Abstract
The present study was performed to investigate the effect of piracetam on neuroinflammation induced by lipopolysaccharide (LPS) and resulting changes in cognitive behavior. Neuroinflammation was induced by a single dose of LPS solution infused into each of the lateral cerebral ventricles in concentrations of 1 μg/μl, at a rate of 1 μl/min over a 5-min period, with a 5-min waiting period between the two infusions. Piracetam in doses of 50, 100, and 200 mg/kg i.p. was administered 30 min before LPS infusion and continued for 9 days. On ninth day, the behavioral test for memory and anxiety was done followed by blood collection and microdissection of the hippocampus (HIP) and prefrontal cortex brain regions. Piracetam attenuated the LPS-induced decrease in coping strategy to novel environment indicating anxiolytic activity. It also reversed the LPS-induced changes in the known arm and novel arm entries in the Y-maze test indicating amelioration of spatial memory impairment. Further, piracetam moderated LPS-induced decrease in the mitochondrial complex enzyme activities (I, II, IV, and V) and mitochondrial membrane potential. It ameliorated changes in hippocampal lipid peroxidation and nitrite levels including the activity of superoxide dismutase. Piracetam region specifically ameliorated LPS-induced increase in the level of IL-6 in HIP indicating anti-neuroinflammatory effect. Further, piracetam reduced HIP Aβ (1–40) and increased blood Aβ level suggesting efflux of Aβ from HIP to blood. Therefore, the present study indicates preclinical evidence for the use of piracetam in the treatment of neuroinflammatory disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation is considered to be one of the principal causes of neurodegenerative disorders such as Alzheimer (AD), Parkinson (PD), and Multiple sclerosis (MS) (Owens et al. 2005). Thus, there is always a question whether inhibition of neuroinflammation can slow down these disease conditions. Several signaling molecules mediate neuroinflammation which includes cytokines, chemokines, oxygen free radicals, reactive nitrogen species, and prostaglandin E2 (McGeer et al. 2003). Lipopolysaccharide (LPS) is a bacterial endotoxin which induces microglia for the production of tumor necrosis factor (TNF) and other cytokines. It causes inflammation and accelerates the appearance and severity of AD pathology in several animal models (Qin et al. 2007). It is used to produce neuroinflammation and administered either by systemic injection, intracerebroventricular (ICV) microinjection, or chronic infusion (Qin et al. 2007; Hauss et al. 1998). Activation of TNF and interleukins (IL-6) increases Aβ in AD brains and decreases efficient plaque removal by resident microglia (Koenigsknecht et al. 2005). Increased microglia activation occurs in brain regions that ultimately demonstrate the greatest concentration of senile plaques and brain atrophy in AD patients (Cagnin et al. 2001). An earlier study reported that LPS induced region-specific expression of neuroinflammatory markers, which is due to differential changes in oxidative stress, mitochondrial activities, and oxidative phosphorylation. Oxidative stress may, in turn, influence mitochondrial membrane potential and mitochondrial complex activity (Jin et al. 2014). Similarly, an earlier study demonstrated mitochondrial electron transfer chain dysfunction due to intracerebroventricular injection of LPS in rat brain (Joshi et al. 2014).
Piracetam is a nootropic drug derived from γ-aminobutyric acid. Clinical application of piracetam includes age-related cognitive disorders, vertigo, cortical myoclonus, dyslexia, sickle cell anemia, and post-stroke aphasia (Winblad 2005; Greener et al. 2001). Its mechanism of action is not clearly understood. However, it binds to AMPA receptor and acts as its positive allosteric modulator (Ahmed et al. 2010). It has been reported to inhibit peripheral cytokine production including TNF-α and IL-1β (Croisile et al. 1993; Waegemans et al. 2002; Mehta et al. 2014). Piracetam is reported to protect mitochondrial complexes and also significantly increased glutathione peroxidase and glutathione reductase activity in aged rat brain (Keil et al. 2006). Further, piracetam is reported to slow down the erythrocyte adhesion at a vascular level and increases microcirculation (Nalbandian et al. 1983). This may be due to anti-platelet effect of piracetam (Evers et al. 1999; Moriau et al. 1993). Therefore, increased microcirculation tends to increase the blood flow and thus facilitates removal of Aβ plaques from the brain. This could result in enhanced cognition in a case of dementia. Further, inhibition of cytokine production could slow down the neuroinflammation process.
Based on available information, we presume that piracetam may ameliorate learning and memory deficits of neuroinflammation, and may facilitate the efflux of Aβ from brain region to blood in LPS-induced neuroinflammatory model. Several behavioral observations have been carried out to confirm the AD-like learning and memory deficits. The level of Aβ has been estimated in the plasma and brain regions to know the extent of efflux of Aβ. Further, the level of IL-6 as a marker of neuroinflammation and mitochondrial function has been estimated in different brain regions to establish the mechanism of action of piracetam.
Materials and Methods
Drug
Piracetam was procured from Sigma (St. Louis, MO, USA) and was dissolved in 0.9% saline.
Chemicals
Lipopolysaccharides (E. coli, L3129), DTNB (29309099990), tetramethylrhodamine methyl ester (TMRM), and Griess reagent were procured from Sigma-Aldrich (St. Louis, MO, USA). Thiobarbituric acid (TBA), cytochrome oxidase, NADH, 2-[4-(2-hydroxyethyl) 1-piperazinyl] ethane sulphonic acid (HEPES buffer, acid-free) phenazine methanesulphonate (PMS), sodium succinate, sodium azide, and nitro blue tetrazolium were purchased from Merck (Darmstadt, Germany). All other chemicals and reagents were purchased from local suppliers and were of analytical grade.
