 1993b; Matsuyama et al. 1996; Miranda et al. 1997; Pedrosa et al. 2000; Cornélio et al. 2003). This divergence is probably due to the superior sensibility of FISH to identify small clusters of repetitive DNA in comparison with CMA banding (Winterfeld and Röser 2007). Small clusters of repetitive sequences like this may occasionally be amplified generating an extra CMA band and changing the chromosome types of homologous or homeologous chromosomes of Citrus species (Guerra 2009).
Although most CMA+ band hybridized with CsSat or rDNA probes, there were a few small bands in C. hystrix, C. maxima and in the three closely related mandarin species, C. deliciosa, C. sunki, and C. reshni (Cornélio et al. 2003; Moraes et al. 2007b), which were not related to any of these sequences. In C. hystrix, this kind of band was found in a large D and in a G chromosome, both of which seem to be homeologous to the FL type observed in other species (Carvalho et al. 2005; Moraes et al. 2007a). This assumption is based on the fact that these three chromosomes are among the largest chromosomes of each complement, only two of them have been found per karyotype (observe in Fig. 5g that a typical FL pair was not found in C. hystrix) and their terminal CMA+ band did not hybridized with the CsSat probe. This CMA+ band may be formed by a far related variant of the CsSat-like sequence or by another satellite DNA family, as the one reported for Citrus, Poncirus, and Fortunella species by De Felice et al. (2006).
In F. obovata, the small terminal bands of three chromosome pairs, including a heteromorphic D/E pair, did not hybridize with any of the probes. Noteworthy, the terminal CMA+ bands of the C chromosome pair of F. obovata did not hybridize with the CsSat probe, whereas both CMA+ bands of each C chromosome of C. sinensis, C. maxima, C. deliciosa hybridized with this probe. These data demonstrate once again that chromosomes of the same CMA+ banding type may represent homoplasy rather than orthology (Guerra 2009).
Curiously, the karyotypes of C. reshni, C. deliciosa, and P. trifoliata displayed one or two D chromosomes with an rDNA signal closely linked to a CsSat satellite site, suggestive of simple translocation between these sites. Additionally, in P. trifoliate, a 45S rDNA site substituted the CsSat site observed in all B chromosomes, whereas the rDNA site of a D chromosome was partially substituted by CsSat sequences, indicating a possible reciprocal translocation between B and D chromosomes.
The evolution of banding patterns, CMA+ bands, and CsSat sequence
In general, a repetitive DNA family is restricted to closely related species or genera (Hemleben et al. 2007), although several examples of largely distributed satellite sequences are known in plants (Sharma and Raina 2005). The phylogenetic proximity of Citrus, Poncirus, and Fortunella (Morton et al. 2003) may explain the conservation of the main satellite DNA of C. sinensis throughout this group. The other Aurantioideae genera investigated here, Atalantia, Severinia, Triphasia, and Murraya, which were more distantly related to the Citrus–Poncirus–Fortunella clade (Morton et al. 2003), showed no signals of CsSat probe after FISH. However, in Southern blot experiments, the HaeIII digested genomic DNA of M. paniculata, S. buxifolia, and T. trifolia hybridized to CsSat probe, although only under low stringency conditions. This result suggests that CsSat-like sequences may still be present in these genera, although conserving only low sequence similarity to the main satellite repeats of C. sinensis.
Southern blot hybridization of genomic DNA of C. sinensis (1, 2), S. buxifolia (3), T. trifolia (4), and M. paniculata (5) with the CsSat probe. The membrane was first hybridized in high stringency (82%) (a) and afterward in low stringency (58%) (b). Amount of genomic DNA per blot: C. sinensis (1 = 400 ng, 2 = 15 ng), S. buxifolia (6.5 μg), T. trifolia (11 μg), and M. paniculata (22 μg).
The highly variable CMA banding pattern, the wide distribution of CsSat and CsSat-like sequences, and their position restricted to chromosome termini, as revealed by FISH, suggest a general model for the evolution of GC-rich heterochromatin in Citrus and related genera, which can explain the parallel chromosome evolution observed in different tribes of Aurantioideae (Guerra et al. 2000). Looking at the vast diversity of CMA banding patterns in Citrus accessions and related genera it becomes clear that some patterns occur two or more times among species of Citrus and other 20 Aurantioideae genera investigated (Guerra et al. 2000; Yamamoto et al. 2008). For example, among the few species investigated here, T. trifolia and C. sunki (15D + 3F and 14D + 4F, respectively), and M. paniculata and C. deliciosa (2C + 14D + 2F and 2C + 12D + 4F, respectively), displayed similar CMA banding patterns. At least in these cases, we know that the similar banding was promoted by amplification of different satellite DNA families or by distantly related members of the CsSat family.
