Abstract
Activation of astrocytes in central nervous system inflammation leads to a disturbance of crosstalk between astrocytes and neurons, and that this may contribute to the death of neurons. CDK11p58 is a member of the large family of p34cdc2-related kinases. It specifically expresses in G2/M phase of the cell cycle and is closely related to cell cycle arrest and apoptosis. Here, we show that astrocyte-conditioned medium stimulated by lipopolysaccharide upregulates CDK11p58 expression and meanwhile causes neuronal apoptosis. CDK11p58 knockdown in PC12 cells represses neuronal apoptosis. CDK11p58 overexpression in PC12 cells promotes neuronal apoptosis. AKT signaling pathway is involved in CDK11p58-induced neuronal apoptosis process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation, a common denominator among the diverse list of neurodegenerative diseases, has been implicated recently as a key mechanism responsible for the progressive nature of neurodegeneration (Minghetti et al. 2005). The hallmark of brain inflammation is the activation of glial cells (Ridet et al. 1997). These cells constitute essential partners of neurons, providing functional support through a myriad of intercellular links (Seifert et al. 2006). However, during inflammation, glial cells may play a double-edged role because, depending on the course of the inflammatory process, glial-derived factors may result in being either beneficial or detrimental to neurons (Wyss-Coray and Mucke 2002).
At least 10 CDK11 isoforms have been cloned in eukaryotic cells, with molecular weight varying from 46 to 110 kDa (Choi et al. 2012). Major isoforms are CDK11p110, CDK11p58 (Bajic et al. 2011; Drogat et al. 2012), and CDK11p46 (Mikolajczyk and Nelson 2004). Previous research (Cai et al. 2002) showed that CDK11p58 enhanced the apoptosis induced by cycloheximide in SMMC-7721 hepatocarcinoma cells and it was also processed to a 50 kDa isoform. Yun et al. 2007 found that overexpression of CDK11p58 not only reduced the protein level of Bcl-2 itself, leading to Bcl-2/Bax ratio decline, but also reduced the Ser70 phosphorylation of Bcl-2. Both of these two mechanisms might contribute to the pro-apoptotic activity of CDK11p58.
Many studies have identified Ras-phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) as a major cell survival signaling pathway (Chalhoub et al. 2009; Engelman et al. 2006; Wagner-Golbs and Luhmann 2012). Akt is a serine/threonine protein kinase that plays a key role in multiple cellular processes such as cell proliferation, apoptosis, and transcription (Datta et al. 1997).
In this study, we show that ACM stimulated by lipopolysaccharide (LPS) upregulates CDK11p58 expression in neuronal cells and causes neuronal apoptosis. CDK11p58 knockdown in PC12 cells represses neuronal apoptosis. CDK11p58 overexpression in PC12 cells promotes neuronal apoptosis. AKT signaling pathway is involved in CDK11p58-induced neuronal apoptosis process.
Materials and Methods
Materials and Reagents
LPS, rabbit anti-PARP (Cleaved-Gly215) antibody, and mouse anti-GAPDH antibody were purchased from Sigma Chemicals. Rabbit polyclonal anti-PITSLRE, rabbit anti-cleaved-caspase-3, horseradish peroxidase (HRP)-conjugated goat anti-rabbit, and HRP conjugated goat anti-mouse IgG secondary antibodies were from Santa Cruz Biotechnology. Mouse anti-proliferating cell nuclear antigen (PCNA), rabbit anti-Bcl-2, rabbit phosphor-Ser473 Akt, and rabbit anti-total AKT antibodies were from Cell Signaling Technology.
Cell Culture and Treatment
C6, a rat astrocyte cell line, and PC12 cells which are a rat neuron cell line were obtained from the American Type Culture Collection (Rockville, MD, USA). C6 glioma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma) supplemented with 10 % (v/v) heat-inactivated fetal bovine serum (FBS), HEPES (3.6 g/L), penicillin (100 IU/ml), and streptomycin (100 μg/ml). At about 60 % confluence, culture medium was switched to DMEM supplemented with 1 % FBS. PC12 cells were cultured in DMEM supplemented with 5 % FBS, penicillin (100 IU/ml), and streptomycin (100 μg/ml). All cells were maintained in a humidified incubator at 37 °C and 5 % CO2. PC12 cells, cultured in plates and dishes until about 70 % confluence, were used for treatment. At the time of treatment, LPS was added to the C6 culture medium to indicated concentrations. For studies with ACM, the culture medium was replaced with ACM from PC12 cultures.