Animals
Adult male albino Wistar rats 220–260 g were procured from the Central Animal House, Institute of Medical Sciences, Banaras Hindu University. The animals were acclimatized for 4 days at 25 ± 1 °C with a 12-h light–dark cycle and were allowed free access to food (Amrut Laboratory Animal feed, Sangli, India) and water throughout the experiment. All efforts were made to minimize the number of animals used, and all experiments were performed following the principles of laboratory animal care (National Research Council US Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011) guidelines. Before performing the experiment on an animal, approval from the animal ethics committee was taken (Ref No. Dean/12-13/CAEC/28).
Animal Treatment
Animals were randomly assigned to five groups (n = 9 in each group); group 1- control, group 2- LPS treated, group 3, 4, and 5 were treated with 50 mg/kg (P-50), 100 mg/kg (P-100), and 200 mg/kg (P-200) piracetam, respectively. LPS solution was prepared in 0.9% saline.
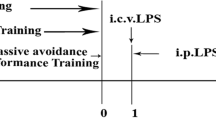
Sodium pentobarbitone (45 mg/kg) intraperitoneally was used as an anesthetic agent (Bamber et al. 1999). After attainment of surgical level, anesthetic rats were placed in stereotaxic apparatus (Stoelting, USA). The skin of the skull was shaved and sterilized conventionally. Two small holes for needle insertion were drilled in the parietal bone posterior to Bregma on either side of the midline (coordinates: posterior −0.8 mm, medial/lateral ± 1.5 mm relative to Bregma, dorsal/ventral −3.8 mm below dura). A single dose of LPS was injected into bilateral intracerebroventricular using Quintessential Stereotaxic Injector. ICV infusion has the advantage of generating inflammatory processes in defined brain areas. However, the progression of neuroinflammation may not be similar to the normal pathophysiological process leading to brain disorders. Nevertheless, it is commonly used as an animal model of brain inflammation (Hauss et al. 1998). LPS solution was injected into each of the lateral cerebral ventricles at a concentration of 1 μg/μl, at a rate of 1 μl/min over a 5-min period with a 5-min waiting period between the two infusions. To limit local infection, betadine was applied prior to the suturing of incision followed by application of neomycin onto the sutured surface. 5 ml of 0.9% saline was injected subcutaneously to prevent dehydration during recovery. Rats were closely monitored during recovery and kept in a room at 22−26 °C. The control group also underwent all surgical steps except that saline was administered rather than LPS. A single ICV infusion of LPS solution was administered to groups 2, 3, 4, and 5 on the first day of the experiment. Just half an hour prior to LPS infusion, group 2 received 0.9% saline i.p., while groups 3, 4, and 5 received piracetam dissolved in 0.9% saline at the dose of 50 mg/kg, 100 mg/kg, and 200 mg/kg i.p., respectively. This was considered to be day 1, and the treatment with vehicle or drug continued for 8 more days, i.e., up to day 9. Figure 1 shows the schematic of the experimental design. Behavioral experiments were done 30 min after the last dose on day 9. After the behavioral experiments, the animals were immediately killed by decapitation. The HIP and PFC were microdissected on the same day (Paxinos and Watson 1997). The tissues were stored at −80 °C until further mechanistic studies.
Behavioral Tests
All the behavioral tests were performed on day nine after LPS infusion in animal, and the behavioral tests were performed in following sequences:
Open Field Test (OFT)
An open field apparatus was used to study the exploratory behavior in rats. It was made of plywood and consists of a square (61 × 61 cm) with high walls (61 × 61 cm). The complete apparatus was painted black except for 6 mm white lines that divided the floor into 16 squares. The whole room except the open field was kept dark throughout the experiment. The open field was lighted by a 60 W bulb focusing on to the field from a height of about 100 cm from the floor. Each animal was centrally placed in the test apparatus for 5 min, and the number of crossings were noted (Brown et al. 1999).
Y-Maze Test
Spatial recognition memory, general exploratory behavior, and anxiety-like behavior were assessed by Y-maze test (Dellu et al. 1992; Krishnamurthy et al. 2013). Y-maze consists of three similar arms of dimension 50 cm long, 16 cm wide, and 32 cm high. In the first trial, the novel arm was blocked and the animal was allowed to move for 15 min in other two arms. In the second trial, the novel arm was opened and the animal was allowed to move for 5 min in all three arms after 4 h of the first trial. The total number of entries in all arms (for 5 min of trial 1 and 2) is indicative of general exploration attitude (curiosity). The % entries in known and novel arms for the 5 min period of trial 2 were considered as a measure of spatial recognition memory. Coping strategy to the novel environment was estimated by the percentage of the ratio of time spent in the novel arm to time spent in all arms and in the center of the apparatus during trial 2. The decrease in the coping behavior to the novel environment was considered as an increase in anxiety-like behavior (Poimenova et al. 2010).
Mitochondria Respiratory Chain Enzymes Estimation
Mitochondrial Isolation
After the cervical dislocation, the rats were decapitated and brain was dissected out. Mitochondria were isolated from different brain regions by differential centrifugation at 4 °C (Pedersen et al. 1978).The HIP and PFC were dissected and rinsed in ice-cold saline (isotonic) followed by homogenization in (1:10, w/v) ice-cold extraction buffer (250 mM sucrose, 1 mM EGTA, and 10 mM HEPES–KOH, pH 7.2). After the removal of cell debris by centrifugation at 600×g for 5 min, the supernatant obtained above was again centrifuged at 10000xg for 15 min followed by removal of pellets and discarding the supernatant. Pellets were placed in a medium (1 ml) containing 250 mM sucrose, 0.3 mM EGTA, and 10 mM HEPES–KOH, pH 7.2. The resulting solution thus obtained was centrifuged at 14000xg for 10 min. The pellets obtained were then suspended in a medium (1 ml) containing 250 mM sucrose and 10 mM HEPES–KOH, pH 7.2.
