Abstract
Adult neurogenesis originates from neural stem cells (NSCs) in specific regions of the adult brain. The molecular mechanisms that control the self-renewal and multipotency of NSCs have not been fully elucidated. In recent years, emerging evidence has revealed that ten-eleven translocation (TET) family DNA dioxygenases TET1 and TET2 play important roles in the central nervous system. Here, I present evidence that Tet1 and Tet2 are expressed in cultured NSCs derived from adult mouse brain and play an important role in the proliferative self-renewal of NSCs in an undifferentiated state. The investigation of intracellular molecular networks involving both Tet1 and Tet2 by gene knockdown and comprehensive genetic analyses showed that overlapping molecular mechanisms involving TET1 and TET2 regulate the expression of at least 16 genes required for DNA replication and cell cycle control. Interestingly, transcriptional regulation of the selected gene through TET1 and TET2 did not correlate with direct CpG demethylation of the gene promoter. These findings suggest that TET1 and TET2 play an important role in the proliferation of NSCs in the adult mouse brain by specifically regulating common genes for DNA replication and the cell cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neural cells are generated from neural stem cells (NSCs). NSCs undergo self-renewal and differentiation during embryogenesis, and some NSCs remain in distinct regions of the adult brain to continue neurogenesis throughout life (Gage 2000; Alvarez-Buylla and Lim 2004). Newborn neurons are observed in the subventricular zone (SVZ) of lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus in the adult brain, which is referred to as adult neurogenesis driven by self-renewing adult NSCs. Sox2+/glial fibrillary acidic protein (GFAP)+/Nestin+ astrocyte-like cells in the SVZ produce neuronal progenitor cells that mature into interneurons of the olfactory bulb (Doetsch et al. 1999; Doetsch et al. 1997). Radial/horizontal shaped Sox2+/GFAP+/Nestin+/brain lipid-binding protein (BLBP)+ astrocyte-like cells in the SGZ produce neuronal progenitor cells that generate mature dentate granule neurons (Kempermann et al. 2004; Steiner et al. 2006; Lugert et al. 2010). Neurogenesis, especially in the SGZ, plays critical roles in antidepressant actions, learning, and pattern separation of memory (Zhao et al. 2008; Johnston et al. 2016). However, our understanding of the molecular mechanisms of how NSCs are a source of newborn neurons, self-renew at a constant rate, and produce newborn neurons in specific regions of the adult brain is incomplete.
DNA dioxygenase ten-eleven translocation (TET) family proteins, TET1–3, demethylate cytosine in genomic DNA and act as transcriptional activators with high enrichment at the transcriptional start site of target genes (Tahiliani et al. 2009; Cimmino et al. 2011). Conversely, TET1 plays a role as a transcriptional repressor by directly interacting with a co-repressor complex (Wu et al. 2011; Williams et al. 2011). Thus, it has been proposed that TET family genes have dual transcriptional regulatory functions (Cimmino et al. 2011). TET1 might play an important role in the self-renewal mechanism of embryonic stem (ES) cells (Ito et al. 2010; Freudenberg et al. 2012; Neri et al. 2015b) and the cellular transformation of tumors (Neri et al. 2015a; Wu and Brenner 2014). However, recent studies of TET1 functions indicate an important role in neuronal differentiation of the fetal mouse brain (Kim et al. 2016) and cognitive functions in hippocampal neurogenesis of the adult mouse brain (Zhang et al. 2013). Furthermore, several reports show that TET1 is important for the regulation of activity-dependent genes in neurons, memory formation, and extinction in mice (Kaas et al. 2013; Rudenko et al. 2013; Kumar et al. 2015). TET2 is suggested to be a causative gene of myeloma (Langemeijer et al. 2009; Delhommeau et al. 2009) and participate in the self-renewal mechanisms of hematopoietic stem cells (Moran-Crusio et al. 2011; Kunimoto et al. 2012). Furthermore, a recent study has indicated the requirement of TET2 for the cell survival mechanism of mouse neuronal cells (Mi et al. 2015).
The aim of this study was to explain a part of the stem cell-controlling mechanism of NSCs by clarifying the common transcriptional signaling networks of TET1 and TET2. I showed that TET1 and TET2 are expressed in cultured adult mouse NSCs and participate in the proliferation mechanisms of these cells. This study also provides evidence that at least 16 common genes associate with DNA replication and cell cycle-controlling mechanisms that function downstream of TET1 and TET2 as overlapping transcriptional regulation programs potentially not involving CpG demethylation in adult mouse NSCs.
Materials and Methods
Adult Neural Stem Cell Cultures, 5-Bromodeoxyuridine (BrdU) Treatment, and Cell Cycle Analysis
Cultured adult NSCs, which had been prepared from the whole forebrain of an adult mouse as described previously (Ray and Gage 2006; Zhang et al. 2008), were kindly provided by Dr. C.L. Zhang (University of Texas Southwestern Medical Center). The adult NSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 containing N2 supplement (Invitrogen), epidermal growth factor (EGF; 20 ng/ml), basic fibroblast growth factor (bFGF; 20 ng/ml), and heparin (5 µg/ml). To phenotypically analyze gene-depleted cells, pSUPER vectors (Oligoengine) expressing a scrambled sequence of short hairpin RNA (shRNA) or shRNAs against mouse Tet1 (5'-GCAGATGGCCGTGACACAAAT-3') or mouse Tet2 (5'-GGATGTAAGTTTGCCAGAAGC-3') genes driven by the H1 promoter and the puromycin resistance gene (Puro R) or the enhanced green fluorescent protein (EGFP) gene driven by the PGK promoter were transfected into the cultured adult NSCs by the Nucleofector system (Lonza). Transfected cells were selected with puromycin (Sigma-Aldrich). For the BrdU staining assay, the adult NSCs were treated with 10 µM BrdU for 1 or 24 h after transfection for 48 h with control, Tet1, or Tet2 shRNA-expressing vectors that co-expressed EGFP. The BrdU-treated cells were fixed with 4 % paraformaldehyde (PFA) and treated with HCl, followed by immunofluorescence staining with rat anti-BrdU (AhD Serotec) and rabbit anti-EGFP (MBL) antibodies. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The cells were analyzed using a PowerBX PLUS microscope (Olympus). For cell cycle analysis, DNA content in transfected GFP-positive cells stained with 7-AAD (SYTOX AADvanced, Invitrogen) was determined by flow cytometry (FACSCalibur; BD Biosciences) with CellQuest software.
Immunohistochemistry
Adult NSCs were fixed with 4 % PFA, treated with blocking solution (1 % donkey serum and 0.1 % Triton-X-100 in PBS) and then processed for immunostaining as described previously (Shimozaki et al. 2013). Primary antibodies included goat anti-TET1 (C-13; Santa Cruz Biotechnology), rabbit anti-TET2 (S-13; Santa Cruz Biotechnology), rabbit anti-Sox2 (Millipore Bioscience Research Reagents), goat anti-Sox2 (Y-17; Santa Cruz Biotechnology), chicken anti-Nestin (Aves Labs), and rabbit anti-active caspase 3 (BD Pharmingen) antibodies. Secondary antibodies were obtained from the Jackson Laboratory. Nuclei were counterstained with DAPI. Stained sections were visually analyzed using an LSM710 confocal microscope (Zeiss) or the PowerBX PLUS microscope (Olympus).