Preparation of C6 Cells ACM
C6 cells (3 × 105 cells/well) were seeded onto 100 mm dishes. After 24 h, the C6 cells were treated with LPS. The medium was collected after 24 h of incubation and stored at −20 °C until the treatment of PC12 cells. CM0, CM0.001, CM0.01, CM0.1, CM1, and CM10 represent conditioned medium from C6 cultures treated with vehicle, 0.001, 0.01, 0.1, 1, and 10 μg/ml LPS, respectively.
Western Blot Analysis
Cells were thoroughly scraped from the culture dishes with a cell scraper, Lysates were homogenized for 10 s at 6,000 rpm in a homogenizer (Brinkman). Protein content was normalized using protein assay kits (Bio-Rad Laboratories). The samples were subjected to SDS-polyacrylamide gel electrophoresis followed by transfer onto a polyvinylidene difluoride (PVDF) membrane filter (Immobilon, Millipore). The filters were incubated in PBS containing 0.5 % Tween20 (PBST) and 5 % nonfat milk and then with anti-PITSLRE antibody (1:1,000), anti-PCNA antibody (1:1,000), anti-Bcl-2 antibody (1:1,000), anti-cleaved-PARP antibody (1:1,000), anti-cleaved-caspase-3 antibody (1:1,000), anti-phospho AKT antibody (1:1,000), anti-total JNK antibody (1:1,000), and anti-GAPDH antibody (1:1,000) overnight at 4 °C. After being incubated with HRP-conjugated anti-rabbit IgG (1:2,000) and HRP-conjugated anti-mouse IgG (1:2,000), blots were washed and immunoreactive proteins were visualized on a film with an enhanced chemiluminescence kit (NEN Life Science Products, Boston, MA, USA). Optical density on the film was measured with a computer imaging system (Imaging Technology, Ontario, Canada). Setting control as 1, the relative differences between control and treatment groups were calculated and expressed. Values are responsible for at least three independent experiments.
TUNEL Apoptosis Assay
Terminal deoxynucleotide transferase-mediated dUTP Nick End-Labeling (TUNEL) was performed to detect cells undergoing apoptosis. An In situ Apoptosis Detection Kit (Roche, Indianapolis, USA) was employed. For the TUNEL analysis, PC12 cells were fixed with 4 % paraformaldehyde in PBS for 20 min at 4 °C and subjected to permeabilization for 20 min at room temperature with 0.1 % sodium citrate containing 0.1 % Triton X-100. The fixed and permeabilized PC12 cells were labeled with the TUNEL reaction mixture for 60 min at 37 °C. The nuclei of PC12 cells were counter-stained with 4′, 6′-diamino-2-phenylindole (DAPI). Fluorescent-labeled DNA, indicating DNA fragmentation, was analyzed by using a laser scanning confocal microscope (Leica TCS-NT).
Apoptosis Monitored by Flow Cytometry
Cultured cells were collected and fixed in 70 % ethanol for 30 min. After washing with PBS and treatment with Rnase A (50 μg/ml in Phosphate Buffered Saline, Sigma, USA) for 30 min, the cells were incubated with propidium iodide (50 μg/ml, Sigma, USA) for 15 min and analyzed by the flow cytometry machine (BECKMAN-COULTER Co., USA). All the data were analyzed by the research software.
siRNAs and Transfection
Double-stranded RNAs of 19 nucleotides were synthesized by Ambion Research. The targeting sequence of rat CDK11p58 mRNA (5′-AGACCCAAATCTTTTGCGGAGGTT-3′) corresponds to the region 1,306–1,326, relative to the first nucleotide of the start codon (GenBank Accession No. NM_145766.1). Nonspecific control siRNA is an irrelevant siRNA with random nucleotides and no known specificity. Transfection of PC12 cells with duplex synthetic siRNA was performed using lipofectamine2000 reagents (Invitrogen) according to the manufacturer’s instructions. Cells were assayed after 48 h of transfection. For mock transfection, all procedures listed above were performed in the absence of siRNA duplex.
Plasmid CDK11p58 and Transfection
For transient transfection, the CDK11p58 expression vector, the nonspecific vector was carried out using lipofectamine2000 (Invitrogen) and plus reagent in OptiMEM (Invitrogen) as suggested by the manufacturer. Transfected cells were used for the subsequent experiments 48 h after transfection.
