Abstract
This review is on how current knowledge of brainstem control of gastric mechanical function unfolded over nearly four decades from the perspective of our research group. It describes data from a multitude of different types of studies involving retrograde neuronal tracing, microinjection of drugs, whole-cell recordings from rodent brain slices, receptive relaxation reflex, accommodation reflex, c-Fos experiments, immunohistochemical methods, electron microscopy, transgenic mice, optogenetics, and GABAergic signaling. Data obtained indicate the following: (1) nucleus tractus solitarius (NTS)—dorsal motor nucleus of the vagus (DMV) noradrenergic connection is required for reflex control of the fundus; (2) second-order nitrergic neurons in the NTS are also required for reflex control of the fundus; (3) a NTS GABAergic connection is required for reflex control of the antrum; (4) a single DMV efferent pathway is involved in brainstem control of gastric mechanical function under most experimental conditions excluding the accommodation reflex. Dual-vagal effectors controlling cholinergic and non-adrenergic and non-cholinergic (NANC) input to the stomach may be part of the circuitry of this reflex. (5) GABAergic signaling within the NTS via Sst-GABA interneurons determine the basal (resting) state of gastric tone and phasic contractions. (6) For the vagal–vagal reflex to become operational, an endogenous opioid in the NTS is released and the activity of Sst-GABA interneurons is suppressed. From the data, we suggest that the CNS has the capacity to provide region-specific control over the proximal (fundus) and distal (antrum) stomach through engaging phenotypically different efferent inputs to the DMV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Preamble
The stomach has three primary motor functions (Hennig and Spencer 2018). These are accommodation, mixing, and emptying. Accommodation refers to the stomach muscle relaxing to receive and to store ingested food. This function is carried out by the proximal stomach which consists of the fundus and corpus. The musculature is relatively thin and consists of distensible smooth muscle layers (Schulze-Delrieu 1983), displaying mainly tonic contractions. Mixing and emptying functions are carried out primarily by the distal stomach, consisting of the distal corpus, antrum and pylorus. Here, the musculature is thick and generates powerful phasic contractions (Pröve and Ehrlein 1982). Our review is focused on brainstem neurocircuitries controlling tonic contractions (that is, accommodation function), and phasic contractions (that is mixing and emptying functions).
Introduction
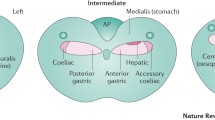
Our journey to understand brainstem neuronal circuitries controlling gastric mechanical activity was initiated by a two-step series of experiments (Pagani et al. 1988). The first step was to use retrograde neuronal tracing techniques to identify potential “control centers” in the brainstem that innervate smooth muscle and sphincters. The second step was to determine whether activation of the brainstem site that contains the most numerous labeled cell bodies influence gastric mechanical and sphincter function. This approach resulted in the following findings: (1) all parts of the stomach receive innervation from the dorsal motor nucleus of the vagus (DMV). The fundus is innervated by the lateral halves of the left and right DMVs, while the antrum is innervated by neurons located primarily in the medial portion of the DMV (Pagani et al. 1988); (2) direct stimulation of the DMVs produces increase in gastric tone and phasic contractions. These are selective effects in the sense that there are no significant changes in heart rate and blood pressure (Pagani et al. 1985; Norman et al. 1985); (3) there is evidence of a dual pathway of preganglionic vagal fibers in the DMV controlling the function of the lower esophageal sphincter (Rossiter et al. 1990). We discovered that the rostral area of the DMV contained neurons which upon activation would increase lower esophageal (LES) pressure. In contrast, the caudal area of the DMV contained neurons which upon activation would decrease LES pressure; and (4) by microinjecting a retrograde tracer into the DMV, we found a population of neurons in the caudal raphe nuclei that project to the DMV. Activation of these neurons causes an increase in gastric motility that is due to excitation of vagal neurons in the DMV (Hornby et al. 1990).
Early on in our studies we became aware that the DMV motoneurons receive an inhibitory GABAergic input (Travagli et al. 1991). This occurred while performing whole-cell current-clamp recordings from anatomically identified DMV neurons in rat brain stem slices. Spontaneously occurring tetrodotoxin-resistent miniature inhibitory synaptic potentials were observed. Electrical stimulation in the tissue surrounding the recording neuron was capable of evoking orthodromic-evoked mixed inhibitory-excitatory postsynaptic potentials, and eventually, action potentials. Whole-cell voltage-clamp recordings of the synaptic currents corresponding to these synaptic potentials in the presence of pharmacological antagonists of the neurotransmitters GABA, glutamate, and glycine receptor subtypes indicated that the inhibitory synaptic currents are mediated by GABA-activated chloride channels, while the excitatory synaptic currents are due to activation of ionotropic glutamate receptors.
In summary, our review is centered on identifying brainstem neuronal circuitries with emphasis on pathways connecting the NTS to the DMV. It also includes information on the importance of local circuit neurons within the NTS and the DMV in the operation of these circuits.
Identification of Brainstem Neural Circuitry Controlling Tonic and Phasic Contractions of the Stomach Using Intravenously Administered Nicotine
Our interest in locating neurons in the brainstem that control stomach mechanical function was magnified by our studies of nicotine (Ferreira et al. 2000, 2001, 2002). For our studies, we used anesthetized rats and recorded tonic—and phasic contractions employing an inflated intragastric balloon placed into the stomach cavity and a strain gauge force transducer sutured to the fundus. Nicotine was given intravenously (iv) in five increasing doses starting with 56.5 nmol/kg and was observed to decrease both gastric tone and phasic contractions. The lower dose range of drug only inhibited fundus tone. With higher doses, both fundus tone and phasic contractions of the antrum were inhibited. Prior bilateral vagotomy prevented the lower dose range of nicotine from reducing fundus tone. This was a provocative observation, that is, noting with low doses of nicotine, decreases in tonic contractions of the fundus without parallel decreases in phasic contractions of the antrum. It suggested an independence of tonic and phasic contractions of the stomach.
In an earlier study, we demonstrated that nicotine microinjected into the medial subnucleus of the tractus solitarius (mNTS) in doses ranging from 0.1 to 300 pmol evoked decreases in intragastric pressure (IGP) (Ferreira et al. 2000). Bilateral cervical vagotomy prevented this effect. The nicotinic receptor antagonist, hexamethonium, microinjected into the mNTS also prevented nicotine from reducing IGP. Based on these findings, we hypothesized that nicotine administered iv was acting in the mNTS to inhibit fundus tone (Ferreira et al. 2002). To test our hypothesis, we first microinjected hexamethonium bilaterally into the mNTS, and then administered nicotine iv. We found that the iv effect of nicotine on fundus tone was prevented signifying that the iv nicotine effect to produce fundus relaxation resides in the mNTS.
To corroborate that iv nicotine was exciting mNTS neurons, doses of this drug that inhibit fundus tone as reflected by IGP—and strain gauge recordings from the fundus were evaluated on c-Fos expression in brain stem nuclei (Ferreira et al. 2002). The lower iv dose range of nicotine stimulated c-Fos expression in the mNTS. In contrast, nicotine had no effect on c-Fos expression in gastric projecting DMV neurons.
Once having established the mNTS as the site of action of iv nicotine, our goals were to identify the subtype of nAchR responsible for the decrease in fundus tone, and the location of the nAchR subtype. To identify the subtype, we tested microinjected nicotine after blocking the α4β2 nAchR in the mNTS with the α4β2 antagonist dihydro -beta-erythroidine (DHBE). In the presence of DHBE, nicotine microinjected into the mNTS had no effect. We also tested cytisine, a drug with its major agonist effect on the α3β4 nAchR but little or no agonistic effect on the α4β2 nAchR. Cytisine microinjected into the mNTS had no effect on fundus tone but did inhibit antral contractions. These data suggested that nicotine acted on mNTS α4β2 nAchRs to inhibit the fundus. In addition, nicotine acted on mNTS α3β4 nAchRs to inhibit the antrum.
To find the location of the nAchRs in the mNTS, we knew from reports of others that nAchRs are located on presynaptic nerve terminals, and when activated will increase release of transmitter such as glutamate (Colquhoun and Patrick 1997; McGehee and Role 1995). Furthermore, glutamate is known to be released by sensory nerve terminals in the NTS (Talman et al. 1980). If nicotine was acting on vagal afferent nerve terminals to release glutamate, pretreatment with an antagonist of glutamate such as kynurenic acid should block nicotine from inhibiting gastric mechanical activity. Hence, we first microinjected kynurenic acid into the mNTS, and followed it with nicotine microinjected into the same site. Kynurenic acid pretreatment prevented nicotine from reducing IGP. Kynurenic acid pretreatment also prevented iv nicotine from inhibiting fundus tone (Ferreira et al. 2002). These data suggested that the location of the α4β2 nAchR in the mNTS is on vagal afferent nerve terminals. We did not pursue studies to locate the α3β4n AchR subtype. Instead, we refer to the report by Xu et al. (2015), indicating both a presynaptic and a somatodendritic location. For the purpose of constructing Fig. 1, we have placed this subtype receptor at a postsynaptic site.

Adapted from Fig. 13 of Ferreira et al. (2002)
Schematic of proposed neurocircuitry involved in nicotine-induced effects on intragastric pressure and phasic contractions in the dorsal medulla.
The possibility the motility of the fundus and the antrum are controlled by different neural pathways was raised by the finding that the lower doses of nicotine administered either iv or locally into the mNTS decreased fundus tone without decreasing antral contractions. Higher doses of the drug affected both areas of the stomach. This possibility was augmented by the finding that activation of α4β2 nAchRs in the mNTS inhibited the fundus, while activation of α3β4 nAchRs in the mNTS inhibited the antrum. To pursue this possibility further we posed the question of what 'downstream' signaling occurs within the mNTS and is this downstream signaling required for both the fundus and antrum to operate.
Ruggiero and colleagues (Ruggiero et al. 1996) reported that NADPH, a marker of nitric oxide synthase (NOS) is present in the mNTS. To assess the role of NO in nicotine-induced fundus relaxation upon microinjection into the mNTS, we microinjected an inhibitor of NOS, L-NAME, bilaterally into the mNTS, and repeated our nicotine microinjection. With inhibition of NO synthesis, nicotine no longer decreased IGP and tonic contractions of the fundus. In contrast, L-NAME microinjected into the mNTS had no effect on nicotine-induced reduction in phasic contractions of the antrum. Thus, based on our nAchR studies at the mNTS, and our studies of inhibiting the synthesis of NO at the mNTS, it is clear that separation of pathways begins at the mNTS.
Is the separation of pathways regulating fundus tone and antrum contractions maintained at the level of the DMV? Communication between the mNTS and the DMV has been suggested to be through noradrenergic (Pickel et al. 1986; Fukuda et al. 1987; Siaud et al. 1989; Bertolino et al. 1997), GABAergic (Davis et al. 2004), and glutamatergic (Willis et al. 1996) projection neurons. To test a role for noradrenergic signaling, we blocked the α2 adrenoreceptors at the DMV. This was accomplished by microinjecting yohimbine into the DMV and activating nicotinic receptors at the mNTS. Blockade of α2 receptors at the DMVsite counteracted nicotine-induced decreases in IGP and fundus tone, but it did not counteract nicotine evoked reduction in antral contractions (Ferreira et al. 2002).
To test the role of GABAergic signaling, we blocked the GABAA receptors at the DMV. This was accomplished by microinjecting bicuculline into the DMV and activating nicotinic receptors at the mNTS. Blockade of GABAA receptors at the DMV site counteracted nicotine evoked reduction in antral contractions but did not counteract nicotine-induced decreases in IGP and fundus tone (Ferreira et al. 2002).
To test for a role of glutamatergic signaling, we blocked the glutamate receptor at the DMV. This was accomplished by microinjecting kynurenic acid into the DMV and activating nicotinic receptors at the mNTS. Blockade of glutamate receptors at the DMV site had no effect on nicotine-induced inhibitory effects at either the fundus or the antrum (Ferreira et al. 2002).
We also assessed whether there was ongoing signaling between the mNTS and the DMV. Blockade of α2 adrenoreceptors at the DMV had no effect per se on gastric mechanical activity. The same was true for blockade of glutamatergic receptors at the DMV. However, blockade of GABAA receptors at the DMV site resulted in significant increases in IGP, fundus tone, and antral contractions indicating ongoing signaling in this pathway (Ferreira et al. 2002).
We have constructed Fig. 1 to summarize our findings and to illustrate how gastric mechanical function is controlled by two distinct pathways. The left side of the figure shows how the stomach communicates information to the brain. Signaling from gastric muscle is relayed via vagal afferent nerves to the nodose ganglion and forwarded on to the mNTS. The transmitter released from these terminals is glutamate, which in turn excites nitrergic neurons. The NO produced excites the circuit that regulates the fundus. Activation of this circuit requires NO which excites noradrenergic projection neurons synapsing at the DMV. The released norepinephrine excites α2 adrenoreceptors resulting in inhibition of DMV neurons projecting to the proximal stomach (right side of the figure). The end result is relaxation of the fundus. Thus, nicotine activates a vago-vago reflex by exciting α4β2 nAchRs on viscerosensory nerve endings in the mNTS causing IGP to drop and fundus tone to decrease (Ferreira et al. 2002). This is circuit number 1.
Signaling in the second circuit starts with nicotine exciting postsynaptic α3β4 nAchRs located on second-order neurons in the mNTS. We suggest that these second-order neurons are GABAergic and project to the DMV. Activation of GABAA receptors in the DMV inhibits output neurons that innervate the distal stomach. Based on our c-Fos data showing no excitation of DMV neurons, we suggest that nicotine acting in the mNTS and engaging GABAergic projection neurons, is suppressing the activity of cholinergic–cholinergic excitatory drive to the antrum.
Additional evidence for a mNTS GABAergic pathway to vagal motor neurons that respond to nicotine was reported by Xu et al. (2015). Gastric-related and GABAergic inhibitory synaptic input to the DMV from the NTS that is activated by nicotine was shown using transgenic mice that express enhanced green fluorescent protein under a GAD 67 promotor in a subset of somatostatin-coexpressing GABAergic neurons. In vivo retrograde pseudo-rabies viral labeling was used to identify gastric-related vagal neurons. Patch-clamp electrophysiology in brain stem slices was then used to show that nicotine application increased excitatory postsynaptic current (EPSCs) in approximately 80% of GABAergic NTS neurons. Previous studies of this group had shown that the majority of nAChRs in the NTS were of the α3β4subtype which is consistent with our earlier conclusion and Fig. 1 of this review (Ferreira et al. 2002).
Identified gastric-related DMV cells were also examined for responses to nicotine application in the NTS. Brief focal application of nicotine in the NTS significantly increased the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in 67% of the cells tested (Xu et al. 2015).