Gene Expression Analyses by Microarray and RT-PCR
Total RNAs were extracted from adult NSCs transfected with control, Tet1, or Tet2 shRNA-expressing vectors and selected with puromycin (Sigma-Aldrich) using an RNeasy kit (Qiagen). For global gene expression analysis, the one-color microarray analysis method was carried out using SurePrint G3 and GeneSpring GX (Agilent Technologies, Santa Clara, CA) by Hokkaido System Science Co., Ltd., Japan, according to the manufacturer’s protocol. Procedures for quantitative real-time RT-PCR (qRT-PCR) were essentially performed according to a previous report (Shimozaki et al. 2013). In brief, total RNAs were reverse transcribed using a ReverTra kit (TOYOBO). qRT-PCR was performed with a SYBR green Q-PCR analysis kit (TaKaRa). All samples were analyzed in quadruplicate for each experiment by a 7900HT (Applied Biosystems). The relative amounts of mRNAs were normalized to the glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA level. For conventional RT-PCR analysis, total RNAs from cultured adult NSCs reverse transcribed with or without reverse transcriptase were amplified with TaKaRa EX Taq HS (TaKaRa) using a GeneAmpR PCR System 9700 (Applied Biosystems) and separated by 1.5 % agarose/TBE gel electrophoresis. The primers used for RT-PCR analyses are listed in the Supplementary material 2.
Gene-Specific CpG Methylation Analysis by Bisulfite Genomic Sequencing
Bisulfite conversion of genomic DNA purified from the cultured adult NSCs was performed using an EZ DNA Methylation™ Kit (ZYMO RESEARCH) according to the manufacturer’s instructions. The target regions were amplified by the hot start-based PCR method with AmpliTaq GoldR (Thermo Fisher) and sub-cloned into a pCRR2.1-TOPOR vector (Invitrogen). The primers used for PCR in bisulfite sequencing were as follows: Ng2: 5′-TTTGGGGGTTTAAAGATTTAAG-3′, 5′-CCAAAATAAAAACCAAAACCA-3′ and Rad51: 5′-TAGAAGYGGAGTAGGTATGAGG-3′, 5′-RAACAACCRACTCTACACACACCACACTCC-3′. Randomly selected 10 clones were sequenced with M13-M3 and M13-RV primers.
Neurosphere Assay
The procedure for the neurosphere assay (NSA) has been reported previously (Louis et al. 2008). In brief, pSUPER.puro vectors (Oligoengine) expressing a scrambled sequence of shRNA or shRNAs against Tet1 or Tet2 with the Puro R gene were transfected into the cultured adult NSCs by the Nucleofector system. The transfected cells were selected with puromycin for 48 h in DMEM/F12 with N2 supplement, EGF (20 ng/ml), bFGF (20 ng/ml), and heparin (5 µg/ml). The selected cells were harvested using TrypLE™ Express (Gibco) at 3 days after transfection and plated at a density of 500 cells per 35-mm poly(2-hydroxyethyl methacrylate) (Sigma-Aldrich)-coated dish. The cells were clonally cultured for 7 days. Primary neurosphere colonies were harvested as described above, and 500 cells were cultured as secondary neurospheres in the NSA culture condition for 7 days. The number and diameter of primary and secondary neurospheres were scored and measured using AxioCam HRc/AxioVision 3.1 (Zeiss) with an ECLIPSE TS100 microscope (Nikon).
Statistical Analysis
Statistical analysis of the data was performed with one- or two-way analysis of variance (ANOVA) followed by Dunnett’s or Sidak’s multiple comparison test using GraphPad Prism 7. Data are shown as the mean ± standard deviation (SD) or standard error of mean (SEM). ****P ≤ 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05 denote statistical significance.
Results
TET1 and TET2 Participate in the Proliferation Mechanism of Cultured NSCs Derived from an adult Mouse Brain
A previous study that analyzed the role of TET1 in ES cells showed the expression of all TET family genes (Tet1–3) in the mouse brain and NSCs (Ito et al. 2010). To investigate how TET1 and TET2 may be related to the stemness of adult NSCs, monolayer-cultured NSCs were used in this study. The cells were isolated from the whole forebrain of an adult Tlx f/Z mouse by density gradient centrifugation and β-gal-based fluorescence-activated cell sorting, and then selected by G418 treatment. These cells contained the lacZ and neomycin resistance genes under the control of the endogenous promoter of the Tlx/Nr2e1 gene, an essential nuclear factor for the stemness of NSCs (Zhang et al. 2008). The expression of all TET family genes was confirmed in the cultured adult NSCs by conventional RT-PCR analysis, which had been selected according to the activity of the Tlx/Nr2e1 gene promoter (Supplementary material 1). Immunostaining and confocal microscopy of TET1 and TET2 proteins were performed in the NSCs to determine their cellular localization. As shown in Fig. 1a (left), TET1 proteins were expressed in the nuclei and cytosol of all Sox2+/Nestin+ adult NSCs. In addition, TET2 proteins were co-localized with Sox2 and Nestin proteins in the adult NSCs (Fig. 1a, right). These data indicate the expression of Tet1 and Tet2 genes in cultured NSCs derived from an adult mouse brain and their relatively broad localization.
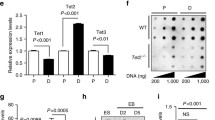
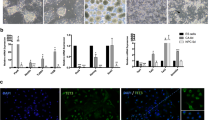
TET1 and TET2 control the self-renewal of mouse NSCs. a Expression of TET1 and TET2 proteins in cultured adult NSCs derived from an adult mouse brain. In the merged image, TET1 and TET2 (white, red, or green) proteins were expressed in both the cytoplasm and nuclei of adult NSCs, together with the intermediate filament protein Nestin (red) and nuclear protein Sox2 (green). Nuclei were visualized by DAPI (blue) staining. Scale bars, 20 µm. b Expression of Tet1 and Tet2 mRNAs in cultured adult NSCs and gene knockdown by sequence-specific shRNAs. Expression vectors for scrambled control (CTRL), Tet1 (Tet1KD), or Tet2 (Tet2KD) shRNAs were transfected into cultured adult NSCs, and then the cells were selected for 48 h with puromycin. Total RNA from these selected cells was analyzed by qRT-PCR. Data are represented as mean ± SD. ****P < 0.0001; two-way ANOVA with Sidak’s multiple comparison test. Quantitative analysis of the number (c) and diameter (d) of primary and secondary neurospheres in an NSA. Knockdown vectors for control (CTRL), Tet1 (Tet1KD), or Tet2 (Tet2KD) shRNAs with the Puro R gene were transfected into the cultured adult NSCs, followed by selection with puromycin for 48 h. The selected cells were harvested and clonally cultured under floating conditions. Assays were performed in triplicate, and the numbers of primary and secondary neurospheres were scored. The diameter of each neurosphere was measured (n = 10). Data are represented as mean ± SD. ***P < 0.001, **P < 0.01, *P < 0.05; one-way ANOVA with Dunnett’s multiple comparison test. e Quantitative analysis of early cell death after gene knockdown. Expression vectors for CTRL, Tet1KD, or Tet2KD shRNAs were transfected into cultured adult NSCs. The cells were fixed with 4 % PFA at 48, 72, and 96 h after transfection and then stained with anti-EGFP and anti-active caspase 3 antibodies, and DAPI. Each positive cell was then counted in six independent microscopic fields. The percentage of GFP and active caspase 3 double-positive cells to GFP-positive cells is shown. White, gray, and light gray bars indicate CTRL, Tet1KD, and Tet2KD, respectively. Data are represented as mean (n = 6) ± SEM. ****P ≤ 0.0001, *P < 0.05; two-way ANOVA with Dunnett’s multiple comparison test. f Quantification of BrdU labeling in NSCs. Expression vectors for CTRL, Tet1KD, or Tet2KD shRNAs were transfected into cultured adult NSCs for 48 h, and then the cells were labeled with 10 μM BrdU for 1 or 24 h. The cells were fixed with 4 % PFA and then stained with anti-EGFP (green) and anti-BrdU (red) antibodies, and DAPI (blue). Each positive cell was then counted in three independent microscopic fields. Arrows indicate GFP and BrdU double-positive cells in the representative views. Insets are enlargements of stained cells. The percentage of GFP and BrdU double-positive cells to GFP-positive cells is shown. Data are represented as mean ± SD. ****P ≤ 0.0001; one-way ANOVA with Dunnett’s multiple comparison test
To investigate the functions of TET1 and TET2 in cultured adult NSCs, expression vectors were constructed to coexpress EGFP or Puro R genes and a scrambled control shRNA (CTRL) or shRNA sequences against Tet1 (Tet1KD) or Tet2 (Tet2KD), according to a previous report (Ito et al. 2010). The vectors were transfected into the cultured adult NSCs, followed by selection with puromycin under the NSC culture condition for 48 h. Total RNA in each transfectant was extracted, and quantitative measurement of the expression of endogenous Tet1/2 mRNAs was carried out by qRT-PCR to determine the knockdown efficiency in the cultured adult NSCs. As shown in Fig. 1b, both Tet1 and Tet2 mRNAs were reduced by the specific shRNAs. As a result, the knockdown efficiencies of Tet1 and Tet2 mRNAs were 68.9 ± 2.8 and 90.7 ± 0.6 %, respectively (both P < 0.0001; Fig. 1b).