Complex I (NADH Dehydrogenase) Activity Estimation
This involves measurement of catalytic oxidation of NADH using spectrofluorophotometer. The reaction mixture consisted of 200 μl of 10 mM potassium ferricyanide, 60 μl of 1 mM NADH in 2 mM potassium phosphate buffer, and 2.64 ml of 0.12 M potassium phosphate buffer. Here, potassium ferricyanide acts as the artificial electron acceptor (Shapiro et al. 1979). In the experiment, 350 and 470 nm were the excitation and emission wavelengths for NADH, respectively.
Complex II (Succinate Dehydrogenase) Activity Estimation
The mitochondrial succinate: acceptor oxidoreductase was determined by the progressive reduction of nitro blue tetrazolium (NBT) to an insoluble colored compound, diformazan (dfz), which is used as a reaction indicator. The reaction of NBT was mediated by H+ released in the conversion of succinate to fumarate. The concentration of NBT–dfz produced was determined at 570 nm. The mean SDH activity of HIP and PFC was expressed as micromole formazan produced/min/microgram of the protein (Sally and Margaret 1989).
Complex IV (Cytochrome c Oxidase) Activity Estimation
Cytochrome oxidase was assayed in mitochondrial preparation (Storrie and Amadden 1990). Briefly, cytochrome c was first reduced by the addition of a few crystals of sodium borohydride and then neutralized to pH 7.0 with 0.1 M HCl. Reduced cytochrome c (0.3 mM) was added to 0.075 M phosphate buffer (pH 7.4), and the reaction was initiated by the addition of appropriate amount of mitochondrial suspension. The decrease in absorbance was measured at 550 nm for 3 min. Results were expressed as nmol cytochrome c oxidized/min/mg protein, using molar extinction coefficient of cytochrome c (19.6 mM −1cm−1).
Complex V (F0–F1 Synthase) Activity Estimation
The level of F0–F1 synthase was expressed as nmol ATP hydrolyzed/mg protein (Griffiths and Houghton 1974). The mitochondrial sample was incubated at 37 °C for 10 min with 500 ml of ATPase buffer (containing 50 mM tris and 5 mM MgCl2, pH 7.5) and 5 mM ATP. This was followed by addition of 500 ml of 10%w/v trichloroacetic acid solution to the above mixture. The content was then centrifuged at 3000xg for 20 min and 500 ml supernatant was mixed with 500 ml water. Phosphate thus produced was measured (Fiske and Subbarow 1925).
Mitochondrial Membrane Potential (MMP) Estimation
The mitochondrial suspensions along with assay buffer containing 80 mM NaCl, 75 mM KCl, 25 mM d-glucose, 25 mM HEPES, pH 7.4 was firstly mixed with TMRM solution followed by incubation at 37 °C for 15 min. Fluorescence (excitation at 535 nm, emission at 580 nm) was measured using Shimadzu fluorescence spectrophotometer (model F-2500, Japan). The TMRM (rhodamine analog) taken up by healthy mitochondria was measured in terms of fluorescence intensity per mg of protein (Huang 2002).
Mitochondrial Oxidative Stress
Superoxide Dismutase (SOD) Level Estimation
The inhibition of reduction of NBT to blue colored formazan in the presence of phenazine methosulfate (PMS) and NADH was determined at 560 nm using n-butanol as blank. The results were expressed as units/mg protein, where one unit of enzyme is defined as the amount of enzyme inhibiting the rate of reaction by 50% (Kakkar et al. 1984).
Nitrite Level Estimation
A colorimetric assay using the Griess reagent was used to determine the nitrite level in mitochondrial samples from HIP and PFC (Green et al. 1982). The nitrite amount was calculated in comparison to the standard nitrite curve, and the results were expressed as nanomoles of nitrite formed/mg of protein.
Mitochondrial LPO or Malondialdehyde (MDA) Formation Estimation
Mitochondrial MDA content was measured by following the standard protocol (Ohkawa et al. 1979). Briefly, the chromophore formed in the reaction was determined at 532 nm. The MDA concentrations are expressed as micromoles of MDA per milligram of protein.
Western Blotting Analysis
A standard plot was generated by bovine serum albumin. An aliquot of each sample was electrophoresed in 12 and 15% SDS-PAGE gels for IL-6 (1:500, Abcam, USA) and Aβ (1:500, Covance, Berley, CA, USA) proteins, respectively, transferred to polyvinylidene fluoride membranes, and probed with specific antibodies. The membranes were incubated overnight with rabbit anti-IL-6 and anti-Aβ polyclonal primary antibody at a dilution of 1:400 and 1:1000, respectively. After detection with the desired antibodies against the proteins of interest, the membrane was stripped with stripping buffer (25 mM glycine pH 2.0, 2% SDS for 30 min at room temperature) and reprobed whole night with rabbit anti β-actin polyclonal primary antibody at a dilution of 1:500 to confirm equal loading of protein. Further, the membrane was probed with corresponding secondary antibodies. Immunoreactive band of proteins was detected by chemiluminescence using enhanced chemiluminescence reagents (Amersham Biosciences, USA). Quantification of results was performed by a densitometric scan of films. The immunoreactive area was determined by densitometric analysis using BIOVIS gel documentation software (Bradford 1976).
Statistical Analysis
Data are presented as mean ± Standard Error Mean (SEM). Data for spatial memory recognition, exploratory behavior (Y-maze test), locomotor activity (OFT), and mitochondrial parameters were analyzed by one-way ANOVA followed by student Newman–Keuls test using Graph Pad Prism version 5 (San Diego, CA). A level of p < 0.05 was accepted as statistically significant.
Results
Effect of Piracetam on LPS-Induced Changes in Behavior
Effect of Piracetam on Locomotor Activity in Open Field Test
Figure 2a shows the effect of piracetam on a number of crossings (index of locomotor activity) in OFT. Analysis by one-way ANOVA showed that there were no significant differences in a number of crossings among groups [F (4, 25) = 0.9404, p > 0.05].