Statistical Analysis
At least three repetitive assessments were performed, and for each assessment, all groups were tested in quadruplicate. All data were given in terms of relative values and expressed as mean ± standard error. One-way ANOVA was used to compare differences between the operated groups and the control group. All statistical analyses were conducted with a STATA 7.0 software package (Stata Corp., College Station, TX, USA), and all statistical significance levels were set at p < 0.05.
Densitometric Analyses
The density of specific bands was measured with a computer-assisted image-analysis system (Adobe Systems, San Jose, CA, USA) and normalized against GAPDH level. Differences between the control and treatment groups were calculated and expressed as relative increases setting the control as 1. Values were obtained from at least three independent experiments.
Results
LPS-Treated ACM-Induced PC12 Cells to Express CDK11p58 Protein in a LPS Concentration-Dependent Manner
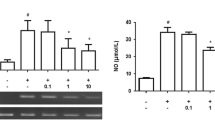
To detect the effect of ACM on CDK11p58 protein expression in PC12 cells, C6 cells, a rat astrocyte cell line were treated by LPS with different concentrations (0, 0.001, 0.01, 0.1, 1, and 10 μg/ml) for 24 h, then ACM was added in PC12 cells for 24 h. PC12 cells treated directly with different concentration LPS have no difference in CDK11p58 protein expression or apoptosis status (data not shown). Western blotting results showed CDK11p58 protein expression in PC12 cells changed in ACM that treated with different dose of LPS in a LPS concentration-dependent manner (Fig. 1a, b).
LPS-treated ACM-induced PC12 cells to express CDK11p58 in a LPS concentration-dependent manner. a CDK11p110 and CDK11p58 protein expressions in PC12 cells after treated by LPS-stimulated ACM at different concentration detected by western blotting. b Quantitation of CDK11p110 and CDK11p58 protein expressions. *p < 0.05 as compared with the control group for each concentration point
LPS-Treated ACM-Induced PC12 Cells Apoptosis in a LPS Concentration-Dependent Manner
To understand whether ACM regulates neuronal apoptosis, proliferation marker PCNA, anti-apoptosis molecule Bcl-2, and pro-apoptosis molecules cleaved-PARP, cleaved-caspase-3 protein expression in PC12 cells after stimulated by ACM was detected by western blotting. The data illustrated that proliferation marker PCNA and anti-apoptosis molecule Bcl-2 decreased (Fig. 2a, left, line 1, line 2) parallel to ACM LPS concentration (0, 0.001, 0.01, 0.1, 1, and 10 μg/ml). On the contrary, pro-apoptosis molecules cleaved-PARP and cleaved-caspase-3 (Fig. 2a, left, line 3, line 4) increased due to ACM LPS concentration (0, 0.001, 0.01, 0.1, 1, and 10 μg/ml). TUNEL stain positive PC12 cells increased after treated with LPS 10 μg/ml ACM compared to that of treated with LPS 0 μg/ml ACM (Fig. 2b). DAPI nuclei stain of PC12 cells showed nuclear fragment and condense in LPS 10 μg/ml ACM treatment group (Fig. 2b). Altogether, the results above indicated that LPS-treated ACM-induced PC12 cells apoptosis in a LPS concentration-dependent manner similar to that of CDK11p58 protein expression in PC12 cells treated with ACM, suggesting that CDK11p58 possibly participates in ACM-induced neuronal apoptosis process.
LPS-treated ACM-induced PC12 cells apoptosis in a LPS concentration-dependent manner. a Cell proliferation marker PCNA, anti-apoptosis molecule Bcl-2 and pro-apoptosis molecules cleaved-PARP, cleaved-caspase-3 protein expression in PC12 cells determined by western blot. *, **, ## p < 0.05 as compared with the control group for each molecule. b TUNEL and DAPI stains of PC12 cells treated with LPS 0 μg/ml and LPS 10 μg/ml ACM. The scale bar represents 50 um
CDK11p58 Upregulates Neuronal Apoptosis in PC12 Cells Treated with ACM
To further certify the function of CDK11p58 in ACM-induced neuronal apoptosis process, CDK11p58 siRNA and CDK11p58 plasmid were constructed and transfected into PC12 cells. Due to CDK11p58 knockdown, CDK11p58 protein level decreased 38 % and 36 % without or with ACM treatment, respectively. Protein expression of cell proliferation marker PCNA, anti-apoptosis molecule Bcl-2 increased, as well as pro-apoptosis molecules cleaved-PARP, cleaved-caspase-3 decreased (Fig. 3a) in PC12 cells after knockdown of CDK11p58. PI and Annexin V double stain showed that knockdown of CDK11p58 repressed PC12 cell apoptosis even after ACM treatment (Fig. 3a). To further identify CDK11p58 regulated neuronal apoptosis after ACM treatment, we constructed HA-CDK11p58 and transfected PC12 with it. Compared with HA vector, CDK11p58 protein expression increased 41 % and 36 % with or without ACM administration. Overexpression of CDK11p58 promoted PC12 cell apoptosis demonstrated by western blotting (Fig. 4a) and flow cytometry results (Fig. 4b). Thus, we drew a conclusion that CDK11p58 acts as a positive regulator during ACM-induced PC12 cell apoptosis.