Next, the growth activity of NSCs was examined after Tet1 or Tet2 knockdowns using the NSA. Untransfected adult NSCs were eliminated by puromycin. The puromycin-selected, knocked down NSCs were then cultured clonally under the floating condition for 7 days. Primary neurospheres were enzymatically dissociated into single cells and cultured again at a clonal cell density. As shown in Fig. 1c, both Tet1 and Tet2 knockdown NSCs showed obvious reductions in the numbers of primary neurospheres (left graph: Tet1KD, P = 0.0056; Tet2KD, P = 0.0004) and secondary neurospheres (right graph: Tet1KD, P = 0.0008; Tet2KD, P = 0.0001) compared with control shRNA transfectants. Neurospheres with knocked down Tet1 or Tet2 also exhibited a significant reduction in their diameters compared with control neurospheres and both types of primary neurospheres (left graph: Tet1KD, P = 0.0235; Tet2KD, P = 0.0091) and secondary neurospheres (right graph: Tet1KD, P= 0.0166; Tet2KD, P = 0.0040) (Fig. 1d). To further investigate the effect of Tet1 or Tet2 knockdown on NSC proliferation, adult NSCs transfected with knockdown vectors were immunostained with the anti-active caspase 3 antibody to measure the apoptosis rate that affects the outcome of cell proliferation analysis. In fact, TET2 has been reported to be a cell survival factor of mouse neuronal cells (Mi et al. 2015). Although approximately <8 % of Tet2KD-transfected cells showed an apoptotic response from 72 h (P = 0.0419) to 96 h (P = 0.0001) after transfection, there were not obvious active caspase 3-positive cells among Tet2KD knockdown vector transfectants within 48 h after transfection (P = 0.8236) (Fig. 1e). Moreover, there was no significant difference between CTRL and Tet1KD (48 h: P = 0.5341; 72 h: P = 0.7951; 96 h: P = 0.1883) transfectants. To further define the roles of TET1 and TET2 in NSC proliferation, the rate of BrdU uptake was measured in NSCs transfected with the knockdown vectors to investigate whether TET1 and TET2 participate in the proliferative self-renewal mechanism of adult NSCs at the point not influenced by cell death. Adult NSCs were transfected with shRNA vectors for 48 h, and then BrdU was added to the culture medium (1 or 24 h). These time points were chosen because the NSCs were unaffected by cell death. The cells were immunostained with anti-BrdU and anti-GFP antibodies after BrdU incorporation (representative images: Fig. 1f, top panel). As shown in the graph in Fig. 1f, the BrdU uptake rate at 1 h in adult NSCs transfected with the scrambled control vector was 16.1 ± 3.0 %. However, there were no BrdU+ cells among GFP-positive, Tet1KD, and Tet2KD cells at 48 h after transfection (P = 0.0001). In addition, adult NSCs transfected with the scramble control vector showed a high rate of BrdU uptake (88.4 ± 8.3 %), whereas Tet1KD (0 %) and Tet2KD (6.5 ± 8.3 %) cells had low rates of BrdU labeling at 24 h (P = 0.0001). These data indicate less possibility of bias in the BrdU labeling, such as limited detection of rapidly dividing cells in this system, and that the reduction of Tet1 and Tet2 mRNAs reduced cell proliferation independent of an apoptotic cellular response. Based on these results, TET1 and TET2 may be involved in the mechanism of cultured adult NSC proliferation.
TET1 and TET2 are Involved in Cell Cycle Control of Cultured Adult NSCs
Next, to further elucidate the involvement of TET1 and TET2 in the proliferation mechanism of cultured adult NSCs, the cell cycle was analyzed after knockdown. The knockdown vectors for coexpression of shRNAs against Tet1 or Tet2 and EGFP were introduced into the cultured NSCs, and then the quantity of DNA was measured in EGFP-positive cells by flow cytometric analysis. As shown in Fig. 2, cells in G0/G1, S, and G2/M phases of the control were 61.8 ± 4.4, 16.0 ± 1.6, and 19.6 ± 1.9 %, respectively. Cells in G0/G1 phase among Tet1 (Tet1KD: 90.3 ± 3.5 %, P = 0.0001) and Tet2 (Tet2KD: 83.4 ± 0.7 %, P = 0.0001) knockdown NSCs were increased in comparison with the control. Conversely, cells in S phase (Tet1KD: 4.3 ± 0.6 %, P = 0.0001; Tet2KD: 7.5 ± 2.3 %, P = 0.0009) and G2/M phase (Tet1KD: 3.7 ± 2.5 %, P = 0.0001; Tet2KD: 8.4 ± 1.9 %, P = 0.0001) were decreased among each type of knockdown cell. These data suggest that TET1 and TET2 participate in execution of S and G2/M phases in cultured NSCs.
TET1 and TET2 are involved in the regulation of cell cycle in cultured adult NSCs. After the introduction of the vectors for control (CTRL), Tet1KD, or Tet2KD shRNAs, which were co-expressed with EGFP, into cultured adult NSCs for 48 h, the DNA contents of the EGFP-positive cells were analyzed by flow cytometry. White, gray, and light gray bars indicate CTRL, Tet1KD, and Tet2KD, respectively. Assays were performed in triplicate. Data are represented as mean ± SD. ****P ≤ 0.0001, ***P < 0.001; two-way ANOVA with Dunnett’s multiple comparison test
Tet1 or Tet2 Gene Knockdowns Influence the Expression of Undifferentiated NSC Marker Genes in Adult NSCs
To assess the undifferentiated state of the NSCs after gene knockdowns, the expression of marker genes expressed in undifferentiated NSCs was measured by qRT-PCR. Cells in specific regions of the adult brain, which express Gfap, Blbp, Nestin, and Sox2, are assumed to be NSCs. Expression of Gfap, Blbp, and Nestin genes represents a state of stemness in NSCs. A lack of Sox2 is known to have a significant effect on the self-renewal mechanisms of adult NSCs and decreases the efficiency of neurogenesis. When Tet1 or Tet2 knockdowns were compared with the control shRNA, the expression of Sox2 was not influenced by the knockdowns (ANOVA between columns, P = 0.0892), but the relative mRNA levels of Gfap (Tet1KD: 21.9 ± 1.9 %; Tet2KD: 21.3 ± 1.6 %), Blbp (Tet1KD: 52.5 ± 1.3 %; Tet2KD: 60.5 ± 1.8 %), and Nestin (Tet1KD: 30.9 ± 0.9 %; Tet2KD2: 60.1 ± 1.8 %) were decreased by the gene knockdowns (Fig. 3, P = 0.0001). These results suggest that reducing the gene dosage of Tet1 and Tet2 changes the undifferentiated state of NSCs, even in the culture condition that maintains the undifferentiated state.
