Abstract
Thymoquinone (TQ), a bioactive constituent of Nigella sativa Linn (N. sativa) has demonstrated several neuropharmacological attributes. In the present study, the neuroprotective properties of TQ were investigated by studying its anti-apoptotic potential to diminish β-amyloid peptide 1–40 sequence (Aβ1–40)-induced neuronal cell death in primary cultured cerebellar granule neurons (CGNs). The effects of TQ against Aβ1–40-induced neurotoxicity, morphological damages, DNA condensation, the generation of reactive oxygen species, and caspase-3, -8, and -9 activation were investigated. Pretreatment of CGNs with TQ (0.1 and 1 μM) and subsequent exposure to 10 μM Aβ1–40 protected the CGNs against the neurotoxic effects of the latter. In addition, the CGNs were better preserved with intact cell bodies, extensive neurite networks, a loss of condensed chromatin and less free radical generation than those exposed to Aβ1–40 alone. TQ pretreatment inhibited Aβ1–40-induced apoptosis of CGNs via both extrinsic and intrinsic caspase pathways. Thus, the findings of this study suggest that TQ may prevent neurotoxicity and Aβ1–40-induced apoptosis. TQ is, therefore, worth studying further for its potential to reduce the risks of developing Alzheimer’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia among the elderly population (Cummings and Cole 2002). One of the pathological hallmarks of AD is senile plaques in the brain, composed of β-amyloid (Aβ) peptide with 39–43 amino acids, derived from cleavage of the amyloid precursor protein (Hardy and Higgins 1992; Yankner 1996). Aβ is reported to be neurotoxic against neuronal primary cultures and cell lines (Irie and Keung 2003; Yu et al. 2005). A few drugs have been approved for managing the cognitive and behavioral symptoms of AD, but side effects (Terry et al. 2011) and an inability to improve the overall symptomatology in AD necessitates the search for newer and better therapies.
There is an ongoing intensive search for potential agents to prevent Aβ neurotoxicity. One promising approach is through the modulation of anti-apoptotic mechanisms. Apoptosis plays a central role in mediating the death of neurons secondary to oxidative damage produced by factors like Aβ (Klein and Ackerman 2003; Kannan and Jain 2000). Extrinsic and intrinsic caspases are important activators of apoptosis, and their modulation by exogenous stimuli could prevent or promote apoptosis. Furthermore, reduced production of free radicals generated by Aβ, and consequently the reduced apoptosis, could therefore prevent oxidative stress, death of neurons, and potentially neurodegenerative diseases (Ferrari 2007; Matteo et al. 2007).
Most studies concerning AD have been conducted on the cerebral cortex; however, there is compelling evidence whereby AD patients exhibit pathological alterations of the cerebellar cortex (Mann et al. 2001). Pieri et al. (2010) showed the protective effects of tachykinin endecapeptide substance P (SP) against Aβ25–35- and Aβ1–42-induced apoptotic on cerebellar granule cells. It has also been demonstrated that treatment of rat cerebellar granule cells with Aβ25–35-induced apoptotic cell death, dependent on depolarization and developmental cell conditions (Scorziello et al. 1996). In addition, Aβ1–42-induced death of PC12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment through the overexpression of Bcl-2 protein (Wei et al. 2000).
Furthermore, the study of cerebellar connectivity and function shows that the cerebellum is not only responsible for motoric function but is also engaged in cognition and learning (Wegiel et al. 1999). As supported by Bloedel and Bracha (1997), Kalashnikova et al. (2005), and Parkins (1997), cerebellum, besides its participation in control of muscle movement accuracy, maintenance of equilibrium and posture, is also engaged in cognition and learning. Studies of the function of patients with defective cerebella reveal deficits in cognitive planning (Appollonio et al. 1993) and cognitive operations in three-dimensional space (Wallesch and Horn 1990). The patients were unable to perform well in the practice-related learning and error-detection (Petersen and Fiez 1993) and learning of arbitrary associations between words (Bracke-Tolkmitt et al. 1989). There were also deficits in judging of time intervals and the velocity of moving stimuli (Ivry and Baldo 1992) and rapidly shifting attention between sensory modalities (Akshomoff and Courchesne 1992). For years, the cerebellum was thought to be unaffected in AD, but neuropathological studies based on more sensitive methods show frequent and varied cerebellar changes in the late stages of the disease (Braak et al. 1989; Joachim et al. 1989; Yamaguchi et al. 1989). Since cerebellum is also engaged in cognition and learning, and its defective functions may cause deficits in cognitive, judgment, and affects AD patients bodily control, therefore it is beneficial to further understand how cerebellum response to Aβ toxicity and search for potential agents to prevent the neurons from further damage.