What remains to be discussed from Fig. 1 is the nature of the efferent projecting pathway out of the DMV affected by nicotine. As shown, two pathways are depicted, one engaged by NTS noradrenergic projection neurons (A2 Neuron) that synapse with preganglionic cholinergic neurons. These preganglionic neurons engage postganglionic neurons synapsing at the fundus. Acetylcholine is the neurotransmitter at both the ganglionic site and the postganglionic neuroeffector junction. Evidence implicating inhibition of this cholinergic-cholinergic pathway in mediating the relaxation of the fundus by microinjected nicotine into the mNTS is our result that ganglionic blockade by iv administered hexamethonium prevents nicotine-induced fundus relaxation. The other pathway is engaged by NTS GABAergic projection neurons that also synapse with preganglionic cholinergic neurons. These preganglionic neurons connect with non-cholinergic–non-adrenergic neurons and is referred to as the NANC pathway (Andrews 1990; Hornby. 2001). Excitation of this pathway leads to relaxation of the stomach (e.g., antrum) due primarily to the release of NO (Takahashi and Owyang 1997). Like the cholinergic–cholinergic pathway, iv hexamthonium would prevent engagement of this system. We did not test hexamethonium on nicotine-induced inhibition of antral contractions. Because nicotine did not increase c-Fos in the DMV, we conclude that only inhibition of the cholinergic–cholinergic excitatory pathway was responsible for nicotine's relaxing effect at both the fundus and the antrum.
A third DMV pathway has been proposed by Krowicki et al. (1997); Zheng et al. (1999) and Krowicki et al. (1999). This efferent pathway consists of DMV nitrergic neurons directly synapsing with gastric smooth muscle. Hexamethonium iv has no effect on this pathway (Krowicki et al. 1999). Since iv hexamethonium fully blocked the nicotine effect, we have no evidence for involvement of DMV nitrergic motor neurons. Consistent with no involvement was the absence of nicotine-induced c-Fos expression in the DMV.
Four important findings emerge with our studies of nicotine. First, is the realization that NTS–DMV noradrenergic pathway may be important for control of gastric mechanical function. Second, second-order nitrergic neurons in the mNTS may be important in the control of fundus tone. Third, two separate brainstem circuits regulate the proximal (fundus) and distal (antrum) stomach. Fourth, a single DMV efferent pathway is involved in brainstem control of gastric mechanical function.
Confirmatory evidence that the fundus could be controlled separate from the antrum and vice versa can be found in studies by Talman and colleagues (Spencer and Talman 1986; Talman et al. 1991) Their experimental set up was the anesthetized rat and gastric pressure was recorded from an inflated balloon inserted at the junction of the antrum and fundus. The balloon registered both tonic and phasic components of mechanical activity. Glutamate was microinjected into the mNTS and decreased tonic gastric pressure as well as inhibited gastric phasic activity (Spencer and Talman 1986). Next, cholecystokinin (CCK) was studied in the NTS in the same manner as glutamate (Talman et al. 1991). CCK decreased tonic gastric pressure and phasic contraction amplitude. The dose–response characteristics were similar for both end points. It was, however, noted that on occasion, decreases in tonic pressure occurred without changes in either amplitude or frequency of contractions. The authors suggest that “tonic and phasic gastric pressure may be independently regulated by central mechanisms”.
Next, we sought additional evidence of nicotine activating noradrenergic neurons in the mNTS (Ferreira et al. 2005). We employed double-label immunocytochemical methods to assess the effect of iv nicotine in the low dose range on c-Fos expression in NTS noradrenergic neurons. The marker of neurons containing norepinephrine is tyrosine hydroxylase (TH). Even though TH also identifies neurons that contain dopamine and epinephrine, it was useful as a marker because neurons in the anatomical area of the NTS we were interested in are primarily noradrenergic (Paxinos 1999). In a 150 µm section of brainstem just rostral to the calamus scriptorius (CS), we observed that 19.7 ± 4.2% of the TH neurons were activated by nicotine (Ferreira et al. 2005). These results appear in Fig. 2 and Table 1.
We also processed brains of rats receiving the low dose range of iv nicotine for NOS immunoreactivity and for c-Fos. We observed little effect of nicotine on c-Fos in NOS immunoreactive neurons. This was surprising because our studies with L-NAME microinjected into the mNTS clearly demonstrated a critical signaling role of NO in the circuit controlling the fundus. We speculate that glutamate release by afferent vagal nerve terminals activate NOS containing nerve terminals in the NTS neuropil. Consistent with this speculation is the report by Lin and Talman (2000), that NMDAR1 immunoreactivity is present in NOS immunoreactive neurons in the NTS. This released NO activates close-by noradrenergic projection neurons that synapse in the DMV (Fig. 3). Nitric oxide could also cause additional release of glutamate from nearby vagal afferent terminals (O'dell et al. 1991).
Schematic of proposed neurocircuitry in the dorsal medulla involved in the esophageal distension and nicotine-induced decreases in IGP. ND nodose ganglion, GluR glutamate receptor, LES lower esophageal sphincter, mAChR muscarinic acetylcholine receptor, nAChR nicotinic acetylcholine receptor, NE norepinephrine, NO nitric oxide, Not nitric oxide at the terminal.
Identification of Brainstem Neural Circuitries Controlling Tonic and Phasic Contractions of the Stomach Using Reflex-Induced Changes in Gastric Reservoir Function
The neural pathways revealed by our study of nicotine led us to hypothesize that these pathways are the same as those that carry out vago-vagal reflexes that occur physiologically. To test this we focused on reflexes that control gastric reservoir function. According to Tack et al. (2012), there are two. After swallowing food, a reflex is evoked by distension of the esophagus. This distension leads to a rapid inhibition of tone and is called the receptive relaxation reflex. Next, as the stomach is filled by food, distension occurs. This distension produces a long lasting relaxation of the proximal gut and the phenomenon is called adaptive relaxation (Desai et al. 1991a, 1991b) or gastric accommodation (Tack et al. 2012).
We will refer to the two reflexes as receptive relaxation and accommodation. The purpose of the receptive relaxation reflex is to prepare the stomach to receive food, and the purpose of the accommodation reflex is to enable the stomach to accept large amounts of food with negligible increases in intragastric pressure.
Brainstem Neuronal Circuitry for the Receptive Relaxation Reflex
To create the receptive relaxation reflex (RRR) in anesthetized rats, intragastric pressure (IGP) was recorded, and a balloon catheter was used to produce distension of the thoracic esophagus (Ferreira et al. 2005). Using this preparation, the general neural circuitry for the RRR was determined. Esophageal distension (ED) consistently decreased IGP. Bilateral cervical vagotomy, bilateral microinjection of tetrodotoxin (a drug known to block nerve conduction) into the mNTS, and iv atropine methyl bromide all prevented the response. Spinal cord transection (to remove the sympathetic nervous system) and iv L-NAME had no effect on ED—induced decreases in IGP. These data suggested that the RRR produced by ED was due to increased neural signaling into the mNTS, inhibition of efferent vagus nerve activity, and withdrawal of cholinergic-cholinergic signaling to fundus smooth muscle. It also suggests that both sympathetic nervous system and the NANC pathway played no role in the RRR.
Next, the effect of ED was evaluated on the expression of c-Fos in brainstem nuclei. As in the case with iv nicotine ED increased c-Fos expression in the NTS, and this expression was significant from − 0.6 mm caudal to CS to + 1.05 mm rostral to CS. Peak expression occurred at CS.
As in our studies with iv nicotine, we sought evidence for ED activating noradrenergic neurons in the mNTS (Ferreira et al. 2005). We used double-label immunocytochemical methods on c-Fos expression in NTS noradrenergic neurons. In a 150 um section of brainstem on either side of CS, as many as 19.7 ± 2.3% of the TH neurons present were activated by ED. Comparing the total number of TH-IR neurons stimulated by ED to data from another laboratory, it was similar to that reported by Willing and Berthoud (1997), for distension of the stomach.
We also processed brains of rats subjected to ED for NOS immunoreactivity. The number of NOS neurons expressing c-Fos was less than the number of TH-immunoreactive neurons expressing c-Fos.
Because other investigators conclude that ED activates a NANC pathway originating in the DMV (Rogers et al. 2003; Hermann et al. 2006) it was important to ask the question of whether ED increases c-Fos in DMV neurons that project to the stomach. Data analysis indicated that ED had no physiological significant effect on c-Fos expression in these neurons. It has been shown by (Willing and Berthoud 1997), that DMV neurons are capable of expressing c-Fos. In their studies, gastric distension produced robust increases in c-Fos expression of DMV neurons.
Communication between the NTS and the DMV in our nicotine experiments involving relaxation of the fundus was through noradrenergic projection neurons. To test for a role of noradrenergic signaling in the ED—induced decreases in IGP, two different α2 adrenoreceptor antagonists were microinjected bilaterally into the DMV in separate experiments. These were yohimbine and SKF86466. Both counteracted ED-induced reductions in IGP. In addition, experiments were performed where the GABAA receptor channel antagonist, picrotoxin, was microinjected bilaterally into the DMV. Blockade of GABAA receptor channels had no significant effect on ED-induced decreases in IGP.
To assess the role of NO in ED-induced decreases in IGP, L-NAME was microinjected bilaterally into the mNTS and ED repeated. With NO synthesis inhibited, ED no longer decreased IGP. Similar experiments were performed with L-NAME microinjected into the DMV. No effect was observed on the RRR.
In summary, our data indicate that a noradrenergic pathway originating in the NTS and projecting to the DMV is part of the hindbrain circuitry for transmitting sensory information from the esophagus to the DMV. Concurring with this finding are the earlier observations of Fukuda et al. (1987), using a rat brain slice preparation. They reported that norepinephrine stimulated α2 adrenoreceptors present on DMV neurons resulting in their inhibition. This finding was corroborated by Valenzuela et al. (2004).
Rogers et al. (2003), were the first to demonstrate that noradrenergic neurons were activated by ED. They showed this using immunohistochemical procedures for detecting changes in c-Fos and TH. ED increase TH neurons in the NTS to colocalize c-Fos. They then performed pharmacological experiments with the α2 adrenoreceptor blocker yohimbine, and the α1 adrenoreceptor blocker, prazosin. ED was tested before and after application of these drugs to the floor of the fourth ventricle. Each blocker antagonized the RRR response by just over 50%, and the combination produced nearly a full blockade of the RRR. In contrast to Rogers and colleagues result, we found that just blockade of α2 adrenoreceptors at the DMV would produce nearly complete blockade of the RRR.
We suggest that Rogers and colleagues were unable to fully block the RRR response with yohimbine because they applied the drug to the floor of the 4th ventricle instead of microinjecting it directly into the DMV. Because floor of the 4th ventricle application would enable yohimbine to diffuse to wide areas of the brainstem, the concentration of the drug reaching the DMV may have been inadequate.
Why did Rogers and colleagues choose to apply the drug to the floor of the 4th ventricle? Their explanation was that “previous work from their laboratory has shown that neurons in NTSc (referring to the NTS subnucleus centralis) area responsive to ED project throughout the entire DMN (DMV),” and this expansive area would not be covered by microinjection of yohimbine. According to Paxinos and Watson (2014), the total length of this columnar structure is about 2.4 mm. The portion of the DMV that sends projections to the fundus is only about 0.65 mm in length, that is, 80% of all labeled DMV neurons are contained in the region of -0.125 to + 0.50 mm on either side of the calamus scriptorius (CS) (Pearson et al. 2007). Therefore, microinjection of drug usually in volumes of 60 nl (Ferreira et al. 2005) should reach and block all α2 adrenoreceptors in that region of the DMV.
Another point of concern in regard to the rationale used by Rogers et al. (2003), in applying drugs to the floor of the 4th ventricle and not microinjecting drugs into the DMV, is that it ignores the high possibility that the entire DMV is not innervated by the noradrenergic input from the NTS. Data of Pearson et al. (2007) indicate that just the area of the DMV projecting to the fundus is innervated by noradrenergic nerve terminals, and this region is about 0.65 mm and not the entire length of the DMV.
Consistent with our finding that blockade of α2 adrenoreceptors at the DMV produces near complete block of the RRR are again the results of Fukuda et al. (1987). These investigators demonstrated that activation of the NTS noradrenergic pathway to the DMV only affects α2 adrenoreceptors on DMV neurons. Alpha1 adrenoreceptors on the same neuron are not affected (presumably, the α1 adrenoreceptor is extrasynaptic). The effect of the α1 adrenoreceptor blocker observed by Rogers et al. (2003), might not have been at the DMV, but at another site reached in the brainstem because prazosin had been applied to the floor of the 4th ventricle.
Data of our study also implicated nitric oxide as an important signaling molecule in the mNTS in carrying out ED—induced decreases in IGP. As with our nicotine study, there was a lack of activation of NOS—containing NTS neurons during ED as assessed by c-Fos expression. How we reconcile the lack of activation of NOS—containing NTS neurons with the L-NAME microinjection data has been discussed (see "Identification of brainstem neural circuitry controlling tonic and phasic contractions of the stomach using intravenously administered nicotine" section).
Most important, these data, in combination with our earlier pharmacological microinjection data with nicotine indicate that both ED and nicotine produce nitric oxide in the NTS, which then activates noradrenergic neurons that terminate on—and inhibit DMV neurons. These neural signaling pathways are illustrated in Fig. 3. Furthermore, both ED and nicotine engage a pathway that appears to affect the proximal part of the stomach.
It was clear that our results differed from Rogers et al. (2003) in several respects. First, blockade of α2 adrenoreceptors almost completely abolished the ED—induced vago-vagal reflex. In their study, blockade of both α2 and α1 adrenoreceptors were required to antagonize the ED—induced vago-vagal reflex. Rogers and colleagues suggested that α2 adrenoreceptors are located on DMV neurons that provide excitatory cholinergic input to the stomach. Upon excitation, there is a decrease in cholinergic—cholinergic drive to the stomach resulting in gastric relaxation. The α1 adrenoreceptors are located on DMV neurons that provide inhibitory NANC input to the stomach. Upon activation, there is an increase in NANC drive to the stomach resulting in gastric relaxation. Thus, the combination of α1 and α2 adrenoreceptor blockade suppresses the RRR by preventing the operation of two independent and parallel pathways emanating from the DMV and give rise to the reflex. It is important to keep in mind that in studying the role of adrenoreceptors at the DMV, Rogers and colleagues applied drugs to the floor of the 4th ventricle while we microinjected drugs into the DMV.
Second, we found that the RRR involves only inhibition of the cholinergic excitatory pathway emanating from the DMV; the NANC inhibitory pathway plays no role in giving rise to this reflex. Our evidence was based on the ability of iv atropine methylbromide to completely block the end organ response. Our evidence was also based on the inability of iv L-NAME to antagonize the end organ response. Additionally, ED, while activating neurons in the NTS area as indicated by c-Fos expression, had no effect on c-Fos expression of DMV neurons that project to the stomach. If activation of α1 adrenoreceptors at the DMV were to be involved in the RRR, an increase in c-Fos expression should be noted. Rogers and colleagues do not report what effect ED has on c-Fos expression in the DMV.
Third, inhibition of NTS NOS in our study abolished the RRR but not in the Rogers et al. (2003) study. Again, it is important to recognize that our data were obtained by microinjecting drug (L-NAME) into the NTS, whereas Rogers et al. data were obtained by application of drug to the floor of the 4th ventricle.