CDK11p58 knockdown represses PC12 cells apoptosis. a CDK11p58, PCNA, Bcl-2, cleaved-PARP, and cleaved-caspase-3 protein expression detected by western blotting after non-transfection, nonspecific siRNA transfection, and CDK11p58 siRNA transfection with or without ACM treatment. *, **, ## p < 0.05 as compared with the control group for each molecule. b Flow cytometry analysis with PI, Annexin V double stain of PC12 cells after non-transfection, nonspecific siRNA transfection, and CDK11p58 siRNA transfection with or without ACM treatment
CDK11p58 overexpression enhances PC12 cells apoptosis. a CDK11p58, PCNA, Bcl-2, cleaved-PARP, and cleaved-caspase-3 protein expression detected by western blotting after non-transfection, HA vector transfection, and HA-CDK11p58 transfection with or without ACM treatment. *, **, ## p < 0.05 as compared with the control group for each molecule. b Flow cytometry analysis with PI, Annexin V double stain of PC12 cells after non-transfection, HA vector transfection, and HA-CDK11p58 transfection with or without ACM treatment
AKT Signaling Pathway is Involved in ACM-Induced PC12 Cell Apoptosis
To determine whether AKT signaling pathway participates in ACM-induced PC12 cell apoptosis, we detected the phosphorylation level of AKT. The phosphorylation level of AKT decreased in PC12 cells treated by ACM in a LPS concentration-dependent manner (0, 0.001, 0.01, 0.1, 1, and 10 μg/ml) (Fig. 5a). Administration of AKT inhibitor LY294002 facilitated PC12 cell apoptosis (Fig. 5b). The data revealed that ACM induces PC12 cell apoptosis via upregulating CDK11p58 expression through inhibited AKT signaling pathway.
CDK11p58 upregulates PC12 cells apoptosis via downregulating AKT signaling pathway. a p-AKT and AKT protein expression in PC12 cells treated by LPS-stimulated ACM in different LPS concentration. *p < 0.05 as compared with the control group for each concentration point. b p-AKT, AKT protein expression, and cell viability detection in PC12 cells treated by LPS-stimulated ACM with or without LY294002. *, **, ## p < 0.05 as compared with the control group
Discussion
In this study, it was observed that ACM-stimulated PC12 cells became dramatically sensitive to ACM-induced neuronal apoptosis via CDK11p58 upregulation through downregulating AKT activation signaling pathway.
Astrocytes are the most numerous cell type within the central nervous system (CNS) and perform a variety of tasks from axon guidance and synaptic support to the control of the blood–brain barrier and blood flow (Benediktsson et al. 2012; Vedam-Mai et al. 2012). However, the major features of brain inflammation are glial cells activation and neuronal apoptosis (Hirsch et al. 2009). Although the pro-inflammatory function of astrocytes is generally acknowledged not to be as prominent as that of microglia (Streit et al. 1999), lots of studies have shown that astrocytes-mediated inflammatory responses also play a leading role in the pathogenesis of various brain injuries (Dietrich et al. 2003; Jiang et al. 2010). Reactive astrocytes may lose neuroprotective functions or gain neurotoxic properties in neurodegenerative diseases. In this study, we first found that conditioned medium from LPS-stimulated C6 cells induces PC12 apoptosis. However, previous studies exposed that astrocyte-conditioned medium protected neurons against some poisons, such as corticosterone (Zhu et al. 2006), copper-catalyzed cysteine (Wang and Cynader 2001), and ethanol (Lamarche et al. 2004). The reason for these differences maybe is that astrocytes possess double-side functions, depending on brain injury situations such as lasting time, happening site, and injury type.