The simplest explanation is that the basic karyotype of Aurantioideae already had tandemly repeated clusters of an ancestral “proto-CsSat” sequence spread on most chromosome termini, which have been amplified or reduced during the evolution of the group, occasionally resulting in similar banding patterns, as proposed in the library hypothesis (Fry and Salser 1977). A phylogenetic analysis of different repeats of the main satellite DNA of three Citrus species and P. trifoliata showed that the similarity between repeats was higher within each species than between species (Fann et al. 2001), indicating that homogenization mechanisms were acting on these sequences followed by species-specific mutations. Therefore, repeated cycles of homogenization and mutation have occurred in parallel to amplification/reduction mechanisms. The species-specific repeat differentiation was less evident within the putative hybrid species, like C. sinensis and C. limon, in which the repeats were not homogenized (Hemleben et al. 2007). Probably, most genera of the subfamily Aurantioideae maintained the terminal distribution of CsSat-like sequences, although the possibility that they have been total or partially substituted by completely independent GC-rich sequences cannot be ruled out. Other telomere-associated sequences, as the CsSat, are nonrandom and highly conserved, probably because they play some structural role in chromosome protection and nuclear organization (Sharma and Raina 2005).
Abbreviations
- CMA:
-
Chromomycin A3
- DAPI:
-
4′,6-diamidino-2-phenylindole
- FISH:
-
Fluorescence in situ hybridization
- FITC:
-
Fluorescein isothiocyanate
- CsSat:
-
Citrus sinensis satellite DNA
- PCR:
-
Polymerase chain reaction
References
Beridze T, Tsirekidze N, Roytberg MA (1992) On the tertiary structure of satellite DNA. Biochimie 74:187–194
Brasileiro-Vidal AC, dos Santos-Serejo JA, Soares Filho WS, Guerra M (2007) A simple chromosomal marker can reliably distinguishes Poncirus from Citrus species. Genetica 129:273–279
Carvalho R, Soares Filho WS, Brasileiro-Vidal AC, Guerra M (2005) The relationship among lemons, limes and citron: a chromosomal comparison. Cytogenet Genome Res 109:276–282
Charlesworth B, Sniegowski P, Stephan W (1994) The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371:215–220
Cornélio MTMN, Figueirôa ARS, Santos KGB, Soares Filho WS, Guerra M (2003) Chromosomal relationships among cultivars of Citrus reticulata Blanco, its hybrids and related species. Plant Syst Evol 240:149–161
CSHL/WUGS/PEB (2000) The complete sequence of a heterochromatic island from a higher eukaryote. The Cold Spring Harbor Laboratory, Washington University Genome Sequencing Center, and PE Biosystems Arabidopsis Sequencing Consortium. Cell 100:377–386
De Felice B, Wilson RR, Ciarmiello L, Scarano MT, Ferrante S (2006) Characterization of a novel satellite DNA sequence from flying dragon (Poncirus trifoliata). Genetica 127:45–53
Fann JY, Kovarik A, Hemleben V, Tsirekidze NI, Beridze TG (2001) Molecular and structural evolution of Citrus satellite DNA. Theor Appl Genet 103:1068–1073
Fry K, Salser W (1977) Nucleotide sequences of HS-alpha satellite DNA from kangaroo rat Dipodomys ordii and characterization of similar sequences in other rodents. Cell 2:1069–1084
Guerra M (1993a) High amount of heterochromatin in a tropical tree species: Genipa americana L. (Rubiaceae). Cytologia 58:427–432
Guerra M (1993b) Cytogenetics of Rutaceae. V. High chromosomal variability in Citrus species revealed by CMA/DAPI staining. Heredity 71:234–241
Guerra M (2009) Chromosomal variability and the origin of Citrus species. In: Mahoney CL, Springer DA (eds) Genetic diversity. Nova Science, New York, pp 51–68
Guerra M, Santos KGB, Barros e Silva AE, Ehrendorfer F (2000) Heterochromatin banding patterns in Rutaceae–Aurantioideae—a case of parallel chromosomal evolution. Am J Bot 87:735–747
Hemleben V, Kovarik A, Torres-Ruiz RA, Volkov RA, Beridze T (2007) Plant highly repeated satellite DNA: molecular evolution, distribution and use for identification of hybrids. Syst Biodivers 5:277–289