It is known that the toxicity effects of Aβ to the neuronal cultures are contributed by its length 25–35 (Pike et al. 1995). Thus, synthetic Aβ25–35 sequence was developed for practical reason. However, still the Aβ25–35 sequence does not exist in the biological system (Yankner et al. 1989). Therefore, Aβ1–40 sequence was chosen as this length is present in the biological system in comparison to Aβ25–35. Even though Aβ1–42 is more prone to aggregate as compared to Aβ1–40, and thus caused the toxicity effects to the neuronal cultures (Selkoe 2001); however, the toxicity effects of Aβ1–40 also cannot be disregarded as Aβ1–40 also prone to aggregate and caused toxicity effects. In consequence, numerous studies have been carried out to understand the roles of Aβ1–40 in vitro (Hirohata et al. 2012; Ono et al. 2005, 2006, 2012) and Aβ1–40 has been used to induce toxicity in rat model of AD (Colom et al. 2013; Wan et al. 2013; Zhang et al. 2013). Also, it has been shown that different structures of Aβ were formed under variety of conditions, which includes aggregation temperatures and incubation period. For instance, Ono et al. (2005) reported that both Aβ1–40 and Aβ1–42 formed fibrils when incubated at 37 °C for 7 days and 24 h, respectively.
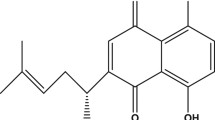
Thymoquinone (TQ) is a bioactive constituent of Nigella sativa Linn (N. sativa), a dicotyledon of the Ranunculaceae family commonly known as black seed. N. sativa was reported in our previous publication as having protective effects against Aβ-induced toxicity in neuronal cells likely due to its antioxidant properties (Ismail et al. 2008). N. sativa was also reported to improve blood flow and reduce neurological deficits in cerebral ischemia (Akhtar et al. 2012). On the other hand, TQ seems promising due to its many biological effects, which include antioxidant, antiinflammatory, and antidiabetic characteristics (Shrivastava et al. 2011) that may be beneficial in the management of AD. TQ was reported to protect against Aβ25–35-induced oxidative stress-associated inflammation in pheochromocytoma (PC12) cells through modulation of endogenous antioxidant status, inflammation-related protein expression, and reactive oxygen species (ROS) level. It also restored abnormal mitochondrial membrane potential (Khan et al. 2012). In PC12 cells, it also protects from glucose deprivation-induced DNA damage (Babazadeh et al. 2012). The combination of TQ and metformin exerted a neuroprotective effect by decreasing ethanol-mediated mitochondria-dependent apoptosis in prenatal rat cortical neurons (Ullah et al. 2012). Moreover, TQ attenuated the oxidative stress-induced vascular endothelial dysfunction, probably resulting from its potent antioxidant capacity (El-Agamy and Nader 2012). TQ is also reported to counteract the induced oxidative stress in rats’ brain tissue by reducing the levels of peroxidation, and enhancing the activities of enzymatic and non-enzymatic antioxidants (Sheikh and Mohamadin 2012).
Considering the growing interest in the use of herbal remedies for chronic diseases, the neuroprotective potentials of TQ appear to be promising in the management of neurodegenerative diseases. Thus, in the present study, we evaluated the neuroprotective effects of TQ on Aβ1–40-induced neurotoxicity, morphological damages, DNA condensation, the production of ROS, and caspase-3, -8, and -9 activations in primary cultured cerebellar granule neurons (CGNs).
Materials and Methods
Primary Culture of CGNs
The primary culture of CGNs was prepared as reported earlier (Ismail et al. 2008), according to requirement of the Universiti Putra Malaysia Use and Care of Animal Ethics Committee. Briefly, the cerebellar tissues from newborn Sprague–Dawley rats (7-days-old) were removed and the meninges were cleaned in phosphate buffered saline (PBS) (pH 7.2). The tissues were then chopped with a scalpel and transferred to a trypsin solution at 37 °C for 7 min. The single cells were obtained through trituration steps using a fire-polished glass pipette in Dulbecco’s modified Eagle’s medium (DMEM) containing 19 mM NaHCO3, 26.2 mM KCl, 7 μM p-aminobenzoic acid, 100 mU/L insulin, and 50 μg/mL gentamicin, and further seeded into 96-well poly-l-lysine-coated plate. The cells were maintained in DMEM supplemented with 10 % fetal bovine serum in a humidified incubator (37 °C, 5 % CO2, and 95 % air). After 48 h of incubation, the non-neuronal cell division was inhibited by adding 10 μM cytosine arabinofuranoside for 24 h. The resulting matured cells were used for the following experiments 4 days thereafter.
Preparation and Dilution of Aβ1–40
The stock solution of Aβ1–40 (Sigma-Aldrich, St. Louis, MO, USA) was prepared by dissolving the lyophilized peptide initially with sterile distilled water at 1 mg/mL (0.23 mM). The lyophilized peptide should not be dissolved directly into saline or buffer as the peptide would not be soluble. The peptide solution was then further diluted with PBS (pH 7.2) to 30 μM and incubated at 37 °C for 4 days. The desired concentration of 10 μM Aβ1–40 was obtained by diluting the peptide with culture medium for cell culture experiments. Our previous study showed that the final concentration of 10 μM Aβ1–40 was sufficient to induce toxicity to the CGNs (Ismail et al. 2008).