Gene knockdowns of Tet1 or Tet2 reduce the expression of some undifferentiated NSC marker genes. qRT-PCR analysis of marker genes for the undifferentiated state of NSCs was performed in adult NSCs with gene knockdowns of Tet1 or Tet2. shRNA expression vectors for control (CTRL), Tet1 (Tet1KD), or Tet2 (Tet2KD) were transfected into cultured adult NSCs, followed by culture for 48 h with puromycin. Total RNA from the selected cells was analyzed by qRT-PCR. Data are represented as mean ± SD. ****P ≤ 0.0001; one-way ANOVA with Dunnett’s multiple comparison test
TET1 and TET2 Control a Part of Overlapping Gene Expression in Cultured Adult NSCs
To search for genes commonly affected by TET1 and TET2 in the regulation of NSC proliferation, cultured adult NSCs with Tet1 or Tet2 knockdown were analyzed by DNA microarray. Although the heat map showed that the patterns of gene expression were considerably different in Tet1KD and Tet2KD cells (Fig. 4a), genes with common changes were observed in both Tet1KD and Tet2KD cells as shown in the Venn diagram by analyzing the genes with Agilent software (1856 upregulated genes and 982 downregulated genes; Fig. 4b). Next, these overlapping genes were subjected to pathway analysis through Gene Ontology. As shown in Fig. 4c, the upper-level genes with common increased expression by Tet1 or Tet2 gene knockdown were classified into the categories of “Endochondral Ossification,” “Focal Adhesion,” “Prostaglandin Synthesis and Regulation,” “Senescence and Autophagy,” and “Osteoclast.” Furthermore, higher genes of the common downregulated genes were in the categories of “DNA replication,” “G1 to S cell cycle control,” “Cell cycle,” “Monoamine GPCRs,” and “Apoptosis” (DNA microarray data can be supplied on request). TET family proteins have been reported to have dual functions in the transcriptional regulatory mechanism (Cimmino et al. 2011). This study focused on the top three categories of common downregulated gene because genes belonging to these categories are factors involved in DNA replication machinery and cell cycle control that could be concerned with the proliferative self-renewal of NSCs. Furthermore, the relative expression of genes listed in the top three categories of the pathway analysis (Fig. 4c) was measured in the cultured adult NSCs after knockdown of Tet1 or Tet2 mRNAs by qRT-PCR. Finally, 16 genes were defined as common genes regulated by TET1 and TET2 (Fig. 5, P = 0.0001). The DNA replication factors, Mcm2 (Tet1KD: 42.8 ± 3.4 %; Tet2KD: 33.6 ± 2.0 %), Mcm3 (Tet1KD: 34.7 ± 2.8 %; Tet2KD: 28.0 ± 1.5 %), Mcm4 (Tet1KD: 36.1 ± 1.3 %; Tet2KD: 36.6 ± 2.5 %), Mcm5 (Tet1KD: 30.9 ± 0.9 %; Tet2KD: 29.0 ± 0.9 %), and Mcm6 (Tet1KD: 49.4 ± 1.6 %; Tet2KD: 35.2 ± 3.0 %), which form a double-hexamer complex with Mcm7, induce the replication of DNA dependent on a cell cycle-controlling system. Mcm10 (Tet1KD: 25.0 ± 0.7 %; Tet2KD: 25.5 ± 2.9 %) is involved in the DNA replication-starting mechanism in which the protein binds to Mcm2 and Mcm6. Ccne1 (Tet1KD: 18.9 ± 0.5 %; Tet2KD: 29.4 ± 3.2 %) is a cyclin-dependent kinase, and Cdt1 (Tet1KD: 19.6 ± 1.2 %; Tet2KD: 24.5 ± 3.5 %) is a DNA replication-licensing factor. Pole (Tet1KD: 30.5 ± 1.0 %; Tet2KD: 29.8 ± 1.7 %) and Pole2 (Tet1KD: 42.7 ± 1.8 %; Tet2KD: 48.5 ± 2.9 %) are DNA polymerase ε, and DNA replication activator Rfc4 (Tet1KD: 50.0 ± 1.2 %; Tet2KD: 33.8 ± 2.0 %) is required for POLE activity with the DNA replication origin recognition complex Orc1 (Tet1KD: 42.2 ± 3.4 %; Tet2KD: 35.5 ± 2.0 %). The results of qRT-PCR also showed the expression of DNA double-strand brake repair factor Rad51 (Tet1KD: 31.8 ± 1.4 %; Tet2KD: 30.0 ± 2.3 %), cell cycle regulator and tumor suppressor E2f2 (Tet1KD: 33.4 ± 4.6 %; Tet2KD: 38.4 ± 4.3 %), M phase regulator Mad2l1 (Tet1KD: 26.0 ± 0.8 %; Tet2KD: 36.0 ± 1.2 %), and proto-oncogene c-Myc (Tet1KD: 38.6 ± 5.8 %; Tet2KD: 45.7 ± 3.6 %).