Rogers and colleagues (see Herman et al. (2009)), continued their studies of the RRR by investigating the peripheral pharmacological basis for the reflex. Studies were carried out in Long-Evans rats anesthetized with 150–200 mg/kg ip thiobutabarbital. ED was used to produce gastric relaxation. Intravenous administration of atropine methyl bromide reduced gastric relaxation to 52 ± 4.4% of the control response. Intravenous administration of L-NAME reduced it to 26.3 ± 7.2% of the control response. Treatment with a combination of the two drugs reduced the gastric relaxation induced by ED to 4.0 ± 2.5% of the control response. They state that “our data provide a clear demonstration that the gastroinhibitory control by the esophagus is mediated via a dual vagal innervation consisting of inhibitory nitrergic and excitatory cholinergic transmission.”
Rogers and colleagues, (Herman et al. 2006), suggest that the significant differences between our studies are that we did not use the same reflex-stimulating technique when we activated mechanoreceptors in the esophagus. They also criticized us for the anesthetic we used (mixture of urethane and chloralose), and for the techniques employed for monitoring gastric tone. Because of their criticisms, we repeated our 2005 study (Ferreira et al. 2005), using the same reflex-stimulating technique as Rogers et al. (2003) and Herman et al. (2008); employed a different anesthetic (isoflurane); and used their method of monitoring gastric tone (Herman et al. 2009). In doing so, we confirmed our earlier findings reported in Ferreira et al. (2005). Specifically, we found that norepinephrine released at the DMV acts on α2 adrenoreceptors to inhibit activity in a cholinergic excitatory pathway to the fundus. We found no evidence suggesting that α1 adrenoreceptors located on a DMV NANC pathway was involved in the operation of the RRR. Our study included a test of blockade of α1 adrenoreceptors at the DMV. Bilateral microinjection of prazosin into the DMV had no effect on the fundus relaxation induced by ED. Additionally, microinjection of norepinephrine into the DMV mimicked the RRR and its effect was prevented by prior administration of an α2 adrenoreceptor antagonist. Finally, we found that intravenous atropine methybromide prevented the reflex-induced fundus relaxation. In contrast, intravenous L-NAME had no effect.
So, how do we reconcile differing results from two independent laboratories? We suggest that the differing results may be due to the flawed design of the experiments performed by Rogers and colleagues. It may be due to a combination of an inadequate atropine dose and a NTS effect of L-NAME. Herman et al. (2006), used a dose of atropine methyl nitrate of 50 ug/kg iv. Their rationale was based on two cited references. They refer to the study of Takahashi and Owyang (1997), who used a similar but not identical dose regimen (50 ug/kg bolus and continuous infusion of 20 ug/kg/h) and reported that atropine had no effect on the gastric accommodation reflex. Since the reflex was not blocked, there is no way of knowing whether the muscarinic receptors were blocked. They refer to P. Millard's chapter in a Textbook on clinical veterinary nursing (Millard 2003). Millard lists the dose range of atropine (0.02–0.05 mg/kg) that is found in the contents of an anesthetic emergency kit. However, no reference is given for how the atropine dose was determined and/or whether the dose range applied to treating an anesthetic emergency in rats. We used a dose of atropine methyl bromide (i.e., 0.1 mg/kg iv) that was twice as high as that used by Herman et al. (2006). In a previous study, we showed that this dose was effective in preventing L-glutamate microinjection in the DMV from increasing IGP (Cruz et al. 2007) In our 2008 study (Herman et al. 2008) we observed that 0.1 mg/kg intravenous atropine methyl bromide counteracted approximately 85% of the RRR.
Regarding the use of L-NAME by Herman et al. (2006), the time interval between its administration and a test of the RRR was 15 min. Ma et al. (1995), showed that in 12–14 mNTS neurons after L-NAME iv decreased their activity, an effect that was maximal 12–15 min after its administration. Thus, L-NAME does affect hindbrain neural activity in one of the two major hindbrain nuclei making up the vago-vagal reflex. In our study, the interval between L-NAME administration and the test of the RRR was 5–7 min. At this time interval, L-NAME appeared to have a maximal effect as indicated by the consistent rise in blood pressure. However, it was ineffective in blocking the reflex response (Herman et al. 2008).
Two other issues need to be mentioned regarding why we are not able to replicate the results of Rogers and colleagues regarding the role of the brainstem NANC pathway in the RRR. One is anesthetic used. We employed isofluorane in our 2008 study (Herman et al. 2008), whereas Rogers and colleagues used a relatively high dose of a barbiturate, thiobutabarbital, 150–200 mg/kg ip. The usual dose of this anesthetic is 80–125 mg/kg ip bulk (Buelke-Sam et al. 1978; Ellison et al. 1987; Laiprasert et al. 2001; Laycock et al. 1979; Sababi and Nylander 1996; Shirley and Walter 1995; Tucker et al. 1982; Vitela et al. 2005). The concern here is that there are data indicating that barbiturates are vagolytic (Koppanyi et al. 1935; Morrison et al. 1951; Korner et al. 1968; Jackson and Richards 1977; Murthy et al. 1982). It is possible that the heavily anesthetized rat with thiobarbiturate had reduced cholinergic drive to the gut prior to studies of the RRR. Our use of isoflurane for anesthesia (Herman et al. 2008), can also be criticized. After performing our study we became aware of published data showing that isoflurane anesthesia produces a significant depressant effect on GI motility (Torjman et al, 2005). Thiobutabarbital may be the better anesthetic to use for GI motility studies, but only if it is used in the recommended anesthetic dose of 100 mg/kg and not the high dose used by Rogers et al. (2003). We suggest this based on data reported by Qualls-creekmore et al (2010), showing that 100 mg/kg ip has no effect on gastric emptying. Since it is well known that babiturates exhibit dose-dependent effects (e.g., Heyer and Macdonald (1982)), we anticipate that higher doses of thiobutabarbital would reduce gastric emptying.
The second issue is not a flaw in the experimental design but a difference in the strain of rats used between the two independent laboratories. We use Sprague–Dawley rats, whereas the Rogers laboratory use the Long–Evans rats, and there are data indicating differing physiological phenomena between the two strains (Faraday 2002; Tan et al. 2009; Oltra-Noguera et al. 2015).
In addition to Rogers and colleagues finding for a positive role for a NANC pathway in the operation of the RRR are the earlier findings of Abrahamsson and Jansson (1969), obtained in a different species, the anesthetized cat. A balloon was used to distend the esophagus while monitoring gastric motility. Inflation of the balloon was produced by either filling it with 2–20 ml of air or water. According to the legend for Fig. 1, the amount of water used was 18 ml. Distending the esophagus produced relaxation of the stomach. This response was prevented by bilateral cervical vagotomy and was unaffected by iv atropine sulfate 0.5 mg/kg. The response was also not affected by suppressing the sympathetic nervous system with either iv guanethidine or cervical spinal cord transection.
How to reconcile our findings with those of Abrahamsson and Jansson? We suggest that the ED stimulus used by Abrahamsson and Janssen in the cat was excessive, possibly activating pain reflexes. In the experimental example shown (Fig. 1), ED was produced by infusing 18 ml of water. We inflated our balloon in the rat with either 0.7 ml of fluid (Ferreira et al. 2005), or 0.10 ml of fluid (Herman et al. 2008).
Brainstem Neuronal Circuitry for the Accommodation Reflex
According to Rogers and Hermann (2012), “the concept of dual vagal effectors controlling cholinergic and NANC input to the stomach is now well accepted.” Their statement refers to dual vagal effectors originating in the DMV as pictured in Figs. 31.3 and 31.4 of their review. Based on our study of the RRR and our study with nicotine, we disagree with the conclusion of Rogers and Hermann (2012). Furthermore, we have sought evidence for dual vagal effectors emanating from the DMV and controlling cholinergic and NANC input to the stomach under many different experimental conditions. These conditions in addition to RRR and nicotine exposure are: (i) excitation of mNTS with microinjected glutamate (Herman et al. 2009); (ii) excitation of mNTS by locally blocking GABAA receptors (Herman et al. 2009); (iii) excitation of mNTS neurons by activating melanocortin-4 receptors (Richardson et al. 2013),; (iv) excitation of mNTS neurons by activating mu opioid receptors (Herman et al. 2010); (v) excitation of mNTS by stimulating GABAB receptors (Cruz et al. 2019); (vi) electrical stimulation of the DMV (Pagani et al. 1985); (vii) excitation of DMV neurons with glutamate (Cruz et al. 2007); (viii) intravenous glucose (Shi et al. 2005).; and (ix) intravenous cholecystokinin (Shi et al. 2005). Under these nine different experimental conditions plus under the experimental conditions of RRR and nicotine exposure, we have not observed convincing evidence for dual vagal effectors originating in the DMV and projecting to the stomach.
One experimental condition that we have not investigated is the brainstem neuronal circuitry for the accommodation reflex. Based on the impressive data of Takahashi and Owyang (1997), we are convinced that a NANC pathway originating in the brainstem is responsible for the gastric relaxation produced by gastric distension. They used the anesthetized rat to test the hypothesis that the accommodation reflex is carried out by the vago-vagal reflex and requires release of NO by postganglionic neurons innervating the stomach. To activate the reflex, they infused saline into the stomach to produce gastric distension while monitoring intragastric pressure (IGP) and stomach volume. A saline volume of 6 ml was infused over 6 min. The choice of 6 ml was based on an approximation of the volume of gastric contents of rats weighing 225–250 g. Gastric distension produced an increase in IGP of 9.0 ±1.0 cm H2O. After vagotomy, gastric distension produced an increase of 16.8 ±1.9 cm H2O. This suggested that a vago-vagal reflex was eliciting the response of the stomach to gastric distension. Specifically, it suggested the vago-vagal reflex involved release of an inhibitory substance that relaxed the gastric smooth muscle, preventing a rise in IGP. Pretreatment with iv L-NAME came close to mimicking the effect of vagotomy. Blockade of NO synthase with this drug resulted in an IGP of 13.8 ±1.9 cm H2O during gastric distension. Prior administration of L-arginine to prevent the L-NAME effect counteracted gastric distension-induced elevation of IGP.
To document the vago-vagal reflex nature of the gastric distension response, hexamethonium was given iv prior to distending the gut. Blockade of nicotinic ganglionic receptors with this drug mimicked the effect of vagotomy (and L-NAME). IGP increased to 14.5 ±1.8 cm H2O upon distension of the stomach with 6 ml of saline. These data provide strong evidence that a vago-vagal reflex consisting of an efferent pathway originating in the DMV and engaging nitrergic neurons in the gastric myenteric plexus exerts an important role in the accommodation reflex.
An important concept that emerges from the study of Takahashi and Owyang (1997) is that the magnitude of gastric distension may be a determinant of the degree to which the NANC arm of the vago-vagal reflex operates. With minimal distension of the gut, the NANC pathway plays a minor role (see Fig. 2 of Takahashi and Owyang (1997), but with large distension, the role of the NANC pathway is magnified (again see Fig. 2). Likewise, an interaction between resting IGP and neural induced responses were described by Andrews and Lawes 1985). They reported that vagal nerve-induced relaxation was minimal at low resting IGP (analogous to minimal distension). At an IGP of 2.6 cm H2O no gastric relaxation was observed in the anesthetized ferret. But at pressures of 8.0 and 12.0 cm H2O, relaxation of 2.3 and 3.5 cm H2O occurred, respectively.
The accommodation reflex and the relationship between gastric distension and/or IGP and neurally induced gastric relaxation probably explains why Rogers and Travaglis' laboratories are able to obtain evidence for NANC input to the stomach. Both of these investigators and their colleagues use iv administration of the muscarinic receptor agonist bethanechol to increase baseline gastric motility prior to testing for a role of NANC pathways in their experimental design. For example, Lewis et al. (2002) reported evidence that CRF reduced gastric motility by activation of NANC pathways. Indeed, part of the evidence documenting that the RRR is mediated by NANC pathways was obtained by increasing baseline gastric motility and tone with bethanechol (Hermann et al. 2006). Travagli and colleagues (Holmes et al. 2013) obtained evidence that oxytocin utilizes the NANC pathway by testing this peptide in anesthetized rats given iv bethanechol. We have administered bethanechol iv to anesthetized rats and found that IGP more than doubles (Cruz et al. 2007). It is our contention that iv bethanchol by increasing IGP induces the accommodation reflex and this is the reason that the Rogers and Travagli laboratories often observe a role for NANC pathways in their studies. Additionally, it should be noted that muscarinic receptors are located on nitrergic neurons of the myenteric plexus (Kortezova et al. 2004; Harrington et al. 2007). Activation of these muscarinic receptors stimulates NOS (Kortezova et al. 2004), and presumably engages the intrinsic nitrergic axonal reflex described by Desai et al. (1991a) and Desai et al. (1991b). Thus, prior to testing CRF, RRR, and oxytocin, NANC pathways to gut smooth muscle could be active because of the accommodation reflex and because of the presence of the intrinsic nitrergic axonal reflex.
Ultrastructural Evidence for Separate Vago-Vagal Reflex Circuits Controlling the Fundus and Antrum
As described in "Identification of brainstem neural circuitry controlling tonic and phasic contractions of the stomach using intravenously administered nicotine" and "Identification of brainstem neural circuitries controlling tonic and phasic contractions of the stomach using reflex-induced changes in gastric reservoir function" sections of our review, data from our studies (Ferreira et al. 2002, 2005; Herman et al. 2008) addressing the question of CNS region-specific control over gastric mechanical function, indicate that inhibition of gastric tone involved norepinephrine (NE)-induced inhibition of DMV gastric projecting neurons, whereas inhibition of phasic contractions involved GABA-induced inhibition of DMV gastric projecting neurons (Ferreira et al. 2002). Approximately 80% of the inhibition of gastric tone was blocked by microinjecting α2-adrenoreceptor antagonists into the DMV, and approximately 80% of the inhibition of phasic contractions was blocked by microinjecting a GABAA receptor channel antagonist into the DMV (Ferreira et al. 2002).
Anatomical Evidence for Selective Noradrenergic Innervation of CNS Vagal Projections to the Fundus
In view of our physiological data identifying noradrenergic projection neurons originating in the mNTS and selectively influencing the proximal stomach, specifically, the fundus, we hypothesized that noradrenergic nerve terminals synapse with DMV fundus-projecting neurons. To test our hypothesis, we used electron microscopy to examine whether noradrenergic terminals form synapses specifically with fundus-projecting neurons.
In one group of rats, the retrograde tracer, CTB-HRP was injected into the gastric smooth muscle of the fundus (Pearson et al. 2007). We observed that fundus-projecting neurons were located in a lateral position in the DMV, and from the CS to + 0.5 mm rostral to the CS. We focused our attention on this part of the DMV and obtained ultrathin sections for analysis of catecholaminergic synapses by electron microscopy. In another group of rats, CTP-HRP was injected into the gastric smooth muscle of the antrum. We observed that antrum-projecting neurons were located in a medial position in the DMV, just medial to the fundus-projecting neurons. The peak number of DMV neurons that projected to the antrum was also at CS to + 0.5 mm rostral to the CS. Thus, the same ultrathin sections taken for EM analysis for the fundus, were used for the antrum study.