Neurotoxicity Assays
The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and lactate dehydrogenase (LDH) release assays are indicators of cell viability and cell death, respectively. The aggregated Aβ1–40 was prepared, as described in “Preparation and Dilution of Aβ1–40 ” section, in the absence of cells. Meanwhile, the CGNs were cultured in 96-well plates at a density of 1 × 105 cells/well and were let to mature for 4 days before use. Pretreatment of the CGNs with TQ for 5 h was subsequently followed by exposure to 10 μM Aβ1–40. After 24 h incubation, 50 μL of the sample media was transferred to a new 96-well plate to assess LDH release.
For MTS assay, 20 μL of MTS reagent (CellTiter 96® AQueous One Solution Reagent, Promega, Southampton, UK) was added into MTS assay plate and incubated for 4 h at 37 °C in a humidified, 5 % CO2. The optical density (OD) of the wells was determined using a microplate reader (Opsys MR, Thermo Labsystems, Franklin, MA, USA) at 490 nm wavelength. For LDH assay, LDH released into the media was determined using the CytoTox 96 Non-radioactive Cytotoxicity Assay (Promega, Southampton, UK). Briefly, 50 μL of assay buffer was added to 50 μL of media removed from each well prior to MTS assay. The plate was further incubated in the dark at room temperature for 30 min, followed by adding 50 μL of stop solution into each well and the absorbance was measured using a microplate reader (Opsys MR, Thermo Labsystems) at 490 nm.
Morphological Assessment by Light Microscope
The morphology of CGNs, treated and untreated, was assessed and captured by light microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany) at 40× magnification using Motic Image Software (Wetzlar, Germany).
Hoechst 33258 Staining Assay by Epifluorescence Microscopy
CGNs were grown in chamber slides (Labtek II Chamber Slide, Nunc, Thermo Fisher Scientific, Germany) at a density of 1 × 105 cells/well and pretreated with 0.1 μM TQ for 5 h followed by exposure to 10 μM Aβ1–40. After 24 h incubation, cells were fixed with 2 mL of 4 % paraformaldehyde and washed three times with PBS (pH 7.2). Cells were then stained with 60 μg/mL Hoechst 33258 in PBS (pH 7.2) for 30 min at room temperature, and thereafter washed three times with PBS (pH 7.2). The excess PBS was dried-out and the slide was observed under an epifluorescence microscope (Leica DMLB, Leica Microsystems CMS GmbH, Wetzlar, Germany) with a UV-2 filter.
Detection of ROS by Electron Spin Resonance (ESR) Spectrometer
CGNs were grown in 6-well plates at a density of 1 × 105 cells/well and pretreated with 0.1 μM TQ before exposure to 10 μM Aβ1–40 for 24 h. The cells were washed and harvested with PBS (pH 7.2). Lysis buffer (9 % (v/v) Triton® X-100) was added to cell pellets and then incubated on ice for 10 min. The pellets were then washed, resuspended, and centrifuged at 1,000 rpm. Three hundred microliters of supernatant were added to 30 μL of 5,5-dimethyl-1-pyrroline-1-oxide (DMPO). The mixture was then transferred to a quartz flat cell and analyzed by an ESR spectrometer (Jeol FA100; Tokyo, Japan).
Caspase-3, -8, and -9 Activation Assays
CGNs were grown in 6-well plates at a density of 1 × 105 cells/well and pretreated with 0.1 μM TQ before exposure to 10 μM Aβ1–40 for 24 h. The cells were then harvested, centrifuged at 1,000 rpm for 10 min and the pellets were resuspended in 1 mL of cell lysis buffer for 1 h. The supernatant was collected for the following caspase-3, -8, and -9 assays. The protein content in each sample was determined using Bradford assay.
In the caspase-3 assay, the supernatant was added to DEVD-pNA substrate (10 mM stock) (Caspase™ Assay System Kit, Promega, Southampton, UK). The solution was swirled slowly, further incubated at 37 °C for 4 h and the absorbance was measured by microplate reader at 405 nm (Opsys MR, Thermo Labsystems, Franklin, MA, USA).
On the other hand, the caspase-8 activity was determined using Caspase-8 Assay Kit (Sigma-Aldrich, St. Louis, MO, USA) with the Ac-IETD-pNA as colorimetric specific substrate labeled with the chromophore p-nitroaniline (pNA). Free pNA was cleaved by caspase-8 enzyme and monitored by a spectrophotometer. The amount of pNA released is proportional to the amount of caspase-8 activity present in the sample. The collected supernatant was added with 50 μL of 2× Reaction Buffer (containing 10 mM DTT), followed by addition of 5 μL of 4 mM IETD-pNA substrate. The absorbance was measured after 4 h incubation at 37 °C by microplate reader (Opsys MR, Thermo Labsystems, Franklin, MA, USA) at 405 nm wavelength.