Comparative analysis by DNA microarray between Tet1 and Tet2 gene knockdowns in cultured adult NSCs. a Hierarchical clustering showing the relationship of control (C), Tet1 (T1)- or Tet2 (T2)-depleted adult NSCs. Red and blue bars indicate up- and downregulation of gene expression, respectively. b Venn diagram after DNA microarray comparative analysis of upregulated genes (Tet1KD: 2584; Tet2KD: 2758) and downregulated genes (Tet1KD: 1710, Tet2KD: 1884 genes) after Tet1 or Tet2 gene knockdowns in cultured adult NSCs. Red and blue circles indicate Tet1KD and Tet2KD, respectively. The number of overlapping genes (indicated in the purple area) is shown in the diagram. c Pathway categories of overlapping genes between Tet1KD and Tet2KD in comparative analysis by DNA microarray
Gene expression of Tet1- and Tet2-dependent common molecular signaling pathways in cultured adult NSCs. Expression vectors for control (CTRL), Tet1 (Tet1KD), or Tet2 (Tet2KD) shRNAs were transfected into cultured adult NSCs, followed by selection with puromycin for 48 h. The common downregulated genes between Tet1KD and Tet2KD cells identified by comparative DNA microarray analysis in the categories “DNA Replication,” “G1 to S cell cycle control,” and “Cell cycle” were analyzed by qRT-PCR. Data are represented as mean ± SD. ****P ≤ 0.0001; one-way ANOVA with Dunnett’s multiple comparison test
The Decline of Transcriptional Activity by Tet1 or Tet2 Knockdown is not Correlated with CpG Methylation Status on the 5′ Proximal Promoter Region in Cultured Adult NSCs
A relationship between transcriptional activity and CpG methylation status in the 5′ proximal promoter region of galanin, neural/glial antigen 2 (Ng2), and neuroglobin (Ngb) genes has previously been reported in nestin-GFP-positive progenitor cells from Tet1 knockout mice (Zhang et al. 2013). Ng2 and the galanin gene are involved in the regulation of neurogenesis (Abbosh et al. 2011; Kucharova and Stallcup 2010), and Ngb is thought to be a neuroprotective hypoxia-inducible factor during ischemic injury (Sun et al. 2001). To assess the effect of Tet1 or Tet2 knockdown in this study, relative mRNA amounts of galanin, Ng2, and Ngb were quantified by qRT-PCR in cultured adult NSCs after Tet1 or Tet2 knockdown. As shown in Fig. 6a, the relative amount of Ng2 mRNA decreased after Tet1 or Tet2 knockdown (Tet1KD: 42.7 ± 5.7 %; Tet2KD: 32.2 ± 2.8 %, P < 0.0001). However, galanin mRNA expression was only reduced after Tet1KD (55.4 ± 5.2 %, P = 0.0008) but not Tet2KD (124 ± 12 %, P = 0.0273), while Ngb mRNA showed no reduction in either Tet1KD (P = 0.5800) or Tet2KD (236 ± 16 %, P = 0.0001) cells. Therefore, the CpG methylation status was examined by bisulfite sequencing in the 5′ proximal promoter region of Ng2 (spanning from −276 to +149), which was the region referred to in the Tet1KO study (Zhang et al. 2013). Additionally, the CpG methylation status was examined in the 5′ proximal promoter region (spanning from −61 to +177) of Rad51, which was selected as one of the 16 genes commonly regulated by TET and TET2 in this study and significantly reduced in both Tet1KD and Tet2KD NSCs (Fig. 5). The CpG sites in the 5′ proximal promoter are represented as circles, and each line represents the bisulfite sequencing subclones. The positions of methylated (closed circle) and unmethylated (open circle) CpGs are indicated in Fig. 6b, and the sum of methylated CpGs in the analyzed subclones was as follows: Ng2 (CTRL: 0.8 %; Tet1KD: 12.8 %; Tet2KD: 3.6 %) and Rad51 (CTRL: 0.4 %; Tet1KD: 0.4 %; Tet2KD: 0.4 %). Based on these data, a statistical assessment of the ratio of CpG methylation in the 10 bisulfite sequencing subclones was performed. Although the expression rates for Ng2 and Rad51 showed a marked decrease after Tet1 or Tet2 knockdown (ANOVA between columns, P < 0.0001), no significant difference was detected between control and knockdown samples for both Ng2 (ANOVA between columns, P = 0.2743) and Rad51 (ANOVA between columns, P = 0.3811) in these specimens (Fig. 6c).
Analysis of transcriptional activity and CpG methylation status of the selected genes in cultured adult NSCs. Adult NSCs transfected with expression vectors for control (CTRL), Tet1KD, or Tet2KD shRNAs were selected by the administration of puromycin for 48 h. Purified total RNA or genomic DNA from selected cells was analyzed by qRT-PCR or gene-specific bisulfite sequencing. a Evaluation of assumed TET1 regulatory factors by qRT-PCR in Tet1 or Tet2 knockdown NSCs. Data are represented as mean ± SD. ****P ≤ 0.0001, ***P < 0.001, *P < 0.05; one-way ANOVA with Dunnett’s multiple comparison test. b Schematic of CpG sites in each promoter. Open and closed circles represent unmethylated and methylated CpG sites after Tet1 or Tet2 knockdown, respectively. c Heat map comparison between gene expression and CpG methylation. The data for Rad51 and Ng2 expression in Figs. 5 and 6a together with CpG methylation rates calculated from the data in Fig. 6b were used to construct the heat maps. The left and right blocks show Ng2 and Rad51, respectively (top panels: gene expression; bottom panels: CpG methylation). Relative expression rates of Ng2 and Rad51, and CpG methylation rates of each bisulfite sequencing subclone in the 5′ proximal promoter region of Ng2 and Rad51 after Tet1 of Tet2 knockdown were tested with one-way ANOVA
Discussion
Adult neurogenesis is involved in multiple critical physiological processes such as learning, pattern separation of memory, and antidepressant actions (Clelland et al. 2009; Sahay et al. 2011; Nakashiba et al. 2012; Santarelli et al. 2003; Snyder et al. 2011). Neurogenesis is strictly controlled by NSCs. NSCs possess multipotency to differentiate into neuronal, astrocyte, and oligodendrocyte lineages while maintaining the ability for self-renewal (Gage 2000; Yao et al. 2012). A promising approach to elucidate the mechanisms underlying adult neurogenesis is to determine the molecular mechanisms by which NSCs maintain steady self-renewal or differentiate into neurons when appropriate. The aim of this study was to reveal the mechanism underlying proliferative NSC self-renewal. An important issue in achieving this aim is whether various stem cell types, such as NSCs, ES cells, and hematopoietic stem cells, share a common mechanism for self-renewal while maintaining their differentiation potency. The characteristics of NSCs also change depending on the developmental stage. For example, NSCs primarily generate neurons during early embryogenesis, astrocytes during mid to late embryogenesis, and oligodendrocytes after birth (Hitoshi et al. 2011; Takizawa et al. 2001; Namihira et al. 2009; Kondo and Raff 2004). A key mechanism involved in this developmental program-dependent plasticity of NSCs has been proposed to be DNA methylation, especially 5-methylcytosine (5-mC) of CpG islands, which leads to qualitative changes in the control of gene expression (Coskun et al. 2012; Olynik and Rastegar 2012; Montalban-Loro et al. 2015). A recent report indicated the existence of 5-hydroxymethylcytosine (5-hmC) and the importance of conversion of 5-mC to 5-hmC in the brain, and TET family proteins catalyze this reaction (Kriaucionis and Heintz 2009; Tahiliani et al. 2009). Analyses of 5-hmC in the mouse hippocampus and cerebellum, and human cerebellum revealed that 5-mC has crucial roles in the regulation of postnatal neural development, aging, and neurological disorders (Szulwach et al. 2011).
TET1 and TET2 are oxidases with DNA demethylase and transcriptional control activities. This study provides evidence that they are expressed in NSCs and involved in stem cell proliferation (Figs. 1 and 2). As shown in Fig. 1a, both TET1 and TET2 were expressed in the nucleus and cytoplasm. Although TET2 protein has been shown to shuttle between the nucleus and cytoplasm of cancer cells (Huang et al. 2016), this shuttling of TET proteins may reflect a common mechanism for the proliferative self-renewal of cultured NSCs and the proliferation of cancer cells. Tet1 or Tet2 knockdown resulted in reduced neurosphere formation (Fig. 1c, d) and BrdU incorporation (Fig. 1f). Tet2 knockdown slightly affected cell viability, but the suppression of cell proliferation was rapid because it occurred within 48 h after knockdown, which was before the apoptotic response. Although reports indicate that morphogenesis is not significantly affected in Tet1 or Tet2 single- or double-knockout mice, some effect has been observed on cell proliferation (Wiehle et al. 2015; Zhao et al. 2015; Dawlaty et al. 2013). Others have reported impairment of proliferative self-renewal in ES cells by Tet1 knockdown (Freudenberg et al. 2012; Ito et al. 2010). The difference in phenotypes between gene knockdown and knockout is an interesting phenomenon. The cellular context must differ between abrupt blockade of gene expression and the absence of the gene from the beginning. Stem cells may have great flexibility to respond to the loss of a gene.