The effect of piracetam (50, 100, and 200 mg/kg) on LPS-induced changes in a number of crossings in the open field test. b Known arm entries (%) and c novel arm entries (%) in Y-maze to determine altered arm discrimination (spatial recognition memory). d Coping behavior to a novel environment. e Curiosity trial 1 and f curiosity trial 2 to determine curiosity behavior. All Values are Mean ± SEM. a p < 0.05 compared to control, b p < 0.05 compared to LPS, c p < 0.05 compared to P-50, d p < 0.05 compared to P-100. All results were analyzed using one-way ANOVA followed by Student Newman–Keuls test
Effect of Piracetam on Alterations in Arms Discrimination Behavior in Y-Maze Test
The effect of piracetam on spatial memory impairment after 9 days of LPS infusion in the Y-maze test is depicted in Fig. 2b, c. Statistical analysis by one-way ANOVA revealed that there were significant differences among groups in % entries in novel [F (4, 25) = 9.811, p < 0.05] and known [p (4, 25) = 35.46, p < 0.05] arms. Post hoc analysis revealed that there were a significant increase and a decrease in the known and novel arm entries, respectively, in LPS-treated rats. Piracetam treatment dose dependently reversed the known and novel arm entries in the Y-maze test paradigm.
Effect of Piracetam on Coping Behavior to Novel Environment in Y-Maze Test
Figure 2d shows the coping behavior in rats in terms of percentage of time spent in the novel arm of the apparatus during trial 2. Statistical analysis revealed that there were significant differences among groups [F (4, 25) = 20.88, p < 0.05]. Student Newman–Keuls test suggests that there was a significant loss of coping behavior in LPS-treated group animals compared to control (vehicle injected) group rats. Piracetam at 100 and 200 mg/kg doses attenuated the LPS-induced decrease in coping strategy to a novel environment. Moreover, piracetam (200 mg/kg) was more effective than other two doses in terms of facilitating coping strategy to a novel environment.
Effect of Piracetam on Curiosity Behavior in Y-Maze Test
Figure 2e, f shows the total number of entries in trial 1 and 2, i.e., curiosity behavior in rats subjected to the Y-maze test. Statistical analysis showed that there were no significant differences in trial 1 [F (4, 25) = 0.8781, p > 0.05] and trial 2 [F (4, 25) = 1.386, p > 0.05] among the groups.
Effects of Piracetam on LPS-Induced Changes in Mitochondria
Effect of Piracetam on LPS-Induced Changes in Mitochondrial Electron Transport Chain Enzyme Activity in Different Brain Regions
Complex I Activity
Figure 3a shows the effect of LPS and piracetam treatment on complex I activity. Analysis by one-way ANOVA revealed that there were significant differences in complex I activity in HIP [F (4, 20) = 38.74, p < 0.05] and PFC [F (4, 20) = 3.185, p < 0.05] among the groups. Post hoc analysis showed that LPS administration caused a significant decrease in complex I activity in both the brain regions. However, piracetam at a dose of 100 and 200 mg/kg attenuated the LPS-induced decrease level of complex I activity in HIP only.
Effect of piracetam (50, 100, and 200 mg/kg) on LPS-induced changes in mitochondrial electron transport chain enzyme activity in different brain regions: a complex I activity, b complex II activity, c complex IV activity, and d complex V activity. All Values are Mean ± SEM. a p < 0.05 compared to control, b p < 0.05 compared to LPS, c p < 0.05 compared to P-50, d p < 0.05 compared to P-100. All results were analyzed using one-way ANOVA followed by Student Newman–Keuls test
Complex II Activity
The effect of piracetam treatment on LPS-induced alterations in the complex II activity is illustrated in Fig. 3b. Statistical analysis revealed that there were significant differences in SDH activity in HIP [F (4, 20) = 14.61, p < 0.05] and in PFC [F (4, 20) = 6.448, p < 0.05] among the groups. Post hoc analysis revealed that LPS administration caused a significant decrease in SDH activity in both HIP and PFC. However, piracetam in a dose of 100 and 200 mg/kg attenuated the LPS-induced decrease in the level of complex II activity in HIP only.
Complex IV Activity
Figure 3c shows the effect of piracetam treatment on LPS-induced changes in complex IV activity. Analysis by one-way ANOVA showed that there were significant differences in complex IV activity in HIP [F (4, 20) = 40.98, p < 0.05] and PFC [F (4, 20) = 5.643, p < 0.05] among the groups. Student Newman–Keuls test revealed that LPS infusion decreased cytochrome c oxidase activity significantly in HIP and PFC. However, P-100 and P-200 attenuated the LPS-induced decrease level of complex IV activity in both HIP and PFC.
Complex V Activity
The effect of piracetam on LPS-induced alterations in the complex V activity is depicted in Fig. 3d. Analysis by one-way ANOVA showed that there were significant differences in complex V activity in HIP [F (4, 20) = 13.04, p < 0.05] and in PFC [F (4, 20) = 4.468, p < 0.05] among the groups. Post hoc analysis revealed that LPS administration caused a significant decrease in ATPase activity in both HIP and PFC. However, piracetam in all doses attenuated the LPS-induced decrease level of complex V activity in HIP only.
Effect of Piracetam on Mitochondrial Membrane Potential (MMP)
Figure 4 depicts the effect of piracetam on LPS-induced altered mitochondrial membrane potential (MMP) in different brain regions. Analysis by one-way ANOVA revealed that there were significant differences in mitochondrial membrane potential in HIP [F (4, 20) = 3.544, p < 0.05] and PFC [F (4, 20) = 3.814, p < 0.05] among the groups. Post hoc analysis showed that LPS administration decreased the MMP in both HIP and PFC significantly. However, piracetam in a dose of 100 and 200 mg/kg attenuated the LPS-induced decrease in MMP in HIP only.