The caspase-9 activity was determined using Colorimetric Activity Assay Kit (Chemicon Inc., PA, USA) with the Ac-LEHD-pNA as colorimetric specific substrate labeled with the chromophore pNA. p-Nitroaniline was released from the substrate upon cleavage by caspase-9 enzyme. Free pNA was monitored by a spectrophotometer. The amount of pNA released is proportional to the amount of caspase-9 activity present in the sample. The supernatant was added to 2 μL of the LEHD-pNA substrate (10 mM stock) for each sample. The absorbance of caspase-9 activity was measured after 4 h incubation at 37 °C by microplate reader (Opsys MR, Thermo Labsystems, Franklin, MA, USA) at 405 nm.
Statistical Analyses
The means of groups were used for the analyses; where error bars are shown, they represent the SEM. One-way analysis of variance (ANOVA) was performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) to assess the level of significant differences at P < 0.01.
Results
Protective Effects of TQ on Aβ1-40-induced Neurotoxicity in CGNs
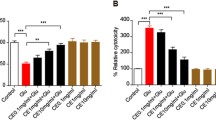
After incubating Aβ1–40 for 96 h, its neurotoxic effects were determined by exposing the CGNs to 10 μM Aβ1–40 at 37 °C for 24 h. The cell viability of 10 μM Aβ1–40 alone, as assessed by MTS assay, was 54 ± 0.98 % (P < 0.01) (Fig. 1a). Pretreatment of the CGNs with TQ attenuated the neurotoxic effects of Aβ1–40. TQ at 0.1 and 1 μM restored the cell viability of CGNs exposed to Aβ1–40 to 99 ± 2.31 and 91 ± 1.92 %, respectively (P < 0.01). Pretreatment with TQ alone did not affect the growth of CGNs within tested concentrations. On the other hand, exposure of CGNs to 10 μM Aβ1–40 increased LDH activity to 84.18 ± 4.62 % (Fig. 1b), while pretreatment with TQ prevented it. At 0.1 and 1 μM concentrations, TQ significantly reduced Aβ1–40-induced increase in LDH to 61 ± 2.05 and 65 ± 3.09 %, respectively.
TQ Preserved the Intact Cell Bodies and Extensive Neurite Network on Aβ1–40-induced Morphological Damages in CGNs
In phase-contrast observations, control cells (Fig. 2a-i) appeared generally healthy, with round cell bodies (thick arrow) and with a well-developed network of neurites (thin arrow). After 24 h exposure to 10 μM Aβ1–40 (Fig. 2a-ii), a significant reduction in the number of viable cells, displayed by damaged and shrunken cell bodies as well as clumped cells (thick arrow) with a disruption of neurites (thin arrow), was observed. Neurons with intact neurites and round soma were considered viable, whereas those with degenerated neurites and irregular soma were considered non-viable. By contrast, cells incubated with 10 μM Aβ1–40 in presence of 0.1 μM TQ (Fig. 2a-iii) were better preserved with intact cell bodies (thick arrow) and neurite network (thin arrow) than cells exposed to Aβ1–40 alone. CGNs treated with 0.1 μM TQ alone (Fig. 2a-iv) did not cause any reduction in the number of viable cells and retained a phenotype similar to untreated control cells.
Phase-contrast micrograph observation on CGNs at ×40 magnification. a The intact cell bodies (white arrow) and neurite network formation (black arrow). i 4-day-old CGNs in medium (control). ii CGNs treated with 10 μM Aβ1–40 alone. iii CGNs pretreated with 0.1 μM TQ and subsequent exposure to 10 μM Aβ1–40. iv CGNs treated with 0.1 μM TQ alone. b The percentage of dead cells. Values represent mean ± SEM # P < 0.01 versus control, *P < 0.01 versus Aβ1–40
TQ Reduced Condensed Chromatin on Aβ1–40-induced DNA Condensation in CGNs
The number of Hoechst-positive nuclei increased in Aβ1–40-treated CGNs (Fig. 3a-ii) as compared to control (Fig. 3a-i). In untreated cells, little fluorescence was observed in the nucleus. On the other hand, condensed and fragmented nuclei and apoptotic bodies were observed in Aβ1–40 treated alone. However, the amount of condensed chromatin in Aβ1–40 treated CGNs decreased significantly in the presence of 0.1 μM TQ (Fig. 3a-iii). CGNs treated with 0.1 μM TQ alone (Fig. 3a-iv) retained a phenotype similar to untreated control cells.