Comprehensive examination of gene expression was conducted by DNA microarray analysis of NSCs after single Tet1 or Tet2 knockdowns. Among the altered genes in both types of knockdown cells, many that had reduced expression relative to control cells belonged to pathways involved in DNA replication and cell cycle control (Fig. 4c). In this study, expression of the selected representative 16 genes showed comparable reductions in Tet1 or Tet2 knockdown cells relative to control cells (Fig. 5). DNA replication and recombination repair require DNA helicase and polymerase. These enzymes form the replication fork at the initiation of replication and cooperatively function with cell cycle control machinery. Minichromosome maintenance (Mcm) proteins 2−7 form a heterohexamer that functions as a DNA helicase in eukaryotic DNA replication (Lei and Tye 2001; Lygerou and Nurse 2000; Forsburg 2004; Tye and Sawyer 2000; Labib et al. 2000). The complex of Mcm2, Mcm6, and Mcm10 plays a critical role in the formation of the replication fork and is controlled by cell cycle-dependent protein degradation (Watase et al. 2012; Kanke et al. 2012; Izumi et al. 2000; Chattopadhyay and Bielinsky 2007). The origin recognition complex (ORC) forms a DNA replication complex with Cdc6 and Mcm proteins during G1 phase (Kneissl et al. 2003; Eki et al. 1996). A DNA polymerase that plays an important role in DNA replication is Pol ε (a member of the B polymerase family) encoded by POLE1–3 genes (Johnson et al. 2015; Edwards et al. 2003). Rfc4 is the 37 kDa subunit of Rfc, which, along with proliferating cell nuclear antigen, is required for DNA synthesis by Pol δ and ε (Okumura et al. 1995). DNA damage can be caused by extrinsic factors in the environment, including radiation, and by cell-intrinsic factors such as DNA replication errors. Upon DNA damage, Rad51 contributes to genome stability as a part of the DNA damage repair pathway (Shinohara et al. 1992; Shinohara et al. 1993). Mad2 and 1 are components of the M phase checkpoint control machinery in the cell cycle (Li and Benezra 1996). Vascular endothelial growth factor (VEGF) also plays an important role in the self-renewal of adult NSCs (Fabel et al. 2003; Kirby et al. 2015). Studies indicate that VEGF facilitates G1/S transition by upregulation of E2f1, E2f2, and E2f3 (Zhu et al. 2003). Cdt1 and its binding partner, geminin, are essential regulators of the Mcm2−7 complex binding to the replication origin during G1 phase (Lygerou and Nurse 2000). Cdt1 is expressed in Sox2-positive neural precursor cells in the mouse central nervous system from embryogenesis to adulthood (Spella et al. 2007). The increase of G0/G1 transition in NSCs by Tet1 or Tet2 knockdown shown in Fig. 2 presumably resulted from reduced levels of molecules that drive G1 phase. For the proliferative self-renewal of NSCs, efficient DNA replication requires adequate expression levels of genes involved in stemness. If intracellular concentrations of necessary factors are too low, the replication efficiency would be impaired. In addition, DNA repair enzymes are necessary to minimize errors associated with replication. The intracellular concentrations of these enzymes need to be maintained at adequate levels. It is possible that TET1 and TET2 maintain high expression levels of these genes in NSCs by broadly regulating DNA replication and repair enzymes, thereby contributing to the fidelity of proliferative NSC self-renewal.
TET1 and TET2 oxidize 5-mC to form 5-hmC. The oxidation product 5-hmC can be further converted to cytosine. To determine how TET1 and TET2 control DNA replication and cell cycle regulatory factors in NSCs, the CpG methylation status was analyzed on the promoter regions of Ng2 and Rad51 as shown in Fig. 6. Interestingly, while Ng2 and Rad51 mRNA reduced efficiently within 72 h after gene knockdown, the change in the CpG methylation pattern of the promoter regions seemed to be insufficient to correlate with gene reduction during this period (Fig. 6b, c). Therefore, these results implicate the involvement of TET1 and TET2 in the transcriptional control of DNA repair and cell cycle regulatory factors through mechanisms not involving direct CpG demethylation on these promoters in cultured adult NSCs. Considering the molecular mechanism, one hypothesis is that TET1 and TET2 directly function as a transcriptional activator without a DNA demethylation activity in an undefined fashion. Another possibility is that they function as a modifying enzyme with other proteins such as a suppressor complex. In fact, TET1 interacts with co-repressor Sin3a and recruits polycomb repressive complex 2 (PRC2) to function as a transcriptional suppressor (Williams et al. 2011; Wu et al. 2011; Xu et al. 2011). Thus, it could be speculated that the TET1/Sin3a/PRC2 complex controls factors that inhibit the transcription of cell cycle regulatory genes. The precise mechanisms by which TET1 and TET2 regulate the promoters of DNA replication factors remain unknown. However, it would be worth studying how TET1 and TET2 control the proliferation of NSCs by direct or indirect transcriptional control. Investigation of demethylation-independent transcriptional control by TET1 and TET2 with a focus on the regulatory factors will contribute to a greater understanding of the mechanisms of TET1- and TET2-mediated regulation of neurogenesis.
The expression of marker genes for NSCs, such as Nestin, Gfap, and Blbp, was decreased after knockdowns of Tet1 or Tet2 even in the culture condition to maintain the undifferentiated state (Fig. 3). These data suggest a transition of the character of NSCs from undifferentiated to a prematurely differentiated state after knockdown. As shown in the DNA microarray analysis (Fig. 4a, b), large numbers of genes were regulated independently between TET1 and TET2. I showed that at least 16 genes were commonly controlled by TET1 and TET2 in the regulation of NSC proliferation in this research, but the molecular mechanisms for inhibition of differentiation processes in NSCs by TET1 and TET2 remain to be elucidated. Moreover, common molecular pathways between TET1 and TET2 for asymmetric cell division during differentiation of NSCs should be analyzed in detail in the future. Although further studies are needed to fully understand the mechanisms by which TET1 and TET2 control DNA replication and the cell cycle in NSCs, drug discovery based on the molecular targets found in this study could lead to treatments for depression and impaired memory by pharmacologically controlling adult neurogenesis.