Brainstems were processed histochemically for CTB-HRP, and immunocytochemically for either dopamine beta hydroxylase (DBH) or phenylethanolamine N-methyltransferase (PMNT) by dual-labeling electron microscopic methods. Analysis of 482 synapses on 238 neurons that projected to the fundus informed us that 17.4 ± 2.7% (n = 4) of synaptic contacts were with DBH-IR terminals. Of 165 fundus-projecting neurons, 4.4 ± 1.5% (n = 4) formed synaptic contacts with PNMT-IR terminals. Analysis of 384 synapses on 223 antrum-projecting neurons revealed no synaptic contact with DBH-IR terminals. These results provide proof that norepinephrine containing nerve terminals synapse with DMV fundus-projecting neurons but not with DMV antrum-projecting neurons. The data also suggest that brainstem circuitry controlling the fundus is different from circuitry controlling the antrum.
Our findings prompted the question of whether noradrenergic afferent input to DMV fundus-projecting neurons that comprise only 17.4 ± 2.7% of the total synapses is physiologically significant. To address this question, we determined the effect of bilateral microinjection of α2 adrenoreceptor antagonists on ED-induced decreases in fundus tone (Herman et al. 2008). Antagonism of α2 adrenoreceptors at the DMV prevented approximately 85% of the fundus relaxation produced by ED. The magnitude of antagonism noted with α2 adrenoreceptor antagonists was similar to the magnitude of antagonism with either iv atropine methyl bromide or bilateral cervical vagotomy.
Anatomical Evidence for Selective GABAergic Innervation of CNS Vagal Projections to the Antrum
Using methods similar to those for establishing that noradrenergic terminals synapsing onto DMV fundus-projecting neurons, we tested the hypothesis that GABAergic terminals form synapses with DMV antrum-projecting neurons, but not with DMV fundus-projecting neurons. Brainstems were processed histochemically for CTB-HRP, and immunocytochemically for glutamic acid carboxylase isoenzye 67 immunoreativity (GAD67-IR) by dual -labeling electron microscopic methods. Analysis of 214 synapses on 195 neurons that projected to the antrum informed us that 23.0 ± 3.6% (n = 4) of synaptic contacts were with GAD67-IR terminals. The analysis of 220 synapses on 203 fundus-projecting neurons indicated that only 7.9 ± 3.1% (n = 4) of synaptic contacts were with GAD67-IR terminals. The difference between GAD67-IR synaptic contacts with antrum- and fundus-projecting neurons was statistically significant (p < 0.05). Our results confirm that brainstem circuitry controlling the antrum involves GABAergic transmission.
The origin of GABAergic input to antrum-projecting neurons was not investigated in our study. Knowledge of the source would allow us to seek the afferent inputs to GABAergic neurons synapsing onto DMV antrum-projecting neurons. A likely source is the mNTS. Immunohistochemical localization studies of GAD67-expressing neurons in the rat brainstem have informed us that the most labeled cells are found in the mNTS (Fong et al. 2005). In support of this, Davis and colleagues, (Davis et al. 2004), performed whole-cell patch-clamp recordings from DMV neurons in rat brain slices and used glutamate photostimulation to activate distinct areas of the NTS. Upon stimulation Davis and colleagues noticed that 65% of the DMV neurons showed IPSCs. The IPSCs were blocked by a GABAA receptor channel antagonist, picrotoxin, indicating that they were mediated by GABA. Davis and colleagues (Davis et al. 2004) suggested “There is a predominance of inhibitory projections to the DMV originating from intact neurons of the NTS.” Their suggestion was based on the observation that IPSCs were found more frequently than EPSCs in DMV recordings.
Thus, our results indicate that DMV antrum-projecting neurons receive significant GABAergic afferent input. This input, according to reports of others (Fong et al. 2005; Davis et al. 2004; Wang et al. 2001) originates in the mNTS. This anatomical evidence along with our pharmacological evidence (Ferreira et al. 2002, 2005; Herman et al. 2008) provide compelling evidence that the GABAergic synapses at the DMV are involved in mediating inhibition of contractility of the antrum evoked by activation of nAChRs.
NTS GABAergic Interneurons: Critical Regulators of the Vago-Vagal Reflexes
Introduction
Our own finding (Travagli et al. 1991) that informed us that DMV motoneurons receive an inhibitory GABAergic input compelled us to question the importance of brainstem GABAergic signaling controlling gastric function. However, it wasn't until we became aware of the seminal findings of Smith et al. (1998) and Fong et al. (2005) that we concentrated our efforts on addressing this question.
Smith et al. (1998) used whole-cell patch-clamp recordings of neurons in the dorsomedial NTS in what they describe as a “modified brainstem-cranial nerve explant preparation”. The unique feature of this preparation is that vagal sensory nerves synapsing in the NTS can be excited a distance away from the recording site thereby eliminating the potential problem of directly activating NTS neurons. Constant latency (interpreted as a monosynaptic response) excitatory postsynaptic currents (EPSCs) were the most common response observed upon stimulating the viscerosensory input. These EPSCs were shown to be mediated by glutamate released from vagal afferent nerves. In about 50% of the NTS cell recordings, the constant latency EPSC was followed by variable latency inhibitory postsynaptic currents (IPSCs), and on occasion, a 'barrage' of IPSC's of variable latency and duration. The IPSC's were always preceded by a constant latency EPSC, and both were blocked by a glutamate receptor antagonist. Neurons that displayed IPSC's also exhibited spontaneous IPSCs. All evoked IPSCs were blocked by the GABAA receptor antagonist, bicuculline. Finally, in some recordings, the constant latency EPSP was followed by a variable latency EPSC. Our interpretation of these data is as follows: electrical stimulation of vagal sensory nerves released glutamate which directly activated NTS neurons (constant latency EPSCs). This, in turn, excited local circuit GABAergic and glutamatergic neurons that evoked the variable latency responses. Thus, brainstem control of gastric function appears to be due to reflex-induced excitation of local circuits (interneurons) in the NTS and the resulting processed information is transmitted by NTS projection neurons to the DMV.
In the second seminal study, (Fong et al. 2005) examined the distribution of GAD67, a marker for localization of GABAergic cells and terminal processes in the rat brainstem with a focus on the NTS. They looked at twelve distinct regions of the NTS and found that the medial and central subdivisions had the highest number of cells per hemisection and the highest number of varicosities. The cells were described as “small, round immunoreactive cell”. Fong et al. (2005) concluded “the findings from the current study support the notion that GABAergic neurons are an integral part of the intrinsic NTS circuitry”.
With this as background, we set out to determine the effect of removing local circuit GABAergic NTS signaling on motor output from the DMV to the stomach.
Experimental Model for Evaluating Drug Effects at the mNTS (Herman et al. 2009)
To perform our study, we required an experimental preparation where blockade of GABAA receptors would be restricted to the area of the mNTS and blockade of GABAA receptors at the nearby DMV would not be a complication. This was important because blockade of GABAA receptors is well known to have potent effects on gastric mechanical events (Sivarao et al. 1998). We developed an anesthetized rat model (a gastric balloon catheter was used to monitor IGP) where drugs would be unilaterally microinjected into the mNTS and the ipsilateral vagus nerve would be sectioned. The contralateral vagus nerve remained intact. Drug effect evoked from the mNTS would be observed because neurons of the mNTS cross the midline and engage the contralateral DMV. Gastric motility responses obtained are prevented by sectioning the contralateral vagus nerve. We had demonstrated this is our earlier studies publications (Ferreira et al. 2000; Cruz et al. 2007). An excitatory substance was microinjected unilaterally into the mNTS and produced inhibition of gastric mechanical activity. Ipsilateral vagotomy was performed and repeat microinjection of the excitatory substance produced a response of the same magnitude. Section of the contralateral vagus nerve prevented the response. Thus, the vago-vagal reflex circuitry stays functional after sectioning of one of the vagus nerves) (Fig. 4).
Microinjection experiments were conducted in the left and right hemispheres, and the left hemisphere is shown only as an example. Ach, acetylcholine; NO, nitric oxidee; DMV, dorsal motor nucleus of the vagus; mNTS, medial subnucleus of the tractus solitarius; + denotes activation; − denotes inhibition. Adapted from Fig. 1 from Herman et al. (2009).
Validation of the Experimental Model for Assessing Neural Activity in the Area of the mNTS and Characterization of DMV Pathways Providing Baseline Neural Activity to the Stomach (Herman et al. 2009 )
We microinjected glutamate into the area of the mNTS with both cervical vagus nerves intact. Glutamate produced a decrease in IGP (Fig. 5A). Following ipsilateral cervical vagotomy, glutamate was re-microinjected into the same site. Glutamate evoked similar decreases in IGP as that observed prior to ipsilateral vagotomy (Fig 5a). Glutamate was re-microinjected into the same site of rats subjected to sectioning of the remaining (contralateral) cervical vagus nerve. Bilateral cervical vagotomy prevented glutamate from decreasing IGP (Fig. 5a). A representative experiment appears as part of Fig. 5a. The micropipette tip in each case was located in the area of the mNTS.
a Histograms of averaged intragastric pressure (IGP) following unilateral microinjection of L-glutamate (glut; 500 pmol/30 nl) into the area of the mNTS with both vagi intact (left histogram, left trace), after ipsilateral vagotomy (ipsi vx; middle histogram, middle trace) and after bilateral vagotomy (bilat vx; right histogram, right trace)*P < 0.05 1-way ANOVA, #P < 0.05 1-sample t-test; n = 4. b histogram of averaged IGP responses (left) and representative experimental traces (right) depicting changes in IGP following administration of NG-nitro-arginine methyl ester (L-NAME; 10 mg/kg iv; left histogram, left trace) and atropine methylbromide (atropine; 0.1 mg/kg iv; right histogram, right trace). #P < 0.05 by 1-sample t-test; n = 4.
To determine the DMV output pathways to the gut that are active under basal conditions, either atropine methyl bromide or L-NAME were administered iv to rats with the ipsilateral cervical vagus nerve sectioned. Intravenous administration of L-NAME to five rats had no significant effect on baseline IGP (Fig. 5b). In contrast, iv administration of atropine methylbromide to rats produced a significant reduction in IGP (Fig. 5b). Representative experiments appear as part of Fig. 5b.
Studies of the Effects of GABAA Receptor Blockade in the Area of the mNTS on Gastric Mechanical Activity (Herman et al. 2009)
Our data indicate that in the ipsilateral vagotomized rat, we have a viable model in which to assess (1) the nature of the synapse(s) where vagal afferent terminals engage NTS inhibitory neurons that project to the DMV, and (2) the nature of output from the DMV to the gut during baseline conditions. Using this model, we microinjected the GABAA receptor channel antagonist bicuculline into the area of the mNTS. The dose used was 20 pmol/30 nl and was based on dose–response data obtained from an earlier study (Wasserman et al. 2002). Microinjection of bicuculline in rats produced a robust decrease in IGP (− 1.6 ±0.2 mmHg, P < 0.05; Fig. 6a) that could be repeated after a 30 min recovery period. In four rats the vehicle for bicuculline microinjected into the area of the mNTS had no effect on IGP (Fig. 6a). We also studied the GABAA receptor antagonist, gabazine. Similar to bicuculline, microinjection of gabazine in rats produced significant decreases in IGP (Fig. 6c).
a Histograms of averaged IGP responses to unilateral microinjection of bicuculline methiodide (BMI; 20 pmol/30 nl) into the area of the mNTS compared with vehicle (Veh; 0.9% saline). *P < 0.05 unpaired t-test, #P < 0.05 1-sample t-test; n = 23 for BMI and n = 4 for vehicle. b Representative experimental tracing depicting changes in IGP following unilateral microinjection of BMI (20 pmol/30 nl) into the area of the mNTS. c Histogram of averaged IGP responses to unilateral microinjection of gabazine (GBZ (20 pmol/30 nl) into the area of the mNTS compared with vehicle (0.9% saline), *P < 0.05 unpaired t-test, #P < 0.05 1-sample t-test; n = 5. d representative experimental tracing depicting changes in IGP following unilateral microinjection of GBZ (20 pmol/30 nl) into the area of the mNTS.
Next, we studied the effect of sectioning the remaining (contralateral) cervical vagus nerve. Microinjection of bicuculline was then repeated and had no effect on IGP (Fig. 7a, b).
a Histograms of averaged IGP responses to unilateral microinjection of BMI (20 pmol/30 nl) into the area of the mNTS after ipsilateral vagotomy compared with microinjection of BMI performed after contralateral (bilateral) vagotomy (contr vx). *P < 0.05 unpaired t-test, #P < 0.05 1-sample t-test; n = 4. b Representative experimental traces depicting IGP responses following unilateral microinjection of BMI (20 pmol/30 nl) into the area of the mNTS after ipsilateral vagotomy (left trace) and after contralateral (bilateral) vagotomy (right trace).
In summary, both bicuculline and gabazine microinjected into the mNTS produced large decreases in gastric mechanical activity upon microinjection into the mNTS. This response could not occur if the only signaling in the NTS was from vagal afferent terminals releasing L-glutamate onto second-order inhibitory NTS neurons that project to the DMV. Neural signaling of this nature would not be altered by blockade of GABAA receptors, and therefore gastric mechanical activity would not be affected. The powerful effect on gastric mechanical activity observed with blockade of GABAA receptors in the area of the mNTS proves that GABA is present in this area and is producing a significant activation of GABAA receptors. Thus, GABAergic signaling in the area of the mNTS is an important component in regulating functional activity within the vago-vagal reflex circuitry.
Studies of the Effects of Glutamate Receptor Blockade in the Area of the mNTS on Gastric Mechanical Activity (Herman et al. 2009)
We microinjected the glutamate receptor antagonist, kynurenic acid, unilaterally into the mNTS. Kynurenic acid microinjection had no effect on IGP. This was unexpected! Why? Because with afferent vagal terminals releasing glutamate onto second-order NTS neurons plus a barrage of EPSCs occurring due to activity of local circuit glutamate neurons (Smith et al. 1998), a profound change in NTS signaling should ensue. This did not happen!
What was of great interest was our observation that unilateral microinjection of kynurenic acid prevented bicuculline from decreasing IGP after microinjecting it into the same site as kynurenic acid. Based on this result, we concluded that the decrease in IGP produced by GABAA receptor blockade was due to unopposed glutamate released from vagal afferent terminals in the mNTS stimulating NTS inhibitory projection neurons to the DMV. This led us to further conclude that activity within the vago-vagal reflex circuitry was controlled not by sensory input but by intra-NTS GABAergic signaling. This signaling has to be overwhelming because of the high level of glutamate present in the NTS caused by viscerosensory input and to activity in local circuit glutamate neurons.
Studies on the Effects of Glutamate Receptor Blockade in the Area of the mNTS on Gastric Mechanical Activity After GABAA Receptors have been Blocked (Herman et al. 2009)
Once GABAA receptors have been blocked at the mNTS, unopposed glutamate released at vagal afferent nerve terminals can excite NTS GABAergic projection neurons to the DMV. This would inhibit vagal motor outflow to gastric smooth muscle and IGP would exhibit a significant decrease. Under these conditions, blockade of glutamate receptor in the mNTS should now exert an effect to increase IGP. Indeed, this is exactly what happens (see Fig. 8), confirming the powerful control that GABAergic tone at the mNTS has in suppressing viscerosensory information at the NTS.
a Histograms of averaged changes in IGP following unilateral microinjection of vehicle, microinjection of Kyn (1 nmol/30 nl) before unilateral microinjection of GBZ (20 pmol/30 nl), and microinjection of Kyn after microinjection of GBZ into the area of the mNTS, *P < 0.05 ANOVA, #P < 0.05 1-sample t-test; n = 4. b Representative experimental tracing depicting changes in IGP following unilateral microinjection of Kyn (1 nmol/30 nl) into the area of the mNTS after unilateral microinjection of GBZ, (20 pmol/30 nl). c Representative experimental tracing depicting IGP responses following unilateral microinjection of Kyn (1 nmol/30 nl).