Hou MH, Robinson H, Gao YG, Wang AH (2004) Crystal structure of the [Mg2+-(chromomycin A3)2]-d(TTGGCCAA)2 complex reveals GGCC binding specificity of the drug dimmer chelated by a metal ion. Nucleic Acids Res 32:2214–2222
Ingle J, Pearson GG, Sinclair J (1973) Species distribution and properties of nuclear satellite DNA in higher plants. Nature 242:193–197
Kang SK, Lee DH, An HY, Park JH, Yun SH, Moon YE, Bang JW, Hur Y, Hoe Koo DH (2008) Extensive chromosomal polymorphism revealed by ribosomal DNA and satellite DNA loci in 13 Citrus species. Mol Cells 26:319–322
Kazama Y, Sugiyama R, Suto Y, Uchida W, Kawano S (2006) The clustering of four subfamilies of satellite DNA at individual chromosome ends in Silene latifolia. Genome 49:520–530
Lim KY, Kovarik A, Matyasek R, Chase MW, Knapp S, McCarthy E, Clarkson JJ, Leitch AR (2006) Comparative genomics and repetitive sequence divergence in the species of diploid Nicotiana section Alatae. Plant J 48:907–919
Moraes AP, Soares Filho WS, Guerra M (2007a) Karyotype diversity and the origin of grapefruit. Chromosome Res 15:115–121
Moraes AP, Lemos RR, Brasileiro-Vidal AC, Soares Filho WS, Guerra M (2007b) Chromosomal markers distinguish hybrids and non-hybrid accessions of mandarin. Cytogenet Genome Res 119:275–181
Matsuyama T, Akihama T, Ito Y, Omura M, Fukui K (1996) Characterization of heterochromatic regions in ‘Trovita’ orange (Citrus sinensis Osbeck) chromosomes by the fluorescent staining and FISH method. Genome 39:941–945
Miranda M, Ikeda F, Endo T, Moriguchi T, Omura M (1997) Comparative analysis on the distribution of heterochromatin in Citrus, Poncirus and Fortunella chromosomes. Chromosome Res 5:86–92
Morton CM, Grant M, Blackmore S (2003) Phylogenetic relationships of the Aurantioideae inferred from chloroplast DNA sequence data. Am J Bot 90:1463–1469
Pedrosa A, Schweizer D, Guerra M (2000) Cytological heterozygosity and the hybrid origin of sweet orange [Citrus sinensis (L.) Osbeck]. Theor Appl Genet 100:361–367
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schwarzacher T (2003) DNA, chromosomes, and in situ hybridization. Genome 46:953–962
Sharma S, Raina SN (2005) Organization and evolution of highly repeated satellite DNA sequences in plant chromosomes. Cytogenet Genome Res 109:15–26
Swingle WT, Reece PC (1967) The botany of Citrus and its wild relatives. In: Reuther W, Weber HJ, Batchelor LD (eds) The Citrus industry. History, world distribution, botany and varieties, vol I. University of California Press, Berkeley, pp 190–430
Tek AL, Song J, Macas J, Jiang J (2005) Sobo, a recently amplified satellite repeat of potato, and its implications for the origin of tandemly repeated sequences. Genetics 170:1231–1238
Wanzenböck EM, Schöfer C, Schweizer D, Bachmair A (1997) Ribosomal transcription units integrated via T-DNA transformation associate with the nucleolus and do not require upstream repeat sequences for activity in Arabidopsis thaliana. Plant J 11:1007–1016
Winterfeld G, Röser M (2007) Chromosomal localization and evolution of satellite DNAs and heterochromatin in grasses (Poaceae), especially tribe Aveneae. Plant Syst Evol 264:75–100
Yamamoto M, Abkenar AA, Matsumoto R, Kubo T, Tominaga S (2008) CMA staining analysis of chromosomes in Citrus relatives, Clymenia, Eremocitrus and Microcitrus. J Jpn Soc Hortic Sci 77:24–27
Zellinger B, Akimcheva S, Puizina J, Schirato M, Riha K (2007) Ku suppresses formation of telomeric circles and alternative telomere lengthening in Arabidopsis. Cell 27:163–169
Acknowledgements
This research was supported by grant of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE). We thank Dr. Walter dos Santos Soares Filho, from EMBRAPA/CNPMF, who kindly send us seeds from several species, Andrea Pedrosa-Harand for critical comments, and Gabriela Cabral for improving the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Pat Heslop - Harrison.
Rights and permissions
About this article
Cite this article
Barros e Silva, A.E., Marques, A., dos Santos, K.G.B. et al. The evolution of CMA bands in Citrus and related genera. Chromosome Res 18, 503–514 (2010). https://doi.org/10.1007/s10577-010-9130-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-010-9130-2