References
Abbosh C, Lawkowski A, Zaben M, Gray W (2011) GalR2/3 mediates proliferative and trophic effects of galanin on postnatal hippocampal precursors. J Neurochem 117(3):425–436. doi:10.1111/j.1471-4159.2011.07204.x
Alvarez-Buylla A, Lim DA (2004) For the long run: maintaining germinal niches in the adult brain. Neuron 41(5):683–686
Chattopadhyay S, Bielinsky AK (2007) Human Mcm10 regulates the catalytic subunit of DNA polymerase-alpha and prevents DNA damage during replication. Mol Biol Cell 18(10):4085–4095. doi:10.1091/mbc.E06-12-1148
Cimmino L, Abdel-Wahab O, Levine RL, Aifantis I (2011) TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell 9(3):193–204. doi:10.1016/j.stem.2011.08.007
Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325(5937):210–213. doi:10.1126/science.1173215
Coskun V, Tsoa R, Sun YE (2012) Epigenetic regulation of stem cells differentiating along the neural lineage. Curr Opin Neurobiol 22(5):762–767. doi:10.1016/j.conb.2012.07.001
Dawlaty MM, Breiling A, Le T, Raddatz G, Barrasa MI, Cheng AW, Gao Q, Powell BE, Li Z, Xu M, Faull KF, Lyko F, Jaenisch R (2013) Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev Cell 24(3):310–323. doi:10.1016/j.devcel.2012.12.015
Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, Kosmider O, Le Couedic JP, Robert F, Alberdi A, Lecluse Y, Plo I, Dreyfus FJ, Marzac C, Casadevall N, Lacombe C, Romana SP, Dessen P, Soulier J, Viguie F, Fontenay M, Vainchenker W, Bernard OA (2009) Mutation in TET2 in myeloid cancers. N Engl J Med 360(22):2289–2301. doi:10.1056/NEJMoa0810069
Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A (1997) Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17(13):5046–5061
Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97(6):703–716
Edwards S, Li CM, Levy DL, Brown J, Snow PM, Campbell JL (2003) Saccharomyces cerevisiae DNA polymerase epsilon and polymerase sigma interact physically and functionally, suggesting a role for polymerase epsilon in sister chromatid cohesion. Mol Cell Biol 23(8):2733–2748
Eki T, Okumura K, Amin A, Ishiai M, Abe M, Nogami M, Taguchi H, Hurwitz J, Murakami Y, Hanaoka F (1996) Mapping of the human homologue (ORC1L) of the yeast origin recognition complex subunit 1 gene to chromosome band 1p32. Genomics 36(3):559–561. doi:10.1006/geno.1996.0511
Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD (2003) VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18(10):2803–2812
Forsburg SL (2004) Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev 68(1):109–131
Freudenberg JM, Ghosh S, Lackford BL, Yellaboina S, Zheng X, Li R, Cuddapah S, Wade PA, Hu G, Jothi R (2012) Acute depletion of Tet1-dependent 5-hydroxymethylcytosine levels impairs LIF/Stat3 signaling and results in loss of embryonic stem cell identity. Nucleic Acids Res 40(8):3364–3377. doi:10.1093/nar/gkr1253
Gage FH (2000) Mammalian neural stem cells. Science 287(5457):1433–1438
Hitoshi S, Ishino Y, Kumar A, Jasmine S, Tanaka KF, Kondo T, Kato S, Hosoya T, Hotta Y, Ikenaka K (2011) Mammalian Gcm genes induce Hes5 expression by active DNA demethylation and induce neural stem cells. Nat Neurosci 14(8):957–964. doi:10.1038/nn.2875
Huang Y, Wang G, Liang Z, Yang Y, Cui L, Liu CY (2016) Loss of nuclear localization of TET2 in colorectal cancer. Clin Epigenetics 8:9. doi:10.1186/s13148-016-0176-7176
Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y (2010) Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466(7310):1129–1133. doi:10.1038/nature09303
Izumi M, Yanagi K, Mizuno T, Yokoi M, Kawasaki Y, Moon KY, Hurwitz J, Yatagai F, Hanaoka F (2000) The human homolog of Saccharomyces cerevisiae Mcm10 interacts with replication factors and dissociates from nuclease-resistant nuclear structures in G(2) phase. Nucleic Acids Res 28(23):4769–4777
Johnson RE, Klassen R, Prakash L, Prakash S (2015) A major role of DNA polymerase delta in replication of both the leading and lagging DNA strands. Mol Cell 59(2):163–175. doi:10.1016/j.molcel.2015.05.038
Johnston ST, Shtrahman M, Parylak S, Goncalves JT, Gage FH (2016) Paradox of pattern separation and adult neurogenesis: a dual role for new neurons balancing memory resolution and robustness. Neurobiol Learn Mem 129:60–68. doi:10.1016/j.nlm.2015.10.013
Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming GL, King JR, Song H, Sweatt JD (2013) TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron 79(6):1086–1093. doi:10.1016/j.neuron.2013.08.032
Kanke M, Kodama Y, Takahashi TS, Nakagawa T, Masukata H (2012) Mcm10 plays an essential role in origin DNA unwinding after loading of the CMG components. EMBO J 31(9):2182–2194. doi:10.1038/emboj.2012.68
Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27(8):447–452. doi:10.1016/j.tins.2004.05.013S0166223604001675
Kim H, Jang WY, Kang MC, Jeong J, Choi M, Sung Y, Park S, Kwon W, Jang S, Kim MO, Kim SH, Ryoo ZY (2016) TET1 contributes to neurogenesis onset time during fetal brain development in mice. Biochem Biophys Res Commun 471(4):437–443. doi:10.1016/j.bbrc.2016.02.060
Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T (2015) Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci USA 112(13):4128–4133. doi:10.1073/pnas.1422448112
Kneissl M, Putter V, Szalay AA, Grummt F (2003) Interaction and assembly of murine pre-replicative complex proteins in yeast and mouse cells. J Mol Biol 327(1):111–128
Kondo T, Raff M (2004) Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev 18(23):2963–2972. doi:10.1101/gad.309404
Kriaucionis S, Heintz N (2009) The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324(5929):929–930. doi:10.1126/science.1169786
Kucharova K, Stallcup WB (2010) The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience 166(1):185–194. doi:10.1016/j.neuroscience.2009.12.014
Kumar D, Aggarwal M, Kaas GA, Lewis J, Wang J, Ross DL, Zhong C, Kennedy A, Song H, Sweatt JD (2015) Tet1 oxidase regulates neuronal gene transcription, active DNA hydroxy-methylation, object location memory, and threat recognition memory. Neuroepigenetics 4:12–27. doi:10.1016/j.nepig.2015.10.002
Kunimoto H, Fukuchi Y, Sakurai M, Sadahira K, Ikeda Y, Okamoto S, Nakajima H (2012) Tet2 disruption leads to enhanced self-renewal and altered differentiation of fetal liver hematopoietic stem cells. Sci Rep 2:273. doi:10.1038/srep00273
Labib K, Tercero JA, Diffley JF (2000) Uninterrupted MCM2-7 function required for DNA replication fork progression. Science 288(5471):1643–1647
Langemeijer SM, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, Stevens-Linders E, van Hoogen P, van Kessel AG, Raymakers RA, Kamping EJ, Verhoef GE, Verburgh E, Hagemeijer A, Vandenberghe P, de Witte T, van der Reijden BA, Jansen JH (2009) Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet 41(7):838–842. doi:10.1038/ng.391
Lei M, Tye BK (2001) Initiating DNA synthesis: from recruiting to activating the MCM complex. J Cell Sci 114(Pt 8):1447–1454
Li Y, Benezra R (1996) Identification of a human mitotic checkpoint gene: hsMAD2. Science 274(5285):246–248
Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, Reynolds BA (2008) Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells 26(4):988–996. doi:10.1634/stemcells.2007-0867
Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C (2010) Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6(5):445–456. doi:10.1016/j.stem.2010.03.017
Lygerou Z, Nurse P (2000) Cell cycle. License withheld–geminin blocks DNA replication. Science 290(5500):2271–2273
Mi Y, Gao X, Dai J, Ma Y, Xu L, Jin W (2015) A novel function of TET2 in CNS: sustaining neuronal survival. Int J Mol Sci 16(9):21846–21857. doi:10.3390/ijms160921846
Montalban-Loro R, Domingo-Muelas A, Bizy A, Ferron SR (2015) Epigenetic regulation of stemness maintenance in the neurogenic niches. World J Stem Cells 7(4):700–710. doi:10.4252/wjsc.v7.i4.700
Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, Perna F, Pandey S, Madzo J, Song C, Dai Q, He C, Ibrahim S, Beran M, Zavadil J, Nimer SD, Melnick A, Godley LA, Aifantis I, Levine RL (2011) Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20(1):11–24. doi:10.1016/j.ccr.2011.06.001
Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S (2012) Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149(1):188–201. doi:10.1016/j.cell.2012.01.046
Namihira M, Kohyama J, Semi K, Sanosaka T, Deneen B, Taga T, Nakashima K (2009) Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev Cell 16(2):245–255. doi:10.1016/j.devcel.2008.12.014
Neri F, Dettori D, Incarnato D, Krepelova A, Rapelli S, Maldotti M, Parlato C, Paliogiannis P, Oliviero S (2015a) TET1 is a tumour suppressor that inhibits colon cancer growth by derepressing inhibitors of the WNT pathway. Oncogene 34(32):4168–4176. doi:10.1038/onc.2014.356
Neri F, Incarnato D, Krepelova A, Dettori D, Rapelli S, Maldotti M, Parlato C, Anselmi F, Galvagni F, Oliviero S (2015b) TET1 is controlled by pluripotency-associated factors in ESCs and downmodulated by PRC2 in differentiated cells and tissues. Nucleic Acids Res 43(14):6814–6826. doi:10.1093/nar/gkv392
Okumura K, Nogami M, Taguchi H, Dean FB, Chen M, Pan ZQ, Hurwitz J, Shiratori A, Murakami Y, Ozawa K et al (1995) Assignment of the 36.5-kDa (RFC5), 37-kDa (RFC4), 38-kDa (RFC3), and 40-kDa (RFC2) subunit genes of human replication factor C to chromosome bands 12q24.2-q24.3, 3q27, 13q12.3-q13, and 7q11.23. Genomics 25(1):274–278
Olynik BM, Rastegar M (2012) The genetic and epigenetic journey of embryonic stem cells into mature neural cells. Front Genet 3:81. doi:10.3389/fgene.2012.00081
Ray J, Gage FH (2006) Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci 31(3):560–573. doi:10.1016/j.mcn.2005.11.010
Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, Faull KF, Jaenisch R, Tsai LH (2013) Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron 79(6):1109–1122. doi:10.1016/j.neuron.2013.08.003
Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472(7344):466–470. doi:10.1038/nature09817
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301(5634):805–809. doi:10.1126/science.1083328301/5634/805
Shimozaki K, Clemenson GD Jr, Gage FH (2013) Paired related homeobox protein 1 is a regulator of stemness in adult neural stem/progenitor cells. J Neurosci 33(9):4066–4075. doi:10.1523/JNEUROSCI.4586-12.2013
Shinohara A, Ogawa H, Ogawa T (1992) Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 69(3):457–470
Shinohara A, Ogawa H, Matsuda Y, Ushio N, Ikeo K, Ogawa T (1993) Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet 4(3):239–243. doi:10.1038/ng0793-239
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476(7361):458–461. doi:10.1038/nature10287
Spella M, Britz O, Kotantaki P, Lygerou Z, Nishitani H, Ramsay RG, Flordellis C, Guillemot F, Mantamadiotis T, Taraviras S (2007) Licensing regulators Geminin and Cdt1 identify progenitor cells of the mouse CNS in a specific phase of the cell cycle. Neuroscience 147(2):373–387. doi:10.1016/j.neuroscience.2007.03.050
Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G (2006) Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia 54(8):805–814. doi:10.1002/glia.20407
Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA (2001) Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci USA 98(26):15306–15311. doi:10.1073/pnas.251466698251466698
Szulwach KE, Li X, Li Y, Song CX, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, Vasanthakumar A, Godley LA, Chang Q, Cheng X, He C, Jin P (2011) 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci 14(12):1607–1616. doi:10.1038/nn.2959
Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324(5929):930–935. doi:10.1126/science.1170116
Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, Taga T (2001) DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell 1(6):749–758
Tye BK, Sawyer S (2000) The hexameric eukaryotic MCM helicase: building symmetry from nonidentical parts. J Biol Chem 275(45):34833–34836. doi:10.1074/jbc.R000018200R000018200
Watase G, Takisawa H, Kanemaki MT (2012) Mcm10 plays a role in functioning of the eukaryotic replicative DNA helicase, Cdc45-Mcm-GINS. Curr Biol 22(4):343–349. doi:10.1016/j.cub.2012.01.023
Wiehle L, Raddatz G, Musch T, Dawlaty MM, Jaenisch R, Lyko F, Breiling A (2015) Tet1 and Tet2 Protect DNA Methylation Canyons against Hypermethylation. Mol Cell Biol 36(3):452–461. doi:10.1128/MCB.00587-15
Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K (2011) TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473(7347):343–348. doi:10.1038/nature10066
Wu BK, Brenner C (2014) Suppression of TET1-dependent DNA demethylation is essential for KRAS-mediated transformation. Cell Rep 9(5):1827–1840. doi:10.1016/j.celrep.2014.10.063
Wu H, D’Alessio AC, Ito S, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y (2011) Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev 25(7):679–684. doi:10.1101/gad.2036011
Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S, Min J, Nicholson T, Chen T, Xu G, Shi Y, Zhang K, Shi YG (2011) Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell 42(4):451–464. doi:10.1016/j.molcel.2011.04.005
Yao J, Mu Y, Gage FH (2012) Neural stem cells: mechanisms and modeling. Protein Cell 3(4):251–261. doi:10.1007/s13238-012-2033-6
Zhang CL, Zou Y, He W, Gage FH, Evans RM (2008) A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451(7181):1004–1007. doi:10.1038/nature06562
Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, Liu W, Xu ZM, Yang L, Ding YQ, Tang F, Meissner A, Ding C, Shi Y, Xu GL (2013) Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell 13(2):237–245. doi:10.1016/j.stem.2013.05.006
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132(4):645–660. doi:10.1016/j.cell.2008.01.033
Zhao Z, Chen L, Dawlaty MM, Pan F, Weeks O, Zhou Y, Cao Z, Shi H, Wang J, Lin L, Chen S, Yuan W, Qin Z, Ni H, Nimer SD, Yang FC, Jaenisch R, Jin P, Xu M (2015) Combined loss of Tet1 and Tet2 promotes B cell, but not myeloid malignancies. Mice. Cell Rep 13(8):1692–1704. doi:10.1016/j.celrep.2015.10.037
Zhu Y, Jin K, Mao XO, Greenberg DA (2003) Vascular endothelial growth factor promotes proliferation of cortical neuron precursors by regulating E2F expression. FASEB J 17(2):186–193. doi:10.1096/fj.02-0515com17/2/186
Acknowledgments
I thank K. Tsurunaga and the members of the Life Science Support Center and Atomic Bomb Disease Institute at Nagasaki University for technical assistance and helpful discussions. Crimson Interactive Pvt. Ltd. (Ulatus) and Edanz Group Japan are acknowledged for their assistance with preparation of the manuscript. This work was supported by a Grant-in-Aid for Scientific Research (C) Grant Number 15K06711 from the Japan Society for the Promotion of Science (JSPS) and a Grant-in-Aid of the Alumni Association of Nagasaki University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10571_2016_432_MOESM1_ESM.tiff
Gene expression of TET family members in cultured adult NSCs analyzed by conventional RT-PCR. Total RNA from cultured adult NSCs was reverse transcribed with [RT (+)] or without [RT (−)] reverse transcriptase and then amplified by the hot start-based PCR method with specific primer sets for Tet1, Tet2, and Tet3 genes. Supplementary material 1 (TIFF 2884 kb)
Rights and permissions
About this article
Cite this article
Shimozaki, K. Ten-Eleven Translocation 1 and 2 Confer Overlapping Transcriptional Programs for the Proliferation of Cultured Adult Neural Stem Cells. Cell Mol Neurobiol 37, 995–1008 (2017). https://doi.org/10.1007/s10571-016-0432-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0432-6