Effect of piracetam (50, 100, and 200 mg/kg) on LPS-induced alterations in the mitochondrial membrane potential in HIP and PFC. All Values are Mean ± SEM. a p < 0.05 compared to control, b p < 0.05 compared to LPS, c p < 0.05 compared to P-50, d p < 0.05 compared to P-100. All results were analyzed using one-way ANOVA followed by Student Newman–Keuls test
Effect of Piracetam on Mitochondrial Oxidative Stress
Superoxide Dismutase Activity
Figure 5a shows the effect of piracetam on LPS-induced changes in superoxide dismutase (SOD) activity. Analysis by one-way ANOVA revealed that there were significant differences in SOD activity in HIP [F (4, 20) = 16.89, p < 0.05] and PFC [F (4, 20) = 3.000, p < 0.05] among the groups. Pos -hoc analysis revealed that LPS administration caused a significant decrease in SOD level in both HIP and PFC. However, P-100 and P-200 attenuated the LPS-induced decrease in SOD in HIP only.
Effect of piracetam (50, 100, and 200 mg/kg) on mitochondrial oxidative stress: a SOD level, b NO level, and c LPO level. All Values are Mean ± SEM. a p < 0.05 compared to control, b p < 0.05 compared to LPS, c p < 0.05 compared to P-50, d p < 0.05 compared to P-100. All results were analyzed using one-way ANOVA followed by Student Newman–Keuls test
Nitrite Level
The effect of piracetam on LPS-induced alterations in NO level is depicted in Fig. 5b. Analysis by one-way ANOVA showed that there were significant differences in NO level in HIP [F (4, 20) = 8.385, p < 0.05] and PFC [F (4, 20) = 3.552, p < 0.05] among groups. Post hoc analysis revealed that LPS administration caused a significant increase in NO level in both HIP and PFC. Piracetam in all the doses attenuated the LPS-induced increase in NO in HIP only.
Lipid Peroxidation
Figure 5C depicts the effect of piracetam on LPS-induced alterations in the extent of LPO. Analysis by one-way ANOVA showed that there were significant differences in LPO level in HIP [F (4, 20) = 5.524, p < 0.05] and in PFC [F (4, 20) = 6.608, p < 0.05] among the groups. Post hoc analysis revealed that LPS administration caused a significant increase in LPO level in both HIP and PFC. However, piracetam in a dose of 200 mg/kg attenuated the LPS-induced increase in the extent of LPO in HIP only.
Effect of Piracetam on IL-6 Production
Figure 6 depicts the effect of piracetam on LPS-induced changes in IL-6 level. Analysis by one-way ANOVA showed that there was a significant difference in IL-6 level in HIP [F (4, 10) = 22.32, p < 0.05] and PFC [F (4, 10) = 14.95, p < 0.05] among the groups. Student Newman–Keuls test revealed that LPS infusion increased the level of IL-6 in both the brain regions. However, P-200 attenuated the LPS-induced increase in the level of IL-6 only in HIP.
Effect of piracetam (50, 100, and 200 mg/kg) on LPS-induced increased IL-6 level in HIP and PFC. The blots are representative of IL-6 (a) in HIP and PFC. The results in the histogram are expressed as the ratio of the relative intensity of levels of protein expression of IL-6 to β-Actin (b). All Values are Mean ± SEM. a p < 0.05 compared to control, b p < 0.05 compared to LPS, c p < 0.05 compared to P-50, d p < 0.05 compared to P-100 (One-way ANOVA followed by Student Newman–Keuls test)
Effect of Piracetam on Aβ in Different Brain Regions and in the Blood
Effect of Piracetam on LPS-Induced Increase in Aβ Level in Brain Regions
The effect of piracetam on LPS-induced alterations in Aβ level in HIP and PFC is depicted in Fig. (7). Analysis by one-way ANOVA showed that there were significant differences in Aβ level in HIP [F (4, 10) = 13.93, p < 0.05] and PFC [F (4, 10) = 4.37, p < 0.05] among the groups. Post hoc analysis showed that LPS administration caused a significant increase in Aβ level in all the brain regions. However, piracetam in a dose of 200 mg/kg significantly attenuated the LPS-induced increase in the level of Aβ in HIP only.
Effect of piracetam (50, 100, and 200 mg/kg) on LPS-induced increased Aβ (1–40) level in HIP and PFC. The blots are representative of Aβ (a) in HIP and PFC. The results in the histogram are expressed as the ratio of the relative intensity of levels of protein expression of Aβ to β-Actin (b). All Values are Mean ± SEM. a p < 0.05 compared to control, b p < 0.05 compared to LPS, c p < 0.05 compared to P-50, d p < 0.05 compared to P-100 (One-way ANOVA followed by Student Newman–Keuls test)
Piracetam Facilitates the Efflux of Aβ from Brain Region to Blood
Figure 8 depicts the effect of piracetam on LPS-induced reduced efflux of Aβ from different brain regions. Statistical analysis revealed that there were significant differences in Aβ level in blood [F (4, 10) = 16.59, p < 0.05] among groups. Post hoc analysis revealed that the level of Aβ in plasma was not altered by LPS treatment. However, piracetam at a dose of 200 mg/kg significantly increased Aβ level in blood, indicating that the Aβ efflux is facilitated by the higher dose of piracetam.
Effect of piracetam (50, 100, and 200 mg/kg) on increased efflux of Aβ (1–40) level from brain regions to blood. The blots are representative of Aβ (a) in plasma. The results in the histogram are expressed as the ratio of the relative intensity of levels of protein expression of Aβ to β-Actin (b). All Values are Mean ± SEM. a p < 0.05 compared to control, b p < 0.05 compared to LPS, c p < 0.05 compared to P-50, d p < 0.05 compared to P-100 (One-way ANOVA followed by Student Newman–Keuls test)
Discussion
The present study for the first time reports the anti-neuroinflammatory activity of piracetam in the LPS-infused rats showing learning and memory deficits. There was also increase in the level of Aβ in plasma with piracetam treatment suggesting that it may enhance the efflux of Aβ.