Apoptotic features of CGNs induced by Aβ1–40. After treatment for 24 h, nuclear chromatin was stained with Hoechst 33258 fluorescent dye. a The thick arrows indicate apoptotic nuclear fragmentation and residual fragments, whereas thin arrows indicate normal cell. i 4-day-old CGNs in medium (control). ii CGNs treated with 10 μM Aβ1–40 alone. iii CGNs pretreated with 0.1 μM TQ and subsequent exposure to 10 μM Aβ1–40. iv CGNs treated with 0.1 μM TQ alone. b The percentage of apoptotic cells. Values represent mean ± SEM # P < 0.01 versus control, *P < 0.01 versus Aβ1–40
TQ Lessen the Production of Free Radical on Aβ1–40 in CGNs
The Aβ peptide has been suggested to be capable of spontaneously generating free radicals. A four-line spectrum was obtained from the incubation of Aβ1–40 alone and DMPO (Fig. 4b). However, the four-line spectrum was slightly inhibited in the presence of 0.1 μM TQ (Fig. 4c). No definite four-line spectrum was observed in the absence of Aβ1–40 peptide (control) (Fig. 4a).
The ESR Spectroscopic spectra obtained after the incubation of 10 μM Aβ1–40 with DMPO. a Control b 10 μM Aβ1–40 alone. c 0.1 μM TQ + 10 μM Aβ1–40. The peaks (shown by red arrows) are indicative of the presence of hydroxyl radicals; higher peaks suggest the presence of more hydroxyl radicals and vice versa (Color figure online)
TQ Reduced the Activation of Caspase-3, -8, and -9 on Aβ1–40 Exposure in CGNs
The activity of caspase-3 in CGNs increased following exposure to 10 μM Aβ1–40 for 24 h (Fig. 5a). However, pretreatment of CGNs with 0.1 μM TQ for 5 h, with subsequent exposure to 10 μM Aβ1–40 was able to reduce caspase-3 activity significantly (from 115 ± 1.09 to 87 ± 5.76 %). Incubation of TQ alone did not increase the caspase-3 activity. A similar pattern of effect was observed when caspase-8 (Fig. 5b) and caspase-9 (Fig. 5c) were assayed; exposure of CGNs to 10 μM Aβ1-40 increased the activities of both (111 ± 6.82 and 148 ± 8.32 %, respectively). TQ reduced caspase-9 activation to 95 ± 5.34 %, whereby caspase-8 activation was reduced to 83 ± 5.08 %, significantly (P < 0.01).
Discussion
Beta-amyloid peptides play a major role in the pathogenesis of AD and compounds that can inhibit pathways related to Aβ-induced neurotoxicity may be of potential therapeutic value in the treatment of AD (Ferrari 2007; Matteo et al. 2007). The present study investigated the neuroprotective mechanism of action of TQ against Aβ1–40-induced neurotoxicity, morphological damages, DNA condensation, the production of ROS, and activation of caspase-3, -8, and -9 in primary cultured CGNs.
It was found that TQ pretreatment increased cell survival (measured by MTS assay) after subsequent exposure to Aβ1–40. LDH release in cell culture supernatant was also determined to further examine this neuroprotective effect. LDH is a stable cytoplasmic enzyme that is released into the surrounding medium when the plasma membrane is damaged by oxidative stress, which is an indication of cell death. In the present study, TQ pretreatment significantly decreased LDH release by CGNs, thereby providing evidence that TQ protects CGNs against Aβ1–40-induced neurotoxicity.
In the morphological observations, TQ preserved the intact cell bodies and extensive neurite network on Aβ1–40-induced morphological damages in CGNs. The morphology of CGNs observed in this study is in agreement with that reported by Ikonomovic et al. (1997) and Fatokun et al. (2007). In addition, TQ reduced condensed chromatin on Aβ1–40-induced DNA condensation in CGNs. This finding was similar to that of Boyd-Kimball et al. (2005), who showed brightly fluoresce and fragmented nuclei that are the indication of apoptosis. Morphologically, apoptotic neurons are characterized by cell shrinkage, the retraction of neuronal processes, a contracted nucleus, chromatin condensation, DNA fragmentation, and plasma membrane blebbing. In the end-stage, the dying cell disintegrates to form apoptotic bodies. This result was also supported by Forloni et al. (1993), who found that Aβ1–40-induced neuronal cell death is typified by the chromatin condensation.
Tabner et al. (2002) suggested that accumulated hydrogen peroxide formed in a metal-dependent mechanism during the incubation of Aβ is readily converted to hydroxyl radicals. Aβ1–40 can form hydrogen peroxide upon incubation in solution at 37 °C (Bush et al. 1999), suggesting that Aβ1–40 becomes toxic to cultured neurons, since the peptide itself could generate free radicals. However, TQ pretreatment inhibited this apparent increase in ROS. Thus, TQ may also prevent Aβ1–40-induced neurotoxicity through its antioxidant ability.
Caspases are implicated in the accomplishment of apoptotic cell death, while the inhibition of their activation is favorable for cell survival. The activation of caspase-3 induces DNA fragmentation, nuclear chromatin condensation, and cell apoptosis (Li et al. 2008). Wai et al. (2009) showed that caspase-3 activation was increased in AD patients, and triggered synaptic failure (D’Amelio et al. 2011) and autophagy, which may contribute to cognitive dysfunction in AD development (Rohn et al. 2011). The increased level of caspase activities induced by Aβ1–40 in our experiments supports the findings of elevated caspase-3, -8, and -9 levels of sporadic AD patients in comparison with non-demented controls (Tacconi et al. 2004), as well as increased caspase-8 expression in the hippocampus of injected rats with Aβ1–40 (Miguel-Hidalgo et al. 2012) and the activation of caspase-3 in SK-N-SH neuroblastoma cells exposed to Aβ1–40 (Li et al. 2012).