Studies to Determine the Vagal Efferent Pathway to the Stomach that Mediates the Gastric Motility Effects Produced by Blocking GABAA Receptors in the Area of the mNTS
To test for the contribution of two parallel efferent DMV pathways we assessed the effect of either iv L-NAME or atropine methyl bromide on decreases in gastric mechanical activity induced by GABAA receptor blockade in the mNTS. Atropine methyl bromide prevented the effects of GABAA receptor blockade on gastric mechanical activity; L-NAME had no effect.
Summary
To visualize how blockade of GABAA receptors in the area of the mNTS results in such a powerful effect on gastric mechanical activity, we suggest the proposed circuitry presented in Fig. 9. Signaling in this circuitry assumes that vagal afferent nerves terminating in the mNTS are active and release glutamate into the neuropil. Without signaling of mNTS GABA interneurons, activation of mNTS GABA projection nerves would not lead to silencing of DMV gastric output neurons. Because of the presence of mNTS GABA interneurons, all signaling from vagal afferent nerves (viscerosensory input) is halted, allowing the spontaneous activity present in DMV neurons to control gastric mechanical activity. With stoppage of intra-nucleus mNTS GABAergic signaling (in the presence of bicuculline or gabazine), the viscerosensory input is now controlling gastric mechanical activity.

Adapted from Fig. 9 of Herman et al. (2009)
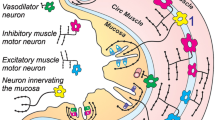
Simplified schematic representation of proposed circuitry for local intra-nucleus tractus solitarius (NTS) GABAergic signaling and the DMV efferent pathway controlling gastric motility. Black circles are GABA interneurons, gray circles are GABA projection neurons, and white circles are DMV preganglionic neurons. Oval is the nodose sensory ganglion. Plus signs in the NTS represent excitatory (ionotropic glutamate receptor-mediated) signaling. Minus signs represent inhibitory (GABAA receptor-mediated) signaling. The question mark placed in the NTS area reflects the lack of knowledge on definite organization of interneurons between the vagal afferent terminals and the NTS projection neurons (question mark pertains to 2009; since then, new information has become available––see Thek et al. (2019).
This schematic representation of proposed mNTS circuitry (Fig. 9) raises questions. First, what is the identity of the GABA interneurons? Second, how are the interneurons organized to integrate and modulate signaling in the mNTS? Additionally, is GABA acting presynaptically to prevent release of glutamate from vagal afferent nerve terminals? Is GABA acting postsynaptically on second-order interneurons synapsing on projection neurons in the mNTS?
Somatostatin-Gaba Interneurons
Sst-GABA Interneurons as Critical Regulators of Neural Signaling at the NTS
Lewin and colleagues (Lewin et al. 2016), reported data using brain stem sections from td Tomato reporter Sst-IRES-Cre mice expressing red fluorescence in Sst-GABA neurons in the vagal complex. These neurons are located both in the DMV and NTS and those in the DMV produce strong direct inhibition of DMV neurons. The demonstration that these Sst-GABA neurons in the NTS are second-order neurons just appeared in the paper of Thek et al. (2019). These investigators mapped Sst neurons within the NTS of rats and found approximately two-thirds that expressed glutamic acid decarboxylase 67 (GAD67) (a marker for the GABAergic neuron). They found that more than 50% of NTS Sst neurons are directly activated by local stimulation of vagal afferent input in the tractus solitarius of horizontal brain slices. They also established with optogenetic studies more details of how Sst neurons are part of the network of neurons within the NTS using channelrhodopsin 2 (ChR2) and yellow fluroescent protein (YFP) transgenic mice that were bred with Sst reporter mice. They proved that Sst neurons use both GABA and glycine to inhibit Sst-negative NTS neurons and modify vagal afferent signaling. These investigators also showed that although NTS Sst neurons represent only a small percentage of NTS neurons, they extensively make synaptic connections within the NTS to gate a majority of non-Sst neurons. The crucial find in the Thek et al. study, thus is that Sst neurons modulate viscerosensory signaling by synaptic gating at the second-order neuron in the sensory reflex processing but without directly affecting presynaptic vagal afferent terminals. This is shown in Fig. 7 of their paper Thek et al. (2019) and reproduced as Fig. 10 below.
Activation of Sst-positive neurons reliably gates sensory primary afferent solitary tract (ST)-evoked action potential (AP) throughout. a Photomicrograph of the horizontal brain stem slice configure with light emitting diode (LED) fiber illuminating the NTS; a bipolar electrical stimulation (ST elec) placed distally on the ST and recording pipette in the NTS; 4 V, fourth ventricle (left). a schematic diagram of the experimental setup where LED pulses activate SST neurons and electric shocks drive sensory afferent input to a second-order NTS neuron is shown on the right.b Traces of whole-cell recording from a representative non-Sst NTS neuron. Electrical ST shocks (arrows evoked action potentials with high reliability (left; 10 traces overlaid in all cases, VH = − 63 mV. The same neuron was hyperpolarized by 10 ms LED pulses (middle trace; note difference in time and voltage scales; LED pulses delivered at the time indicated by the solid blue box). Electrical ST shocks timed with LED pulses effectively reduced action potentials throughout (right and bottom left graph). c Raster plot graphs from six cells show that AP responses to ST stimulation were blocked or decreased during LED stimulation of Sst neuron inputs (AP success represented by black bars in the roster plot). The percentage of ST shocks that elicited APs during control or LED stimulation periods are shown to the right of the raster plot. For each neuron throughput was averaged across the ST only and ST + LED conditions (left graph). Across the group AP throughout was significantly re8duced by Sst input activation (*t(4) = 6.709, P = 0.003; paired t-test data are mean ± SEM). Adapted from Fig. 7 of Thek et al. (2019).
In summary, activation of Sst inputs provide a powerful inhibition of viscerosensory signal throughput postsynaptically, with the ability to disengage second-order NTS neurons from their sensory input. The authors suggest “that the Sst inhibitory input within the NTS is pivotal in coordinating and modulating viscerosensory integration at its initial stages.” This powerful phenomenon fits with the overwhelming role for GABA in blocking viscerosensory input in our in vivo rat model of reflex control of gastric mechanical function.
Sst-GABA Interneurons as Critical Regulators of Neural Signaling at the DMV
Wang and Bradley (2010), described somatostatin positive GABAergic neurons (Sst-GABA) in the brain stem with electrophysiological and functional profiles similar to the GIN neurons in this area (Gao et al. 2009, 2009), as well as in the hippocampus and cortex regions (Oliva et al. 2000; Halabisky et al. 2006; Taniguchi et al. 2011). Sst-GABA neurons are a prominent inhibitor of upregulated network dynamics of the brain (Fanselow et al. 2008; Fanselow and Connors 2010; Gentet et al. 2012; Royer et al. 2012). Taken together, the findings of these investigators prompted us to study these neurons on gastric DMV motor neurons that receive robust local GABA signaling as in the NTS.
To accomplish this, we used patch clamp electrophysiology within brain slice preparations of transgenic adult Sst-IRES-Cre mice expressing td Tomato fluorescence and distinct light-sensitive differential opsins. Optogenetic interrogation and Ca[i] signaling within DMV neurons were established (Lewin et al. 2016). Optogenetic stimulation of Sst-ChR2 transgenic mouse showed prominent inhibition of these gastric DMV neurons. Moreover, Sst-GABA neurons could be detected by the transynaptic tracer, PRV-152 EGRP, in the DMV after injection into the antrum of the stomach. Altogether, this shows that the Sst-GABA neuron plays a seminal role in the vagal gastric circuit that mediate mechanical activity in the antrum (Lewin et al. 2016). Light stimulation in the DMV activated Sst-GABA-ChR2 neurons (Fig. 11b–e) and suppressed action potentials in a majority of DMV gastric antrum-related neurons (Fig. 11f, g).
Optogenetic stimulation of Sst-GABA-ChR2 neurons in the DMV inhibits vagal output neurons in the nucleus that innervate the gastric antrum. a Photomicrograph showing a recording electrode in the DMV and the extent of the area (purple circle) illuminated by optogenetic stimulation. Scale bar = 200 µm. b Direct light stimulation (blue bar) of a Sst-GABA-ChR2 neuron increases its action potential frequency. c Stimulatory effects of light on action potential frequency in Sst-GABA neurons during current-clamp recording (n = 7; **P < 0.01). d and e A similar increase is seen in action potential frequency of Sst-GABA neurons recorded in the cell-attached mode (n = 5;*P < 0.05). f Representative current-clamp recording showing light-induced (blue bar) inhibition of action potential firing in a DMV antrum projection neuron. g Inhibitory effects of Sst-GABA stimulation on action potential frequency of gastric antrum-projecting neurons (n = 14/18; ****P < 0.005). h Current clamp recording demonstrating the repeatability of light-induced inhibition in a DMV antrum projection neuron. i Voltage clamp recording (VH = − 30) from the cell in (f) showing that light stimulation (blue bar) is accompanied by an increase in IPSCs. j Light stimulation robustly increases sIPSCs in antrum-projecting neurons (n = 11;***P < 0.001), which is repeatable (k). l expanded view of regions 1 and 2 in the tracing in (i), showing sIPSCs before (1, top) and during light stimulation of Sst-GABA neurons (2, bottom). m The decrease in light-induced action potentials is prevented by pretreatment with gabazine. n Representative tracing of action potentials in a DMV antrum projection neuron that was unaffected by optogenetic stimulation. o Increase in IPSCs as a result of light stimulation is blocked by gabazine. Blue bar = light with wavelength of 450–490 nm.
To determine the neurotransmitter underlying the light-induced inhibition of DMV neurons, we used the GABAA receptor antagonist, GABAzine, prior to exposure to blue light in Sst-GABA-ChR2 neurons in the transgenic mouse. Before GABAzine, light stimulation produced an increase in frequency and amplitude of IPSCs. Stimulation of IPSCs coincided with the light-induced inhibitory effect on action potentials in DMV neurons (Fig. 2f). Both light-induced inhibition of action potentials and light-induced stimulation of IPSCs were eliminated by GABAzine (Fig. 11h–o).
To assess whether Sst-GABA neurons were the origin of tonic suppression of DMV neurons, recordings were made from brain stem slices obtained from transgenic mice expressing the proton transporting opsin, ArchT in Sst-GABA neurons. Exposure to light inhibited the action potentials of DMV Sst-GABA-Arch T neurons. Recordings were then taken from retrogradely labeled DMV neurons. Optogenetic inhibition of Sst-GABA-ArchT neurons was associated with a decrease in spontaneous postsynaptic currents in DMV neurons that projected to the antrum.
To assess whether Sst-GABA DMV neurons are under tonic inhibition, they were exposed to a low extracellular calcium concentration in the loose cell configuration. In the presence of low calcium the frequency of action potentials increased two-fold. This increase was reduced by the SK channel positive modulator, CyppA. Our results indicate that Sst-GABA neurons are significant regulators of pre-motor neurons that innervate the antrum. This control over vagal output occurs by a tonically active GABAergic pathway. Moreover, our results indicate that the DMV Sst-GABA neurons are under tonic inhibitory drive.
In summary, our studies have revealed an important role for Sst-GABA neurons in local GABA signaling that has strong inhibitory effect on vagal output to the stomach. We show that Sst-GABA neurons are anatomically connected to DMV output neurons that project to the antrum; are functionally connected to DMV output neurons that innervate the antrum; are an important source of tonic inhibition of DMV output neurons that innervate the antrum; and also are under tonic inhibitory drive.
We propose that the inhibitory input onto DMV output neurons arises largely from output neurons in the nucleus and not from those in the NTS. Light stimulation was restricted primarily to the DMV (Fig. 11) and so both cell bodies and their terminals within the nucleus would be stimulated, thereby producing the strongest rhodopsin-mediated effect on output neurons to the antrum. Moreover, our in vivo studies demonstrate opposite intragastric pressure responses induced by local GABA blockade in the two nuclei (Ferreira et al. 2002; Herman et al. 2009). Blockade of GABA in the NTS decreases gastric mechanical activity (Ferreira et al. 2002; Herman et al. 2009), whereas, in the DMV, it increases motility (Ferreira et al. 2002; Herman et al. 2009).
Identifying Ways to Control the Overwhelming Effect of Gabaergic Gating of Viscerosensory Information Arriving at the NTS
In seeking to understand how the CNS could control neural activity in the vago-vagal reflex circuitry, we focused on the powerful influence that GABA interneurons in the mNTS had in blocking out viscerosensory information coming from the stomach. This powerful effect was observed in vivo (Herman et al. 2009) and in vitro (Thek et al. 2019). Based on our studies (Lewin et al. 2016) and studies of Thek et al. (2019)), we refer to these interneurons as Sst-GABA neurons. Since some of these Sst-GABA neurons are second-order neurons (Glatzer and Smith 2005; Glatzer et al. 2007; Thek et al. 2019) we asked what other endogenous substances are active at this 'gating' site? One category of substances that stood out was the endogenous opioids. According to the anatomical studies of Martin-Schild et al., and Pierce and Wessendorf, endorphins are present at sensory afferent terminals and in second-order sensory neurons in the NTS (Martin-Schild et al. 1999; Pierce and Wessendorf 2000). Staining for endomorhin-1 occurs in cell bodies in the dorsomedial NTS. This is the area mainly contacted by gastric sensory afferents (Shapiro and Miselis 1985).
According to the electrophysiological studies of Glatzer et al. (2007), endorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. Whole-cell recordings were made from NTS and DMV neurons in brain stem slices from rats and transgenic mice that expressed enhanced green fluorescent protein (EGFP) under the control of a GAD 67 promoter (EGFP-GABA neurons) to identify opioid mediated effects on GABAergic Circuitry. Their study showed that µ opiod receptors are functional on GABAergic NTS neurons and that they inhibit both local GABAergic transmission and GABA projection neurons to the DMV. Taken together, these findings suggested to us that µ opioid receptor stimulation at the mNTS might mimic the effect that we observed with microinjected GABA receptor antagonists at this site (see our earlier findings, i.e., Herman et al. (2009)).