LPS-induced neuroinflammation leads to learning and memory deficits (Gong et al. 2010). LPS treatment increased known arm and decreased the novel arm entries indicating loss of spatial memory. A similar result has been reported for LPS-induced decrease in alternation behavior in the Y-Maze Test (Joshi et al. 2014). Piracetam is reported to augment learning acquisition, retention of memory, and exhibited anxiolytic activity after subchronic administration in rats (Bhattacharya et al. 1993). Piracetam treatment dose dependently reversed the known arm and novel arm entries in the Y-maze test indicating attenuation of LPS-induced spatial memory impairment. Further, LPS treatment caused significant loss of coping behavior in terms of time spent in the novel environment indicating anxiety-like behavior. Piracetam attenuated the LPS-induced decrease in coping strategy to a novel environment. Thus, piracetam showed anxiolytic activity in the Y-maze paradigm. Therefore, piracetam improves cognitive deficits and exhibits anxiolytic activity during LPS-induced neuroinflammation.
LPS induces a spectrum of cellular insults and host damage as a result of a dysregulated immune inflammatory response to an infective stimulus. A complex molecular network exists which transduces this stimulus and ultimately over expresses the inflammatory mediators by increasing expression of NFkB (Macdonald et al. 2003). LPS administration caused increased production of IL-6, a potent marker of neuroinflammation as reported earlier (Beurel and Jope 2009). It has been reported that piracetam inhibits peripheral cytokine production in peripheral neuropathic pain induced by chronic constriction injury of the sciatic nerve in rats (Mehta et al. 2014).In the present study, piracetam at a dose of 200 mg/kg significantly attenuates LPS-induced increase in IL-6 suggesting anti-neuroinflammatory activity.
The LPS-induced neuroinflammation has been characterized by an increase in production of inflammatory cytokines and oxidative stress (Ben et al. 2001). Formation of ROS and lipid peroxidation products such as superoxide anions and peroxides was increased by LPS (Giralt et al. 1993; Nowak et al. 1993). There was an increase in the level of MDA, an end product of lipid peroxidation indicating oxidative stress (Melchiorri et al. 1996). Oxidative stress may be due to an imbalance between free radical production and ability to scavenge those (Kim et al. 2004). Superoxide dismutase is an enzyme, which acts as the first line of defense against ROS generation by dismutating superoxide to H2O2 (Apel and Hirt 2004). In this present study, LPS increased oxidative stress by increasing the level of mitochondrial MDA (by increasing the activity of LPO) and nitrite levels. Piracetam in a dose of 200 mg/kg attenuated LPS-induced increase level of MDA and nitrites as reported earlier (Kalkan et al. 2011; Akdur et al. 2008). The LPS-induced decrease in SOD level was ameliorated by piracetam indicating antioxidant activity.
LPS administration leads to the breakdown of mitochondrial membrane potential as reported earlier (Noble et al. 2007). Piracetam in a dose of 200 mg/kg attenuated LPS-induced decrease in mitochondrial membrane potential (Keil et al. 2006). The activity of mitochondrial respiratory chain enzymes, i.e., complex I, II, IV, and V was found to be reduced in mitochondria isolated from HIP and PFC of rats treated with LPS infusion (Escames et al. 2003). Treatment with piracetam significantly ameliorated the reduction in activity of the mitochondrial enzyme complexes in HIP. An earlier study has reported that piracetam enhances the mobility and function of the complexes of the respiratory chain (Gupta et al. 2014). Thus, piracetam attenuated the LPS-induced mitochondrial dysfunction in HIP suggesting a region-specific role of piracetam.
Intracerebroventricular infusion of LPS has been previously reported to increase inflammatory cytokines which cause the amyloid genesis through β- and γ-secretases, thereby elevating Aβ level in the hippocampus (Lee et al. 2008). In the present study, the level of soluble Aβ1–40 was measured as a marker of AD-like symptoms. Infusion of LPS significantly increased Aβ level in HIP as well as PFC which is consistent with earlier findings (Kakkar et al. 1984; Lee et al. 2008). The administration of piracetam at a dose of 200 mg/kg attenuated the LPS-induced increase in Aβ level in HIP, suggesting a region-specific role of piracetam. The HIP is the brain region known to be particularly susceptible to AD and has a significant role in learning and memory functions (He et al. 2008). Neuroinflammation of the HIP can lead to learning and memory deficits. In the present study, LPS infusion did not cause any significant increase in the Aβ in blood even though there was a significant increase in HIP and PFC. It has been reported that LPS causes a selective influx of Aβ from blood to the brain and inhibits efflux of Aβ from the brain to blood (Jaeger et al. 2009). This can lead to accumulation of Aβ in the brain tissues. Piracetam at a dose of 200 mg/kg significantly increased the level of Aβ in blood, while there was a concomitant decrease in HIP. Therefore, we can presume that piracetam can facilitate efflux of Aβ from brain to the blood. This could perhaps be possibly due to the reports which suggest an increase in the microvascular blood flow by piracetam (Vernom and Sorkin 1991; Platt et al. 1993). Therefore, the possible mechanism behind the neuroprotective effect of piracetam may be due to increase in the clearance of Aβ from HIP tissues.