Caspase-8 is a member of the initiator family of caspases, making it an important player in the cascade leading up to apoptosis. Once activated, caspase-8 initiates downstream caspases (3, 6, and 7) that eventually cleave key cellular substrates, leading to apoptotic cell death. It is well documented that Aβ may prompt neuronal cell death associated with AD by the induction of apoptosis followed by cross-linking of death-receptors and concomitant activation of caspase-8 and caspase-3 (Rohn et al. 2001), suggesting that the activation of caspase-8 and apoptosis are crucial steps toward the damage caused by Aβ in AD (Rohn et al. 2001; Ivins et al. 1999). Suppression of the activity of caspase-3, -8, and -9, in the presence of TQ, therefore suggests anti-apoptosis as one of the mechanisms by which TQ protected the CGNs from the cytotoxic effects of Aβ1–40.
In this study, the pretreatment of CGNs with TQ (0.1 and 1 μM) increased cell viability, reduced LDH release, preserved cell bodies, promoted neurite network, attenuated condensed chromatin and free radical generation, inhibited caspase-3, -8 and -9 activation compared to those exposed to Aβ1–40 alone. Our results are in agreement with previous findings, which showed that TQ is protective against Aβ-induced toxicity (Babazadeh et al. 2012; El-Agamy and Nader 2012; Khan et al. 2012; Sheikh and Mohamadin 2012; Ullah et al. 2012). This suggests that TQ, like other promising agents reported previously (Ferrari 2007; Matteo et al. 2007) could potentially protect against AD and possibly other neurodegenerative disorders.
Conclusions
It has been demonstrated in the current study that Aβ1–40, which is pathognomonic of AD, will lead to the activation of both extrinsic and intrinsic apoptotic pathways, as shown by activated caspases -8 and -9. TQ, however, may be able to protect CGNs from Aβ1–40 toxic effects through the suppression of caspases and lessen the generation of free radicals with a resultant improvement in cell viability. Our findings suggest that TQ has neuroprotective effects, and may be worth looking into further as a potential agent in lowering the risks of AD.
Abbreviations
- Aβ:
-
Beta-amyloid peptide
- AD:
-
Alzheimer’s disease
- TQ:
-
Thymoquinone
- CGNs:
-
Primary cultured cerebellar granule neurons
- PC12:
-
Pheochromocytoma
- MTS:
-
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- LDH:
-
Lactate dehydrogenase
- DMSO:
-
Dimethyl sulfoxide
- PBS:
-
Phosphate buffered saline
- DMPO:
-
5,5-Dimethyl-1-pyrroline-1-oxide
References
Akhtar M, Maikiyo AM, Khanam R, Mujeeb M, Aqil M, Najmi AK (2012) Ameliorating effects of two extracts of Nigella sativa in middle cerebral artery occluded rat. J Pharm Bioallied Sci 4:70–75
Akshomoff N, Courchesne E (1992) A new role for the cerebellum in cognitive operations. Behav Neurosci 106:731–738
Appollonio IM, Grafman J, Schwartz V, Massaquoi S, Hallett M (1993) Memory in patients with cerebellar degeneration. Neurology 43:1536–1544
Babazadeh B, Sadeghnia HR, Kapurchal ES, Parsaee H, Nasri S, Zahra TN (2012) Protective effect of Nigella sativa and thymoquinone on serum/glucose deprivation-induced DNA damage in PC12 cells. Avicenna J Phytomed 2:125–132
Bloedel JR, Bracha V (1997) Duality of cerebellar motor and cognitive functions. Int Rev Neurobiol 41:613–634
Boyd-Kimball D, Sultana R, Mohammad HA, Butterfield DA (2005) γ-Glutamylcysteine ethyl ester-induced up-regulation of glutathione protects neurons against Aβ(1-42)-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. J Neurosci Res 79:700–706
Braak H, Braak E, Bohl J, Lang W (1989) Alzheimer’s disease: amyloid plaques in the cerebellum. J Neurol Sci 93:277–287
Bracke-Tolkmitt R, Linden A, Canavan AGM, Rockstroh B, Scholz E, Wessel K, Diener HC (1989) The cerebellum contributes to mental skills. Behav Neurosci 103:442–446
Bush AI, Huang X, Fairlie DP (1999) The possible origin of free radicals from amyloid β-peptides in Alzheimer’s disease. Neurobiol Aging 20:335–337
Colom LV, Castaneda MT, Aleman D, Touhami A (2013) Memantine protects cholinergic and glutamatergic septal neurons from Aβ1–40-induced toxicity. Neurosci Lett 541:54–57
Cummings JL, Cole G (2002) Alzheimer disease. JAMA 287:2335–2338
D’Amelio M, Cavallucci V, Middei S et al (2011) Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci 14:69–76