For these studies of the effects of µ opioid receptor stimulation (and blockade) at the mNTS, we used the same rat preparation described in our earlier publications (Cruz et al. 2007; Herman et al. 2008, 2009) Initially, we examined the effects of microinjected DAMGO [ED-Ala2, mePhe4, Gly9ol)5] enkephalin, an agonist of the mu opioid receptor on IGP (Herman et al. 2010). DAMGO, 1–10 fmol, microinjected into the NTS produced dose-dependent reductions in IGP. The endogenous µ opioid receptor agonists endomorpin-1 and endomorphin-2 microinjected in a dose of 20 fmol into the mNTS produced effects similar to those of 10 fmol DAMGO. The effects of 10 fmol DAMGO in the mNTS were blocked by vagotomy and by blockade of µ opiod receptors, GABA receptors and ionotropic glutamate receptors in the mNTS. We also tested the effect of blockade of µ opioid receptors on esophageal distension (ED) induced reductions in fundus tone. Bilateral microinjection of the opioid receptor antagonist naltrexone, while having no effect on fundus tone per se, blocked ED-induced decreases in fundus tone. Our data demonstrate that (1) activation of µ opioid receptors in the mNTS, like bicuculline and gabazine, inhibits gastric mechanical activity, (2) the mechanism of mu opioid receptor effects in the mNTS is through suppressing local circuit GABA activity, and (3) blockade of µ opioid receptors in the mNTS counteracts the ED response. Our data suggest that opioids play an important physiological role in mediating an important vago-vagal reflex, the RRR, through release of an endogenous opioid in the mNTS. In contrast, an endogenous opioid has no role in controlling brainstem reflex circuitry at rest. We therefore speculate that for ED-induced fundus relaxation to occur, release of an endogenous opioid is required in the mNTS to counteract the overwhelming GABAergic drive that normally blocks all viscerosensory input to the mNTS.
As in most of our studies described in our review, we performed experiments to determine whether the efferent pathway involved in the DAMGO-induced decrease in IGP was comprised of cholinergic excitatory, non-adrendergic-non-cholinergic inhibitory, or both types of nerves (the so-called dual pathways). Again, intravenous administered L-NAME failed to alter the DAMGO-induced decrease in IGP. In contrast, intravenous administered atropine methyl bromide prevented DAMGO microinjected into the mNTS from decreasing IGP. Thus, DAMGO-induced inhibition of gastric motility is mediated by the vagus nerves, specifically through withdrawal of the cholinergic-cholinergic excitatory efferent pathway.
Summary and Conclusions
GABAergic Signaling is Essential for the Brainstem to Exert Control Over Gastric Mechanical Activity
Based on our review, GABAergic signaling at the mNTS and DMV, as well as the mNTS/DMV GABA connection, come into view as vitally important to brainstem control of gastric tone and phasic contractions. GABAergic signaling at the mNTS determines the basal (resting) state of gastric tone and phasic contractions and mediates the changes in gastric mechanical function when the vago-vagal reflex becomes operational. This is revealed by blocking GABAA receptors in the mNTS during the basal state which results in robust decreases in both gastric tone and gastric phasic contractions. Activation of the vago-vagal reflex using a physiological stimulus, i.e., esophageal distension [we refer to this as the receptive relaxation reflex (RRR)], requires GABAergic signaling at the mNTS. Thus, blockade of GABAA receptors in the mNTS with bilateral microinjection of gabazine diminishes the RRR [unpublished data cited in Herman et al. (2010)]. In light of this finding, we suggest that the RRR occurs because GABAergic tone is 'dialed down' in the mNTS, allowing the tonically released glutamate from viscerosensory nerve endings to excite mNTS-noradrenergic projection neurons synapsing with DMV fundus projection neurons. The released norepinephrine acts on postsynaptic α2 receptors to produce relaxation of the fundus (see Fig. 3).
What is it that 'dials down' GABAergic signaling at the mNTS? Based on our study results with DAMGO and naltrexone (Herman et al. 2010), we suggest that it is an endogenous opioid released by viscerosensory nerve endings in the mNTS. Opioids have long been known to inhibit the release of GABA in the brain (Christie et al. 2000; Wilson-Poe et al. 2017), and neurons that contain endogenous opioids are present in the mNTS (Martin-Schild et al. 1999; Pierce and Wessendorf 2000). Indeed, Scanlin et al. (2008), report that activation of neurons in the nodose ganglion releases endomorphin-2. We demonstrate that the µ opioid receptor antagonist, naltrexone, administered locally (into the mNTS) and systemically, significantly diminishes the RRR. Taken together, we propose that esophageal distension releases an endogenous opioid at the mNTS which reduces GABA release, enabling the glutamate present at the mNTS to engage the noradrenergic projection system to inhibit fundus tone. Blockade of µ opioid receptors by naltrexone had no effect per se on gastric mechanical activity indicating that opioids in the mNTS are not functional in the basal state. Instead, endogenous opioids are exerting an effect at the onset of the RRR.
Relating our findings in the rat to data obtained in humans and to other reflexes, our results are consistent with the finding that naloxone counteracts the gastric accommodation reflex in humans (Geeraerts et al. 2008). Microinjection of opioid antagonists into the mNTS does not alter cardiovascular reflexes (Viard and Sapru 2006) and does not affect the cough reflex (Mutolo et al. 2008). These findings suggest that endogenous opioids in the mNTS might selectively mediate vago-vagal reflexes.
In addition to neurons that contain endogenous opioids existing in the NTS (Martin-Schild et al. 1999; Pierce and Wessendorf 2000), and having the power to dial down GABAergic signaling, there are a substantial number of pro-opiomelanocortin (POMC)-expressing neurons present in the NTS (Bronstein et al. 1992; Padilla et al. 2012; Wang et al. 2015). POMC neurons release peptides (e.g., α melanocyte stimulating hormone) that can activate melanocortin-4 receptors, and activation of these receptors suppresses GABAergic signaling much like that observed with activation of µ opioid receptors (Richardson et al. 2013; Lewin et al. 2016).
Viewed through the lens of the findings of Thek et al. (2019), we have a greater understanding of how local GABAergic signaling occurs at the mNTS. Sst-GABA interneurons are involved and directly activated by viscerosensory input. All non-Sst-GABA interneurons receive input from Sst-GABA interneurons, and when activated suppress tractus solitarius (ST)—induced action potentials at second-order NTS neurons, indicating robust modulation of viscerosensory input. Thus, Sst-GABA inhibitory network innervates widely within the NTS and has the potential to gate viscerosensory input such as vagal sensory input from the GI Tract.
Sst-GABA interneurons are also important in the DMV (Lewin et al. 2016). They are capable of inhibiting DMV output neurons to the stomach, and they themselves are under inhibitory influence. Their exact role in the operation of the vago-vagal reflex remains to be identified. GABAergic tone is ongoing at the DMV as demonstrated by the finding that blockade of GABAA receptors at this site increases gastric mechanical activity (Sivarao et al. 1998).
The GABA projection neurons presumably originating in the NTS and synapsing onto DMV output neurons, represent a key component of one of the vago-vagal reflex circuits. We discovered this circuit when we performed our studies with nicotine (Ferreira et al. 2002 and Fig. 1). This is the circuit that we propose controls phasic contractions of the antrum. It is well accepted that functional neurons that connect the NTS to the DMV are GABAergic (Travagli et al. 2003). But the evidence that Travagli and colleagues cite is not convincing. Some of the referenced evidence for GABA projection neurons is taken from the study of McCann and Rogers (1992). A reading of this paper reveals no evidence. Evidence is also cited in the study of Sivarao et al. (1998). These investigators report that microinjection of bicuculline into the DMV of the anesthetized rat while monitoring IGP and pyloric motility evoked increases in both endpoints of mechanical activity. The responses were prevented by bilateral vagotomy and by prior microinjection of the GABAA receptor agonist, muscimol, into the DMV. However, the source of GABAergic tone was not studied. Finally, evidence is taken from one of our studies with Travagli as the lead author (Travagli et al. 1991). Using the in vitro brain slice preparation of the rat, we reported that all synaptic activity at vagal motor neurons is abolished by a combination of GABAA and excitatory amino acid antagonists. We did not determine whether the NTS was the source of the GABAA synaptic activity recorded from DMV neurons.
We have been able to obtain convincing evidence that the mNTS is the source of GABA activity in the DMV (Cruz 2006). We accomplished this by using the experimental model shown as Fig. 4. This experimental model required relating drug-induced perturbations in synaptic events in the area of the NTS to downstream changes in the DMV as reflected by changes in IGP. As mentioned in our description of Fig. 4, we needed to avoid the confounding effect of microinjected drugs into the NTS from affecting the nearby DMV. To avoid any possible DMV effects of microinjected drugs, unilateral microinjection into the mNTS was performed in rats with the ipsilateral vagus nerve sectioned but with the contralateral vagus nerve still intact. This was possible because glutamate unilaterally microinjected into the mNTS can evoke vagal—mediated decreases in IGP via activation of an inhibitory pathway originating in the microinjected NTS that projects to the contralateral DMV (Fig. 4).
For our study, glutamate was microinjected into the mNTS of four rats with only the contralateral vagus nerve intact. This evoked an average decrease of 0.28 ± 0.1 mmHg (P < 0.05) in the IGP, and a decrease of 17.0 ± 2.5 mmHg.s in AUC (area under the motility curve recording) (P < 0.05). Bicuculline (40 pmol) was microinjected into the contralateral DMV and glutamate microinjected into the mNTS was then repeated. After injection of bicuculline, the glutamate effect on gastric motility was completely antagonized. Glutamate microinjection after bicuculline now increased IGP by 0.23 ± 0.1 mmHg (P < 0.05) and no effect was observed on AUC (− 1.23 ± 18.1 mmHg.s, P > 0.05). The data indicate that signaling between the NTS and the DMV involves GABAergic projection neurons as depicted in Fig. 1.
Two Separate Circuits Exist for the Brainstem to Selectively Control the Pylorus and the Antrum
Our studies with low iv doses of nicotine resulted in the discovery that there are at least two distinct and separate circuits that control gastric mechanical activity (Ferreira et al. 2002). Low iv doses (doses affecting α4β2 nAChRs) of nicotine selectively affected the fundus without affecting the antrum. These low iv doses were shown to engage noradrenergic (A2) neurons in the mNTS via a nitrergic mechanism (Fig. 3) which resulted in activation of α2 adrenoreceptors on DMV neurons projecting to the fundus. High iv nicotine doses brought into play the second circuit, namely, the circuit that controls the antrum. Nicotine acting at the mNTS engaged GABA projecting neurons directly (no nitrergic mechanism was involved), and activation of GABAA receptors inhibited DMV projecting neurons to the antrum. Comparing and contrasting these two circuits, it was noted that the 'noradrenergic' circuit is not active during the basal or resting state and becomes active in the presence of low iv doses of nicotine and with esophageal distension (Ferreira et al. 2005). In contrast, the 'GABAergic' circuit is active both during the basal or resting state as well as when the vago-vagal reflex is engaged.
Our ultrastructural anatomical data provide the strongest evidence for the existence of two separate brainstem circuits controlling gastric mechanical activity (Pearson et al. 2007, 2011). Using electron microscopy, we observed norepinephrine containing nerve terminals synapsing with DMV fundus-projecting neurons but not with antrum-projecting neurons. Examination of synapses on DMV neurons that projected to the antrum indicated synaptic contacts with GAD67 immunoreactive terminals. Thus, noradrenergic synapses at the DMV appear to mediate decreases in tone of the fundus while GABAergic synapses at the DMV appear to mediate decreases in phasic contractions of the antrum.
Additional evidence for the existence of two different functional circuits operating to produce vago-vagal reflex-induced changes in gastric mechanical activity was obtained from our studies of melanocortin signaling in the brainstem (Richardson et al. 2013). We reported that α-MSH and melanotan II, an agonist of the MC-4 receptors, microinjected into the DMV (unlike glutamate) did not affect gastric tone. Only changes in gastric phasic contractions occurred. We suggest that the reason tone was not affected is because MC-4 R mRNA expression is mainly restricted to the medial portion of the DMV (Mountjoy et al. 1994; Kishi et al. 2003; Liu et al. 2003). Preganglionic neurons in the DMV are topographically organized such that those projecting to the antropyloric region of the stomach are more medially situated than those that project to the fundus (Shapiro and Miselis 1985; Pagani et al. 1988; Pearson et al. 2007). Thus, microinjection of agonists of the MC-4 receptor would exert an effect in the medial DMV (location of antrum-projecting neurons) but not in the lateral DMV (location of fundus-projecting neurons).
In view of our finding describing two separate brainstem circuits, one controlling primarily the fundus, and the other controlling primarily the antrum, we suggest that drug targeting to correct disorders of the fundus could be aimed at the α4β2 receptor in the mNTS (Ferreira et al. 2002). Additionally, since NO is required for the functioning of the reflex involving the fundus but not the antrum (Ferreira et al. 2002), the nitrergic system is a potential second target for drug therapy. For drug targeting to correct disorders of the antrum, one could aim therapy at the MC-4 receptor located in the medial portion of the DMV.
Characterization of the DMV Efferent Vagal Pathways Influencing Mechanical Behavior of the Stomach
Rogers and Hermann (2012), conclude that the concept of dual vagal effectors to the stomach “is now well accepted.” We strongly disagree. Our results do not support dual pathways emanating from the DMV controlling gastric motility. Instead, using multiple different experimental conditions, we have obtained evidence for the functional presence of just the cholinergic-cholinergic excitatory pathway. The different experimental conditions we have used to study the nature of the efferent component of the vago-vagal reflex are as follows: (i) esophageal distension (Ferreira et al. 2005; Herman et al. 2008), (ii) exposure to nicotine (Ferreira et al. 2002), (iii) excitation of mNTS with glutamate (Herman et al. 2009), (iv) excitation of mNTS by locally blocking GABAA receptors (Herman et al. 2009), (v) excitation of mNTS neurons by activating MC-4 receptors (Richardson et al. 2013), (vi) excitation of mNTS neurons by activating mu opioid receptors (Herman et al. 2010), (vii) excitation of mNTS by stimulating GABAB receptors (Cruz et al. 2019), (viii) direct stimulation of the DMV (Pagani et al. 1985), (ix) excitation of DMV neurons with glutamate (Cruz et al. 2007), (x) intravenous glucose (Shi et al. 2005), and (xi) intravenous cholecystokinin (Shi et al. 2005). Our evidence for the role of a single pathway, i.e., cholinergic-cholinergic excitatory pathway was based on the following type of evidence: (i) blockade of the response by a muscarinic receptor antagonist that does not cross the blood brain barrier, atropine methyl bromide, (ii) lack of blockade by L-NAME, an inhibitor of nitric oxide synthase; and (iii) lack of increased c-Fos expression in the DMV with iv nicotine, esophageal distension, and iv cholecystokinin.
There are reports of other investigators who also fail to find a NANC pathway. Some of these reports have to do with intra-cerebroventricular (ICV)—and microinjection of drugs into the brain and assessing whether responses obtained were prevented by either iv administered atropine or L-NAME. Bulbul and colleagues studied apelin which had been shown to inhibit gastric emptying. Apelin, administered either ICV or by microinjection decreased gastric mechanical activity. Atropine, but not L-NAME countered the effect (Bülbül et al. 2018; Bülbül and Sinen 2018). Travagli and colleagues also report lack of evidence of a NANC pathway in two of their studies (Anselmi et al. 2017; Jiang et al. 2018). In a non-drug study, (Kobashi et al. 2002) failed to obtain evidence of dual DMV pathways to the stomach of the rat. In their study, they showed that activation of water-responsive vagal afferents in the superior laryngeal nerve produced gastric inhibition. Neurophysiological recordings revealed a decrease in the firing rat of DMV neurons in response to the administration of water into the larynx. Intravenous atropine abolished the inhibition of gastric motility induced by administration of water into the larynx (Kobashi et al. 2000). No evidence for reflex-induced activation of an inhibitory DMV pathway was obtained.