Conclusions
Piracetam ameliorates the LPS-induced behavioral deficits in rats. Piracetam (200 mg/kg) significantly decreased LPS-induced increase in the level of IL-6 and Aβ in HIP indicating anti-neuroinflammatory effect. It significantly increased Aβ level in blood suggesting efflux of Aβ from HIP to blood. Piracetam also showed significant attenuation of LPS-induced mitochondrial dysfunction and decrease of mitochondrial membrane potential. Thus, piracetam could be considered as a potential drug for pharmacotherapy of diseases related to neuroinflammation.
Abbreviations
- HIP:
-
Hippocampus
- PFC:
-
Prefrontal cortex
- AD:
-
Alzheimer
- PD:
-
Parkinson
- MS:
-
Multiple sclerosis
- LPS:
-
Lipopolysaccharide
- TNF:
-
Tumor necrosis factor
- ICV:
-
Intracerebroventricular
- IL:
-
Interleukins
- TMRM:
-
Tetramethyl rhodamine methyl ester
- TBA:
-
Thiobarbituric acid
- PMS:
-
Phenazine methanesulphonate
- OFT:
-
Open field test
- MDA:
-
Malondialdehyde
- SEM:
-
Standard error means
- MMP:
-
Mitochondrial membrane potential
- NFkB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- LPO:
-
Lipid peroxidation
- SOD:
-
Superoxide dismutase
- HO:
-
Hydroxyl radical
References
Ahmed AH, Oswald RE (2010) Piracetam defines a new binding site for allosteric modulators of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J Med Chem 53(5):2197–2203. doi:10.1021/jm901905j
Akdur O, Kuçuk C, Durukan P (2008) The effect of piracetam on brain damage and serum nitric oxide levels in dogs submitted to hemorrhagic shock. TJTES 14(4):277–283
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. doi:10.1146/annurev.arplant.55.031903.141701
Bamber NI, Li H, Aebischer P, Xu XM (1999) Fetal spinal cord tissue in mini-guidance channels promotes longitudinal axonal growth after grafting into hemisected adult rat spinal cords. Neural Plast 6(4):103–121. doi:10.1155/NP.1999.103
Ben-Shaul V, Lomnitski L, Nyska A, Zurovsky Y, Bergman M, Grossman S (2001) The effect of natural antioxidants, NAO, and apocynin, on oxidative stress in the rat heart following LPS challenge. Toxicol Lett 123(1):1–10
Beurel E, Jope RS (2009) Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation 6(1):9. doi:10.1186/1742-2094-6-9
Bhattacharya SK, Sen AP, Upadhyay SN, Jaiswal AK (1993) Anxiolytic activity of piracetam, a nootropic agent, following subchronic administration in rodents. Indian J Exp Biol 31(11):902–907
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Brown RE, Corey SC, Moore AK (1999) Differences in measures of exploration and fear in MHC-congenic C57BL/6 J and B6-H-2 K mice. Behav Genet 29(4):263–271
Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB (2001) In-vivo measurement of activated microglia in dementia. Lancet 358(9280):461–467. doi:10.1016/S0140-6736(01)05625-2
Croisile B, Trillet M, Fondarai J, Laurent B, Mauguiere F, Billardon M (1993) Long term and high dose piracetam treatment of Alzheimer’s disease. Neurology 43(2):301–305
Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H (1992) A two-trial memory task with automated recording: study in young and aged rats. Brain Res 588(1):132–139
Escames G, León J, Macías M, Khaldy H, Acuña-Castroviejo D (2003) Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J 17(8):932–934. doi:10.1096/fj.02-0692fje
Evers S, Grotemeyer KH (1999) Piracetam and platelets. Pharmacopsychiatry. doi:10.1055/s-2007-979236
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J. Biol Chem 66:375–400
Giralt M, Gasull T, Blanquez A, Hidalgo J (1993) Effect of endotoxin on rat serum, lung and liver lipid peroxidation and on tissue metallothionein levels. Rev Esp Fisiol 49(2):73–78
Gong QH, Wang Q, Pan LL, Liu XH, Huang H, Zhu YZ (2010) Hydrogen sulfide attenuates lipopolysaccharide-induced cognitive impairment: a pro-inflammatory pathway in rats. Pharmacol Biochem Behav 96(1):52–58
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem 126(1):131–138
Greener J, Enderby P, Whurr R (2001) Pharmacological treatment for aphasia following stroke. The Cochrane Library. doi:10.1002/14651858.CD000424
Griffiths DE, Houghton RL (1974) Studies on energy linked reactions: modified mitochondrial ATPase of oligomycin resistant mutants of saccharomyces cerevisiae. Eur J Biochem 46(1):157–167
Gupta S, Verma DK, Biswas J, Raju KS, Joshi N, Singh S (2014) The metabolic enhancer piracetam attenuates mitochondrion-specific endonuclease G translocation and oxidative DNA fragmentation. Free Radic Biol Med 73:278–290. doi:10.1016/j.freeradbiomed.2014.05.014
Hauss-Wegrzyniak B, Lukovic L, Bigaud M, Stoeckel ME (1998) Brain inflammatory response induced by intracerebroventricular infusion of lipopolysaccharide: an immunohistochemical study. Brain Res 794(2):211–224
He Z, Liao Y, Zheng M, Zeng FD, Guo LJ (2008) Piracetam improves cognitive deficits caused by chronic cerebral hypoperfusion in rats. Cell Mol Neurobiol 28(4):613–627. doi:10.1007/s10571-007-9165-x
Huang SG (2002) Development of a high throughput screening assay for mitochondrial membrane potential in living cells. J Biomol Screen 7(4):383–389. doi:10.1089/108705702320351240
Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfiled DA, Banks WA (2009) Lipopolysaccharide alters the blood–brain barrier transport of amyloid β protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun 23(4):507–517. doi:10.1016/j.bbi.2009.01.017
Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG (2014) Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta Mol Basis Dis 1842(8):1282–1294. doi:10.1016/j.bbadis.2013.09.007
Joshi R, Garabadu D, Teja GR, Krishnamurthy S (2014) Silibinin ameliorates LPS-induced memory deficits in experimental animals. Neurobiol Learn Mem 116:117–131. doi:10.1016/j.nlm.2014.09.006
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21(2):130–132
Kalkan E, Keskin F, Kaya B, Esen H, Tosun M, Kalkan SS, Erdi F, Unlu A, Avunduk MC, Cicek O (2011) Effects of iloprost and piracetam in spinal cord ischemia–reperfusion injury in the rabbit. Spinal Cord 49(1):81–86
Keil U, Scherping I, Hauptmann S, Schuessel K, Eckert A, Müller WE (2006) Piracetam improves mitochondrial dysfunction following oxidative stress. Br J Pharmacol 147(2):199–208. doi:10.1038/sj.bjp.0706459
Kim SH, Johnson VJ, Shin TY, Sharma RP (2004) Selenium attenuates lipopolysaccharide-induced oxidative stress responses through modulation of p38 MAPK and NF-κB signaling pathways. Exp Biol Med 229(2):203–213
Koenigsknecht-Talboo J, Landreth GE (2005) Microglial phagocytosis induced by fibrillar β-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci 25(36):8240–8249. doi:10.1523/JNEUROSCI.1808-05.2005
Krishnamurthy S, Garabadu D, Joy KP (2013) Risperidone ameliorates post-traumatic stress disorder-like symptoms in modified stress re-stress model. Neuropharmacology 75:62–77. doi:10.1016/j.neuropharm.2013.07.005
Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT (2008) Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 5(1):1. doi:10.1186/1742-2094-5-37
Macdonald J, Galley HF, Webster NR (2003) Oxidative stress and gene expression in sepsis. Br J Anaesth 90(2):221–232
McGeer EG, McGeer PL (2003) Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27(5):741–749. doi:10.1016/S0278-5846(03)00124-6
Mehta AK, Bhati Y, Tripathi CD, Sharma KK (2014) Analgesic effect of piracetam on peripheral neuropathic pain induced by chronic constriction injury of sciatic nerve in rats. Neurochem Res 39(8):1433–1439. doi:10.1007/s11064-014-1329-8
Melchiorri D, Reiter RJ, Sewerynek E, Hara M, Chen L, Nisticò G (1996) Paraquat toxicity and oxidative damage: reduction by melatonin. Biochem Pharmacol 51(8):1095–1099
Moriau M, Crasborn L, Lavenne-Pardonge E, Von Frenckell R, Col-Debeys C (1993) Platelet anti-aggregant and rheological properties of piracetam. A pharmacodynamic study in normal subjects. Drug Res 43(2):110–118
Nalbandian RM, Henry RL, Burek CL, Diglio CA, Goldman AI, Taylor GW, Hoffman WH (1983) Diminished adherence of sickle erythrocytes to cultured vascular endothelium by piracetam. Am J Hematol 15(2):147–151
Noble F, Rubira E, Boulanouar M, Palmier B, Plotkine M, Warnet JM, Marchand-Leroux C, Massicot F (2007) Acute systemic inflammation induces central mitochondrial damage and mnesic deficit in adult Swiss mice. Neurosci Lett 424(2):106–110. doi:10.1016/j.neulet.2007.07.005
Nowak D, Pietras T, Antczak A, Krol M, Piasecka G (1993) Effect of bacterial lipopolysaccharide on the content of lipid peroxidation products in lungs and other organs of mice. Antonie Van Leeuwenhoek 63(1):77–83
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Owens T, Babcock AA, Millward JM, Toft-Hansen H (2005) Cytokine and chemokine inter-regulation in the inflamed or injured CNS. Brain Res Rev 48(2):178–184
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, vol 2. Academic Press, San Diego
Pedersen PL, Greenawalt JW, Reynafarje B, Hullihen J, Decker GL, Soper JW, Bustamente E (1978) Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol 20:411–481
Platt D, Horn J, Summa JD, Schmitt-Ruth R, Kauntz J, Kronert E (1993) On the efficacy of piracetam in geriatric patients with acute cerebral ischemia: a clinically controlled double-blind study. Arch Gerontol Geriatr 16(2):149–164
Poimenova A, Markaki E, Rahiotis C, Kitraki E (2010) Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience 167(3):741–749
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55(5):453–462. doi:10.1002/glia.20467
Sally LO, Margaret AJ (1989) The method of the micro photometric assay of succinate dehydrogenase and cytochrome-C oxidase activities for use on human skeletal muscle. Histochem J 21:545–555
Shapiro BL, Feigal RJ, Lam LF (1979) Mitrochondrial NADH dehydrogenase in cystic fibrosis. Proc Natl Acad Sci 76(6):2979–2983
Storrie B, Amadden E (1990) Isolation of subcellular organelles. Methods Enzymol 182:203–225
Vernon MW, Sorkin EM (1991) Piracetam An overview of its pharmacological properties and a review of its therapeutic use in senile cognitive disorders. Drugs Aging 1(1):17–35
Waegemans T, Wilsher CR, Danniau A, Ferris SH, Kurz A, Winblad B (2002) Clinical efficacy of piracetam in cognitive impairment: a meta-analysis. Dement Geriatr Cognit Disord 13(4):217–224
Winblad B (2005) Piracetam: a review of pharmacological properties and clinical uses. CNS Drug Rev 11(2):169–182
Acknowledgements
Alok Tripathi has received Postgraduate student fellowship from University Grants Commission (UGC), India through Indian Institute of Technology (Banaras Hindu University), Varanasi- 221005 U.P., India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Tripathi, A., Paliwal, P. & Krishnamurthy, S. Piracetam Attenuates LPS-Induced Neuroinflammation and Cognitive Impairment in Rats. Cell Mol Neurobiol 37, 1373–1386 (2017). https://doi.org/10.1007/s10571-017-0468-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0468-2