Di Matteo V, Pierucci M, Di Giovanni G, Esposito E (2007) Prevention and therapy of neurodegenerative disorders: role of nutritional antioxidants. In: Qureshi GA, Parvez SH (eds) Oxidative stress and neurodegenerative disorders. Elsevier, Amsterdam, pp 621–661
El-Agamy DS, Nader MA (2012) Attenuation of oxidative stress-induced vascular endothelial dysfunction by thymoquinone. Exp Biol Med 237:1032–1038
Fatokun AA, Stone TW, Smith RA (2007) Cell death in rat cerebellar granule neurons induced by hydrogen peroxide in vitro: mechanisms and protection. Brain Res 1132:93–202
Ferrari CKB (2007) Diet, herbs, and nutritional protection against oxidative stress in neurological diseases. In: Qureshi GA, Parvez SH (eds) Oxidative stress and neurodegenerative disorders. Elsevier, Amsterdam, pp 525–541
Forloni G, Chiesa R, Smiroldo S, Verga L (1993) Apoptosis-mediated neurotoxicity neurotoxicity induced by chronic application of beta amyloid fragment 25–35. Neuroreport 4:523–526
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Hirohata M, Ono K, Jun-ichi T, Takahashi R, Ikeda T, Morinaga A, Yamada M (2012) Anti-amyloidogenic effects of soybean isoflavones in vitro: fluorescence spectroscopy demonstrating direct binding to Aβ monomers, oligomers and fibrils. Biochim Biophys Acta 1822:1316–1324
Ikonomovic S, Kharlamov E, Manev H, Ikonomovic MD, Grayson DR (1997) GABA and NMDA in the prevention of apoptotic-like cell death in vitro. Neurochem Int 31:83–290
Irie Y, Keung WM (2003) Rhizoma acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid-[beta] peptide. Brain Res 963:282–289
Ismail N, Ismail M, Latiff LA, Mazlan M, Mariod AA (2008) Black cumin seed (Nigella sativa Linn.) oil and its fractions protect against beta-amyloid peptide-induced toxicity in primary cerebellar granule neurons. J Food Lipids 15:519–533
Ivins K, Thornton P, Rohn T, Cotman C (1999) Neuronal apoptosis induced by β-amyloid is mediated by caspase-8. Neurobiol Dis 6:440–449
Ivry R, Baldo J (1992) Is the cerebellum involved in learning and cognition? Curr Opin Neurobiol 2:212–216
Joachim CL, Morris JH, Selkoe DJ (1989) Diffuse senile plaques occur commonly in the cerebellum in Alzheimer’s disease. Am J Pathol 135:309–319
Kalashnikova LA, Zueva YV, Pugacheva OV, Korsakova NK (2005) Cognitive impairments in cerebellar infarcts. Neurosci Behav Physiol 35:773–779
Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiology 7:153–163
Khan A, Vaibhav K, Javed H, Khan MM, Tabassum R, Ahmed ME et al (2012) Attenuation of Aβ-induced neurotoxicity by thymoquinone via inhibition of mitochondrial dysfunction and oxidative stress. Mol Cell Biochem 369:55–65
Klein JA, Ackerman SL (2003) Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest 111:785–793
Li G, Ma R, Huang C et al (2008) Protective effect of erythropoietin on β-amyloid-induced PC12 cell death through antioxidant mechanisms. Neurosci Lett 442:143–147
Li W, Chu Y, Zhang L, Yin L, Li L (2012) Ginsenoside Rg1 prevents SK-N-SH neuroblastoma cell apoptosis induced by supernatant from Aβ1–40-stimulated THP-1 monocytes. Brain Res Bull 88:501–506
Mann DM, Pickering-Brown SM, Takeuchi A, Iwatsubo T (2001) Amyloid angiopathy and variability in amyloid beta deposition is determined by mutation position in presenilin-1-linked Alzheimer’s disease. Am J Pathol 158:2165–2175
Miguel-Hidalgo JJ, Paul IA, Wanzo V, Banerje PK (2012) Memantine prevents cognitive impairment and reduces Bcl-2 and caspases 8 immunoreactivity in rats injected with amyloid β1–40. Eur J Pharmacol 692:38–45
Ono K, Hasegawa K, Naiki H, Yamada M (2005) Preformed β-amyloid fibrils are destabilized by coenzyme Q10 in vitro. Biochem Biophys Res Comm 330:111–116
Ono K, Hasegawa K, Naiki H, Yamada M (2006) Anti-Parkinsonian agents have anti-amyloidogenic activity for Alzheimer’s β-amyloid fibrils in vitro. Neurochem Int 48:275–285
Ono K, Li L, Takamura Y, Yoshiike Y, Zhu L, Han F, Mao X, Ikeda T, Jun-ichi T, Nishijo H, Takashima A, Teplow DB, Zagorski MG, Yamada M (2012) Phenolic compounds prevent amyloid β-protein oligomerization and synaptic dysfunction by site-specific binding. J Biol Chem 287:14631–14643
Parkins EJ (1997) Cerebellum and cerebrum in adaptive control and cognition: a review. Biol Cybern 77:79–87