In our earliest study of the nature of efferent vagal pathways to the GI tract, we did observe in the cat, that glutamate microinjection into the caudal DMV, i.e., 0.5–1.5 mm caudal to obex [i.e., calamus scriptorius (CS)] produced decreases in IGP and pyloric motility (Rossiter et al. 1990). Decreases in lower esophageal pressure were also noted. We did not at the time attempt to identify whether these responses were mediated by withdrawal of cholinergic excitatory tone, activation of NANC inhibitory pathway, or both. Later, we did an extensive investigation of this issue in the rat (Cruz et al. 2007). In 39 out of 43 experiments, microinjection of glutamate into different areas of the DMV rostral to CS resulted in vagal mediated excitatory effects on motility. We observed little evidence for inhibitory effects. Microinjection of glutamate into the DMV caudal to CS produced vagally mediated gastric inhibition that was resistant to L-NAME.
Could the caudal DMV contain the neurons that are responsible for the NANC pathway? Our answer is no, and for the following reasons: (i) investigators that have concluded that a vagal NANC pathway exists base their conclusion largely on the finding that L-NAME blocked the gastric relaxation response (Hermann et al. 2006; Lewis et al. 2002; Takahashi and Owyang 1997). Because L-NAME does not antagonize glutamate-induced gastric inhibition evoked from the caudal DMV (Niedringhaus et al. 2008; Cruz et al. 2007),we conclude that the neural circuitry studied by the other investigators does not contain the caudal DMV neurons that we studied. (ii) As mentioned above, methods used to inhibit gastric mechanical activity in the rat (e.g., esophageal distension, iv nicotine, iv cholecystokinin, and iv glucose (Shi et al. 2003), does not activate c-Fos in caudal DMV neurons that project to the stomach, (iii) studies of others have shown that excitation of vagal afferent nerves caused by gastric distention inhibits more than 85% of the DMV efferent neurons (McCann and Rogers 1992; Zhang et al. 1998; Zhang and Fogel 2002). If a DMV–NANC pathway was involved, then a significant percentage of DMV neurons should have been excited. (iv) Studies of a vago-vagal reflex involving the superior laryngeal nerve showed that activation of water-responsive afferents led to inhibition of gastric motility, but only inhibition of caudal DMV neuronal discharge (Kobashi et al. 2002). In order for DMV preganglionic neurons synapsing with NANC enteric neurons to play a role in reflex-induced inhibition of gastric motility, caudal DMV neurons would have to exhibit activation. In the same study, lesion of the caudal DMV had no effect on reflex-induced inhibition of gastric motility. However, lesion of the intermediate DMV did inhibit the response.
There are data from one study that differ from our finding that microinjection of L-glutamate into different areas of the DMV result primarily in vagal mediated excitatory effects on motility. Zhou et al. (2008), report findings indicating that microinjection of L-glutamate can change IGP either by activating preganglionic neurons that synapse with intragastric neurons leading to either gastric contraction or gastric relaxation. The latter response was shown to occur through release of both NO or vasoactive intestinal peptide (VIP). Their experimental conditions were similar to ours (i.e., Ferreira et al. (2000) and Cruz et al. (2007) in terms of using Sprague–Dawley rats, measuring IGP, distension of the stomach, and using ipsilateral vagotomy to show the DMV origin of IGP responses. However, one aspect of their study was different. This was the dose of microinjected L-glutamate employed. Zhou and colleagues microinjected 10 pmol L-glutamate in 20 nl into the DMV. Ferreira et al. (2000) determined the dose of L-glutamate to use by performing a dose–response curve. Using a 60 nl volume, it required a dose of 30 pmol to even notice a response, and the ED50 dose was 344 pmols. Spencer and Talman 1986, chose a dose of 300 pmol contained in 25 nl volume to activate the mNTS. Their dose was selected from dose–response data using a range of L-glutamate doses from 3 to 1500 pmol. Cruz et al. (2007) used 500 pmol in 30 nl to obtain repeatable IGP responses from microinjecting L-glutamate into both the mNTS and the DMV. Based on our studies (i.e., Ferreira et al. 2000 and Cruz et al. 2007), and the study of Spencer and Talman (1986), 10 pmol in 20 nl is ineffective in activating brainstem nuclei involved in controlling gastric mechanical activity.
We have tried to understand why we fail to find evidence confirming other investigators of dual DMV output pathways controlling gastric mechanical activity. The reasons we believe are based on different methodology and are described under "Identification of brainstem neural circuitries controlling tonic and phasic contractions of the stomach using reflex-induced changes in gastric reservoir function" section and in the "Discussion" section of several of our papers (Ferreira et al. 2005; Cruz et al. 2007, 2019; Herman et al. 2008). Methodological issues include use of different methods for delivering drugs into the brain [other investigators use microinjection into the dorsal vagal complex (which includes the NTS, the DMV and the area postrema instead of the DMV) and application of drug to the floor of the 4th ventricle]; failure to use appropriate doses (concentrations) of drugs (see “Discussion” section in Cruz et al. (2007)) need to use bethanechol to elevate baseline levels of IGP (see “Identification of brainstem neural circuitries controlling tonic and phasic contractions of the stomach using reflex-induced changes in gastric reservoir function” section); techniques used to record gastric mechanical activity, and anesthetic issues. We suggest that except for the accommodation reflex, the DMV pathway to the gut that functions physiologically is a tonically active vagal cholinergic–cholinergic connection. Excitation and inhibition of gastric tone and gastric contractions occur through increases and decreases in neuronal discharges of these cholinergic–cholinergic vagal neurons, respectively.
References
Abrahamsson H, Jansson G (1969) Elicitation of reflex vagal relaxation of the stomach from pharynx and esophagus in the cat. Acta Physiol Scand 77(1–2):172–178
Andrews PL, Lawes IN (1985) Gastric tone modifies the responses to extrinsic neural stimuli in the anaesthetized ferret. J Physiol (Lond) 366(1):1–16
Andrews PR (1990) Central organization of the vagal drive to the nonadrenergic, noncholonergic neurones controlling gastric motility. Arch Int Pharmacodyn Ther 303:167–198
Anselmi L, Toti L, Bove C et al (2017) Vagally mediated effects of brain stem dopamine on gastric tone and phasic contractions of the rat. Am J Physiol-Gastrointest Liver Physiol 313(5):G434–G441
Bertolino M, Vicini S, Gillis R et al (1997) Presynaptic alpha2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol Gastrointest Liver Physiol 272(3):G654–G661. https://doi.org/10.1152/ajpgi.1997.272.3.G654
Bronstein DM, Schafer MK, Watson SJ et al (1992) Evidence that β-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res 587(2):269–275. https://doi.org/10.1016/0006-8993(92)91007-2
Buelke-Sam J, Holson JF, Bazare JJ et al (1978) Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci 28(2):157–162
Bülbül M, Sinen O (2018) Dual autonomic inhibitory action of central Apelin on gastric motor functions in rats. Auton Neurosci 212:17–22
Christie MJ, Connor M, Vaughan CW et al (2000) Cellular actions of opioids and other analgesics: implications for synergism in pain relief. Clin Exp Pharmacol Physiol 27(7):520–523. https://doi.org/10.1046/j.1440-1681.2000.03291.x
Colquhoun LM, Patrick JW (1997) Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Anon Adv Pharmacol 39:191–220
Cruz MT (2006) Characterization of DMV pathways controlling gastric motility in the rat. Ph.D., Georgetown University Medical Center, Washington, DC
Cruz MT, Murphy EC, Sahibzada N et al (2007) A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol-Regul Integr Comp Physiol 292(1):R291–R307. https://doi.org/10.1152/ajpregu.00863.2005
Cruz MT, Dezfuli G, Murphy E et al (2019) GABAB receptor signaling in the dorsal motor nucleus of the vagus stimulates gastric motility via a cholinergic pathway. Front Neurosci 13:967
Davis SF, Derbenev AV, Williams KW et al (2004) Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017(1–2):208–217
Desai KM, Sessa WC, Vane JR (1991a) Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature 351(6326):477–479
Desai KM, Zembowicz A, Sessa WC et al (1991b) Nitroxergic nerves mediate vagally induced relaxation in the isolated stomach of the guinea pig. Proc Natl Acad Sci 88(24):11490–11494
Ellison DH, Velazquez H, Wright FS (1987) Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol-Renal Physiol 253(3):F546–F554. https://doi.org/10.1152/ajprenal.1987.253.3.F546
Fanselow EE, Connors BW (2010) The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in up-down states of mouse neocortex. J Neurophysiol 104(2):596–606. https://doi.org/10.1152/jn.00206.2010
Fanselow EE, Richardson KA, Connors BW (2008) Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol 100(5):2640–2652. https://doi.org/10.1152/jn.90691.2008
Faraday MM (2002) Rat sex and strain differences in responses to stress. Physiol Behav 75(4):507–522
Ferreira M, Ebert SN, Perry DC et al (2001) Evidence of a functional α7-neuronal nicotinic receptor subtype located on motoneurons of the dorsal motor nucleus of the vagus. J Pharmacol Exp Ther 296(2):260–269
Ferreira M, Sahibzada N, Shi M et al (2005) Hindbrain chemical mediators of reflex-induced inhibition of gastric tone produced by esophageal distension and intravenous nicotine. Am J Physiol-Regul Integr Comp Physiol 289(5):R1482–R1495. https://doi.org/10.1152/ajpregu.00003.2005
Ferreira M, Sahibzada N, Shi M et al (2002) CNS site of action and brainstem circuitry responsible for the intravenous effects of nicotine on gastric tone. J Neurosci 22(7):2764–2779
Ferreira M, Singh A, Dretchen KL et al (2000) Brainstem nicotinic receptor subtypes that influence intragastric and arterial blood pressures. J Pharmacol Exp Ther 294(1):230–238
Fong AY, Stornetta RL, Foley CM et al (2005) Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. J Comp Neurol 493(2):274–290. https://doi.org/10.1002/cne.20758
Fukuda A, Minami T, Nabekura J et al (1987) The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol (Lond) 393(1):213–231
Gao H, Glatzer NR, Williams KW et al (2009) Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res 1291:40–52
Geeraerts B, Mimidis K, Van Oudenhove L et al (2008) Role of endogenous opioids in the control of gastric sensorimotor function. Neurogastroenterol Motil 20(10):1094–1102. https://doi.org/10.1111/j.1365-2982.2008.01144.x
Gentet LJ, Kremer Y, Taniguchi H et al (2012) Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci 15(4):607–612
Glatzer NR, Derbenev AV, Banfield BW et al (2007) Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. J Neurophysiol 98(3):1591–1599. https://doi.org/10.1152/jn.00336.2007
Glatzer NR, Smith BN (2005) Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol 93(5):2530–2540. https://doi.org/10.1152/jn.00429.2004
Halabisky B, Shen F, Huguenard JR et al (2006) Electrophysiological classification of somatostatin-positive interneurons in mouse sensorimotor cortex. J Neurophysiol 96(2):834–845
Harrington AM, Hutson JM, Southwell BR (2007) Immunohistochemical localisation of cholinergic muscarinic receptor subtype 1 (M1r) in the guinea pig and human enteric nervous system. J Chem Neuroanat 33(4):193–201
Hennig GW, Spencer NJ (2018) Chapter 21—Physiology of gastric motility patterns. In: Said HM (ed) Physiology of the gastrointestinal tract, 6th edn. Academic Press, Burlingtonp, pp 469–484
Herman MA, Cruz MT, Sahibzada N et al (2009) GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am J Physiol-Gastrointest Liver Physiol 296(1):G101–G111. https://doi.org/10.1152/ajpgi.90504.2008
Herman MA, Niedringhaus M, Alayan A et al (2008) Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in reflex control of fundus tone. Am J Physiol-Regul Integr Comp Physiol 294(3):R720–R729. https://doi.org/10.1152/ajpregu.00630.2007
Herman MA, Alayan A, Sahibzada N et al (2010) μ-Opioid receptor stimulation in the medial subnucleus of the tractus solitarius inhibits gastric tone and motility by reducing local GABA activity. Am J Physiol-Gastrointest Liver Physiol 299(2):G494–G506
Hermann GE, Alberto Travagli R, Rogers RC (2006) Esophageal-gastric relaxation reflex in rat: dual control of peripheral nitrergic and cholinergic transmission. Am J Physiol-Regul Integr Comp Physiol 290(6):R1570–R1576. https://doi.org/10.1152/ajpregu.00717.2005
Heyer EJ, Macdonald RL (1982) Barbiturate reduction of calcium-dependent action potentials: CORRELATION with anesthetic action. Brain Res 236(1):157–171. https://doi.org/10.1016/0006-8993(82)90042-7
Holmes GM, Browning KN, Babic T et al (2013) Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol (Lond) 591(12):3081–3100
Hornby PJ, Rossiter CD, White RL et al (1990) Medullary raphe: a new site for vagally mediated stimulation of gastric motility in cats. Am J Physiol Gastrointest Liver Physiol 258(4):G637–G647. https://doi.org/10.1152/ajpgi.1990.258.4.G637
Hornby PJ (2001) II. Excitatory amino acid receptors in the brain-gut axis. Am J Physiol Gastrointest Liver Physiol 280(6):G1055–G1060. https://doi.org/10.1152/ajpgi.2001.280.6.G1055
Jackson DM, Richards IM (1977) The effects of pentobarbitone and chloralose anaesthesia on the vagal component of bronchoconstriction produced by histamine aerosol in the anaesthetized dog. Br J Pharmacol 61(2):251
Jiang Y, Browning KN, Toti L et al (2018) Vagally mediated gastric effects of brain stem α2-adrenoceptor activation in stressed rats. Am J Physiol-Gastrointest Liver Physiol 314(4):G504–G516. https://doi.org/10.1152/ajpgi.00382.2017
MorrisonHeymanasRichardson JLCAP et al (1951) The comparative action of certain ganglionic blocking agents. Arch Int Pharmacodyn Ther 86(2):203–213
Kishi T, Aschkenasi CJ, Lee CE et al (2003) Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457(3):213–235. https://doi.org/10.1002/cne.10454
Kobashi M, Koga T, Mizutani M et al (2002) Suppression of vagal motor activities evokes laryngeal afferent-mediated inhibition of gastric motility. Am J Physiol-Regul Integr Comp Physiol 282(3):R818–R827. https://doi.org/10.1152/ajpregu.00180.2001