Petersen SE, Fiez JA (1993) The processing of single words studied with positron emission tomography. Annu Rev Neurosci 16:509–530
Pieri M, Amadoro G, Carunchio I, Ciotti MT, Quaresima S, Florenzano F, Calissano P, Possenti R, Zona C, Severini C (2010) SP protects cerebellar granule cells against b-amyloid-induced apoptosis by down-regulation and reduced activity of Kv4 potassium channels. Neuropharmacology 58:268–276
Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW (1995) Structure–activity analyses of beta-amyloid peptides: contributions of the beta 25–35 region to aggregation and neurotoxicity. J Neurochem 64:253–265
Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH (2001) Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiol Dis 8:1006–1016
Rohn TT, Wirawan E, Brown RJ, Harris JR, Masliah E, Vandenabeele P (2011) Depletion of Beclin-1 due to proteolytic cleavage by caspases in the Alzheimer’s disease brain. Neurobiol Dis 43:68–78
Scorziello A, Meucci O, Florio T, Fattore M, Forloni G, Salmona M, Schettini G (1996) Beta 25–35 alters calcium homeostasis and induces neurotoxicity in cerebellar granule cells. J Neurochem 66:1995–2003
Selkoe DJ (2001) Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis 3:75–80
Sheikh BY, Mohamadin AM (2012) Thymoquinone a potential therapy for cerebral oxidative stress. Asian J Nat Appl Sci 1:76–92
Shrivastava RM, Agrawal RC, Parveen ZJ (2011) A review on therapeutic applications of Nigella sativa. J Chem Chem Sci 1:241–248
Tabner BJ, Turnbull S, El-Agnaf OMA, Allsop D (2002) Formation of hydrogen peroxide and hydroxyl radicals from Aβ and α-synuclein as a possible mechanism of cell death in Alzheimer’s disease and Parkinson’s disease. Free Radic Biol Med 32:1076–1083
Tacconi S, Perri R, Balestrieri E et al (2004) Increased caspase activation in peripheral blood mononuclear cells of patients with Alzheimer’s disease. Exp Neurol 190:254–262
Terry AV Jr, Callahan PM, Hall B, Webster SJ (2011) Alzheimer’s disease and age-related memory decline (preclinical). Pharmacol Biochem Behav 99:190–210
Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO (2012) Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci 13:11
Wai MS, Liang Y, Shi C, Cho EY, Kung HF, Yew DT (2009) Co-localization of hyperphosphorylated tau and caspase in the brainstem of Alzheimer’s disease patients. Biogerontology 10:457–469
Wallesch CW, Horn A (1990) Long-term effects of cerebellar pathology on cognitive functions. Brain Cogn 14:19–25
Wan B, Hu X, Nie J, Zhou M, Yang B, Li Y, Wen W, Lu C (2013) Effects of triptolide on degeneration of dendritic spines induced by Aβ1–40 injection in rat hippocampus. Neurol Sci. doi:10.1007/s10072-013-1463-0
Wegiel J, Wisniewski HM, Dziewiatkowski J, Badmajew E, Tarnawski M, Reisberg B, Mlodzik B, Miller DC (1999) Cerebellar atrophy in Alzheimer’s disease-clinicopathological correlations. Brain Res 818:41–50
Wei H, Leeds PR, Qian Y, Wei W, Chen R, Chuang D (2000) β-Amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur J Pharmacol 392:117–123
Yamaguchi H, Hirai S, Morimatsu M, Shoji M, Nakazato Y (1989) Diffuse type of senile plaques in the cerebellum of Alzheimer-type dementia demonstrated by β protein immunostain. Acta Neuropathol 77:314–319
Yankner BA (1996) Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 16:921–932
Yankner BA, Dawes LR, Fisher S, Vilia-Komaroff L, Oster-Granite ML, Neve RL (1989) Neurotoxicity of a fragment of amyloid precursor associated with Alzheimer’s disease. Science 245:417–420
Yu MS, Leung SKY, Lai SW et al (2005) Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against β-amyloid peptide neurotoxicity. Exp Gerontol 40:716–727
Zhang J, Zhen YF, Pu-Bu-Ci-Ren, Song LG, Kong WN, Shao TM, Li X, Chai XQ (2013) Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav Brain Res 244:70–81
Acknowledgments
Authors are thankful to Government of Malaysia and Universiti Putra Malaysia for providing financial support for this work via Research University Grant.
Conflict of interests
None of the authors have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ismail, N., Ismail, M., Mazlan, M. et al. Thymoquinone Prevents β-Amyloid Neurotoxicity in Primary Cultured Cerebellar Granule Neurons. Cell Mol Neurobiol 33, 1159–1169 (2013). https://doi.org/10.1007/s10571-013-9982-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-9982-z