Kobashi M, Mizutani M, Matsuo R (2000) Water stimulation of the posterior oral cavity induces inhibition of gastric motility. Am J Physiol-Regul Integr Comp Physiol 279(3):R778–R785. https://doi.org/10.1152/ajpregu.2000.279.3.R778
Koppanyi T, Linegar CR, Dille JM (1935) The peripheral action of barbiturates. Science 82(2123):232
Korner PI, Langsford G, Starr D et al (1968) The effects of chloralose-urethane and sodium pentobarbitone anaesthesia on the local and autonomic components of the circulatory response to arterial hypoxia. J Physiol (Lond) 199(2):283–302
Kortezova NI, Shikova LI, Milusheva EA et al (2004) Muscarinic modulation of nitrergic neurotransmission in guinea-pig gastric fundus. Neurogastroenterol Motil 16(2):155–165
Krowicki ZK, Sharkey KA, Serron SC et al (1997) Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol 377(1):49–69
Krowicki ZK, Sivarao DV, Abrahams TP et al (1999) Excitation of dorsal motor vagal neurons evokes non-nicotinic receptor-mediated gastric relaxation. J Auton Nerv Syst 77(2–3):83–89
Laiprasert JD, Hamlin RL, Heesch CM (2001) Afferent baroreceptor discharge in pregnant rats. Am J Physiol-Heart Circ Physiol 281(6):H2456–H2462
Laycock JF, Penn W, Shirley DG et al (1979) The role of vasopressin in blood pressure regulation immediately following acute haemorrhage in the rat. J Physiol (Lond) 296(1):267–275
Lewin AE, Vicini S, Richardson J et al (2016) Optogenetic and pharmacological evidence that somatostatin-GABA neurons are important regulators of parasympathetic outflow to the stomach. J Physiol 594(10):2661–2679. https://doi.org/10.1113/JP272069
Lewis MW, Hermann GE, Rogers RC et al (2002) In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol (Lond) 543(1):135–146. https://doi.org/10.1113/jphysiol.2002.019281
Lin L, Talman WT (2000) N-methyl-D-aspartate receptors on neurons that synthesize nitric oxide in rat nucleus tractus solitarii. Neuroscience 100(3):581–588
Liu H, Kishi T, Roseberry AG et al (2003) Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23(18):7143–7154
Ma S, Abboud FM, Felder RB (1995) Effects of L-arginine-derived nitric oxide synthesis on neuronal activity in nucleus tractus solitarius. Am J Physiol-Regul Integr Comp Physiol 268(2):R487–R491
Martin-Schild S, Gerall AA, Kastin AJ et al (1999) Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405(4):450–471. https://doi.org/10.1002/(SICI)1096-9861(19990322)405:43.0.CO;2-#
McCann MJ, Rogers RC (1992) Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J Physiol (Lond) 453(1):401–411. https://doi.org/10.1113/jphysiol.1992.sp019235
McGehee DS, Role LW (1995) Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol 57(1):521–546. https://doi.org/10.1146/annurev.ph.57.030195.002513
Bülbül M, Sinen O, Gök M et al (2018) Apelin-13 inhibits gastric motility through vagal cholinergic pathway in rats. Am J Physiol-Gastrointest Liver Physiol 314(2):G201–G210. https://doi.org/10.1152/ajpgi.00223.2017
Millard P (2003) Anesthetic procedures. In: Aspinall V (ed) Clininical procedures in veterinary medicine, 2nd edn. Elsevier, Saunders, pp 121–155
Mountjoy KG, Mortrud MT, Low MJ et al (1994) Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8(10):1298–1308
Murthy VS, Zagar ME, Vollmer RR et al (1982) Pentobarbital-induced changes in vagal tone and reflex vagal activity in rabbits. Eur J Pharmacol 84(1–2):41–50
Mutolo D, Bongianni F, Cinelli E et al (2008) Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol-Regul Integr Comp Physiol 295(1):R243–R251. https://doi.org/10.1152/ajpregu.00184.2008
Niedringhaus M, Jackson PG, Pearson R et al (2008) Brainstem sites controlling the lower esophageal sphincter and crural diaphragm in the ferret: a neuroanatomical study. Auton Neurosci 144(1–2):50–60
Norman WP, Pagani FD, Ormsbee HS III et al (1985) Use of horseradish peroxidase to identify hindbrain sites that influence gastric motility in the cat. Gastroenterology 88(3):701–705
O’dell TJ, Hawkins RD, Kandel ER et al (1991) Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci 88(24):11285–11289
Oliva AA, Jiang M, Lam T et al (2000) Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20(9):3354–3368
Oltra-Noguera D, Mangas-Sanjuan V, González-Álvarez I et al (2015) Drug gastrointestinal absorption in rat: strain and gender differences. Eur J Pharm Sci 78:198–203
Padilla SL, Reef D, Zeltser LM (2012) Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology 153(3):1219–1231
Pagani FD, Norman WP, Kasbekar DK et al (1985) Localization of sites within dorsal motor nucleus of vagus that affect gastric motility. Am J Physiol-Gastrointest Liver Physiol 249(1):G73–G84
Pagani FD, Norman WP, Gillis RA (1988) Medullary parasympathetic projections innervate specific sites in the feline stomach. Gastroenterology 95(2):277–288
Paxinos G (1999) Chemoarchitectonic atlas of the rat forebrain. Academic, San Diego
Pearson RJ, Gatti PJ, Sahibzada N et al (2007) Ultrastructural evidence for selective noradrenergic innervation of CNS vagal projections to the fundus of the rat. Auton Neurosci 136(1):31–42. https://doi.org/10.1016/j.autneu.2007.03.003
Pearson RJ, Gatti PJ, Sahibzada N et al (2011) Ultrastructural evidence for selective GABAergic innervation of CNS vagal projections to the antrum of the rat. Auton Neurosci 160(1):21–26. https://doi.org/10.1016/j.autneu.2010.10.010
Pickel VM, Chan J, Park DH et al (1986) Ultrastructural localization of phenylethanolamine N-methyltransferase in sensory and motor nuclei of the vagus nerve. J Neurosci Res 15(4):439–455
Pierce TL, Wessendorf MW (2000) Immunocytochemical mapping of endomorphin-2-immunoreactivity in rat brain. J Chem Neuroanat 18(4):181–207. https://doi.org/10.1016/S0891-0618(00)00042-9
Pröve J, Ehrlein HJ (1982) Motor function of gastric antrum and pylorus for evacuation of low and high viscosity meals in dogs. Gut 23(2):150–156
Qualls-creekmore E, Tong M, Holmes GM (2010) Gastric emptying of enterally administered liquid meal in conscious rats and during sustained anaesthesia. Neurogastroenterol Motil 22(2):181–185
Richardson J, Cruz MT, Majumdar U et al (2013) Melanocortin signaling in the brainstem influences vagal outflow to the stomach. J Neurosci 33(33):13286–13299. https://doi.org/10.1523/JNEUROSCI.0780-13.2013
Rogers RC, Travagli RA, Hermann GE (2003) Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol-Regul Integr Comp Physiol 285(2):R479–R489
Rogers R, Hermann G (2012) Brainstem control of the gastric function. Physiol Gastrointest Tract 1:861–891. https://doi.org/10.1016/B978-0-12-382026-6.00031-2
Rossiter CD, Norman WP, Jain M et al (1990) Control of lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol-Gastrointest Liver Physiol 259(6):G899–G906. https://doi.org/10.1152/ajpgi.1990.259.6.G899
Royer S, Zemelman BV, Losonczy A et al (2012) Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci 15(5):769–775
Ruggiero DA, Mtui EP, Otake K et al (1996) Central and primary visceral afferents to nucleus tractus solitarii may generate nitric oxide as a membrane-permeant neuronal messenger. J Comp Neurol 364(1):51–67
Sababi M, Nylander O (1996) Comparative study of the effects of nitric oxide synthase and cyclo-oxygenase inhibition on duodenal functions in rats anaesthetized with inactin, urethane or a-chloralose. Acta Physiol Scand 158(1):45–52
Scanlin HL, Carroll EA, Jenkins VK et al (2008) Endomorphin-2 is released from newborn rat primary sensory neurons in a frequency- and calcium-dependent manner. Eur J Neurosci 27(10):2629–2642. https://doi.org/10.1111/j.1460-9568.2008.06238.x
Schulze-Delrieu K (1983) Volume accommodation by distension of gastric fundus (rabbit) and gastric corpus (cat). Dig Dis Sci 28(7):625–632
Shapiro RE, Miselis RR (1985) The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol 238(4):473–488. https://doi.org/10.1002/cne.902380411
Shi M, Jones AR, Niedringhaus MS et al (2003) Glucose acts in the CNS to regulate gastric motility during hypoglycemia. Am J Physiol-Regul Integr Comp Physiol 285(5):R1192–R1202. https://doi.org/10.1152/ajpregu.00179.2003
Shi M, Jones AR, Ferreira M Jr et al (2005) Glucose does not activate nonadrenergic, noncholinergic inhibitory neurons in the rat stomach. Am J Physiol-Regul Integr Comp Physiol 288(3):R742–R750
Shirley DG, Walter SJ (1995) A micropuncture study of the renal response to haemorrhage in rats: assessment of the role of vasopressin. Exp Physiol 80(4):619–630
Siaud P, Denoroy L, Assenmacher I et al (1989) Comparative immunocytochemical study of the catecholaminergic and peptidergic afferent innervation to the dorsal vagal complex in rat and guinea pig. J Comp Neurol 290(3):323–335. https://doi.org/10.1002/cne.902900302
Sivarao DV, Krowicki ZK, Hornby PJ (1998) Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil 10(4):305–313
Smith BN, Dou P, Barber WD et al (1998) Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol (Lond) 512(1):149–162
Spencer SE, Talman WT (1986) Modulation of gastric and arterial pressure by nucleus tractus solitarius in rat. Am J Physiol-Regul Integr Comp Physiol 250(6):R996–R1002. https://doi.org/10.1152/ajpregu.1986.250.6.R996
Tack J (2012) Chapter 34—Neurophysiologic mechanisms of gastric reservoir function. In: Johnson LR, Ghishan FK, Kaunitz JD et al (eds) Physiology of the gastrointestinal tract, 5th edn. Academic Press, Boston, pp 951–957
Takahashi T, Owyang C (1997) Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol (Lond) 504(Pt 2):479
Talman WT, Andreasen K, Calvin J et al (1991) Cholecystokinin in nucleus tractus solitarii modulates tonic and phasic gastric pressure. Am J Physiol-Regul Integr Comp Physiol 261(1):R217–R222. https://doi.org/10.1152/ajpregu.1991.261.1.R217
Talman WT, Perrone MH, Reis DJ (1980) Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science 209(4458):813–815
Tan AA, Quigley A, Smith DC et al (2009) Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J Neurotrauma 26(4):539–548
Taniguchi H, He M, Wu P et al (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71(6):995–1013. https://doi.org/10.1016/j.neuron.2011.07.026
Thek KR, Ong SJ, Carter DC et al (2019) Extensive inhibitory gating of viscerosensory signals by a sparse network of somatostatin neurons. J Neurosci 39(41):8038–8050
Torjman MC, Joseph JI, Munsick C, Morishita M, Grunwald Z (2005) Effects of Isoflurane on gastrointestinal motility after brief exposure in rats. Int J Pharm 294(1–2):65–71. https://doi.org/10.1016/j.ijpharm.2004.12.028
Travagli RA, Hermann GE, Browning KN et al (2003) Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes?: III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am J Physiol-Gastrointest Liver Physiol 284(2):G180
Travagli RA, Gillis RA, Rossiter CD et al (1991) Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol-Gastrointest Liver Physiol 260(3):G531–G536. https://doi.org/10.1152/ajpgi.1991.260.3.G531
Tucker BJ, Peterson OW, Ziegler MG et al (1982) Analysis of adrenergic effects of the anesthetics inactin and alpha-chloralose. Am J Physiol-Ren Physiol 243(3):F253–F259
Viard E, Sapru HN (2006) Endomorphin-2 in the medial NTS attenuates the responses to baroreflex activation. Brain Res 1073–1074:365–373. https://doi.org/10.1016/j.brainres.2005.12.102
Vitela M, Herrera-Rosales M, Haywood JR et al (2005) Baroreflex regulation of renal sympathetic nerve activity and heart rate in renal wrap hypertensive rats. Am J Physiol-Regul Integr Comp Physiol 288(4):R856–R862
Wang D, He X, Zhao Z et al (2015) Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat 9:40
Wang J, Irnaten M, Mendelowitz D (2001) Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res 889(1):78–83. https://doi.org/10.1016/S0006-8993(00)03112-7
Wang M, Bradley RM (2010) Properties of GABAergic neurons in the rostral solitary tract nucleus in mice. J Neurophysiol 103(6):3205–3218
Wasserman AM, Ferreira M, Sahibzada N et al (2002) GABA-mediated neurotransmission in the ventrolateral NTS plays a role in respiratory regulation in the rat. Am J Physiol-Regul Integr Comp Physiol 283(6):R1423–R1441. https://doi.org/10.1152/ajpregu.00488.2001
Watson C (2014) Paxinos and Watson’s the rat brain in stereotaxic coordinates, 7th edn. Elsevier, Amsterdam
Willing AE, Berthoud H (1997) Gastric distension-induced c-Fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol-Regul Integr Comp Physiol 272(1):R59–R67
Willis A, Mihalevich M, Neff RA et al (1996) Three types of postsynaptic glutamatergic receptors are activated in DMNX neurons upon stimulation of NTS. Am J Physiol-Regul Integr Comp Physiol 271(6):R1614–R1619. https://doi.org/10.1152/ajpregu.1996.271.6.R1614
Wilson-Poe A, Jeong H, Vaughan CW (2017) Chronic morphine reduces the readily releasable pool of GABA, a presynaptic mechanism of opioid tolerance. J Physiol 595(20):6541–6555. https://doi.org/10.1113/JP274157
Xu H, Boychuk JA, Boychuk CR et al (2015) Nicotine enhances inhibition of mouse vagal motor neurons by modulating excitability of premotor GABAergic neurons in the nucleus tractus solitarii. J Neurophysiol 113(4):1165–1174
y Valenzuela IM, Rogers RC, Hermann GE et al (2004) Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol-Gastrointest Liver Physiol 286(2):G333–G339
Zhang X, Fogel R (2002) Glutamate mediates an excitatory influence of the paraventricular hypothalamic nucleus on the dorsal motor nucleus of the vagus. J Neurophysiol 88(1):49–63. https://doi.org/10.1152/jn.2002.88.1.49
Zhang X, Renehan WE, Fogel R (1998) Neurons in the vagal complex of the rat respond to mechanical and chemical stimulation of the GI tract. Am J Physiol-Gastrointest Liver Physiol 274(2):G331–G341. https://doi.org/10.1152/ajpgi.1998.274.2.G331
Zheng ZL, Rogers RC, Travagli RA (1999) Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience 90(2):685–694. https://doi.org/10.1016/S0306-4522(98)00586-7
Zhou S, Lu Y, Yao H et al (2008) Spatial organization of neurons in the dorsal motor nucleus of the vagus synapsing with intragastric cholinergic and nitric oxide/VIP neurons in the rat. Am J Physiol-Gastrointest Liver Physiol 294(5):G1201–G1209
Acknowledgements
RG expresses great appreciation to Gail J. Price for her wisdom and backing. This work was supported by the National Institutes of Health (NIDDK, United States) Grant R01-DK117508.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gillis, R.A., Dezfuli, G., Bellusci, L. et al. Brainstem Neuronal Circuitries Controlling Gastric Tonic and Phasic Contractions: A Review. Cell Mol Neurobiol 42, 333–360 (2022). https://doi.org/10.1007/s10571-021-01084-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01084-5