To further reveal the machinery in PC12 apoptosis, we detected CDK11p58 protein expression during PC12 apoptosis process. Our data showed that PC12 CDK11p58 protein expression parallels its apoptosis degree. CDK11p58 knockdown and overexpression verified that CDK11p58 promotes neuronal apoptosis. Our previous research (Liu et al. 2012) demonstrated that CDK11p58 promotes rat astrocyte cell C6 activation and inflammatory response via activating p38 and JNK pathways induced by LPS. Taken together, these data illustrated that CDK11p58 is a regulatory molecular with multi-functions in different CNS cells. Besides CDK11p58, neuronal cells also secrete other substances to regulate neuronal apoptosis. For example, Wang et al. 2005 found that proinflammatory factors such as TNF-α, IL-1β, NO, and superoxide, contributed at different degrees to LPS-treated ACM-induced [Ca2+] increase and apoptosis in PC12 cells.
Akt has been recognized as one of the main protein kinases that promote neuronal survival (Yano et al. 2001; Zhang et al. 2004). AKT serves as a pro-survival signaling pathway (Brunet et al. 2001) and inactivation of AKT signaling has been implicated in many apoptotic paradigms (Arboleda et al. 2010; Crowder and Freeman 1998; Lee et al. 2011). Further extending these findings, our data indicated inhibited AKT pathway during neuronal apoptosis. The use of the chemical proteomic platform developed in this study has revealed a new subset of possible targets for LY294002, whose inhibition could affect various cellular processes particularly metabolism, transcription or protein trafficking, and dynamics. The major nonlipid kinases identified in this study have been reported previously (CK2, mTOR, and GSK3β) (protein kinase inhibitors Jacobs et al. 2005; Tolloczko et al. 2004). The serine/threonine kinase CK2 plays a putative role in proliferation and other cellular processes through activation of transcription factors and other cell signaling proteins, and its overexpression in cancer has been reported (Landesman-Bollag et al. 2001). In addition, GSK3 is involved in the regulation of glycogen metabolism through the inactivation of glycogen synthase and it also has an important role in regulating many signaling pathways (Doble and Woodgett 2003). This kinase is a known substrate of Akt (Cross et al. 1995) and is therefore regulated downstream of the class I PI3Ks.
In this study, we also found an extremely interesting phenomenon that LPS treatment directly to PC12 culture has no effect on CDK11p58 expression or neuronal apoptosis whereas it does when applied to C6 culture. It’s perhaps because PC12 cells have no LPS receptors and consequently have no ability to accept LPS stimulation.
In summary, we have delineated a key pathway involved in the regulation of neuronal apoptosis induced by astrocytes stimulated by LPS. In future studies, it will be important to investigate the role of this pathway in vivo models of neuronal injury and neurodegenerative diseases and to explore the therapeutic potential of targeting this pathway.
References
Arboleda G, Cardenas Y, Rodriguez Y, Morales LC, Matheus L, Arboleda H (2010) Differential regulation of AKT, MAPK and GSK3beta during C2-ceramide-induced neuronal death. Neurotoxicology 31(6):687–693
Bajic VP, Su B, Lee HG, Kudo W, Siedlak SL, Zivkovic L et al (2011) Mislocalization of CDK11/PITSLRE, a regulator of the G2/M phase of the cell cycle Alzheimer disease. Cell Mol Biol Lett 16(3):359–372
Benediktsson AM, Marrs GS, Tu JC, Worley PF, Rothstein JD, Bergles DE et al (2012) Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia 60(2):175–188
Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11(3):297–305
Cai MM, Zhang SW, Zhang S, Chen S, Yan J, Zhu XY et al (2002) Different effects of p58PITSLRE on the apoptosis induced by etoposide, cycloheximide and serum-withdrawal in human hepatocarcinoma cells. Mol Cell Biochem 238(1–2):49–55
Chalhoub N, Zhu G, Zhu X, Baker SJ (2009) Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes Dev 23(14):1619–1624
Choi HH, Choi HK, Jung SY, Hyle J, Kim BJ, Yoon K et al (2012) CHK2 kinase promotes pre-mRNA splicing via phosphorylating CDK11(p110). Oncogene. doi:10.1038/onc.2012.535
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378(6559):785–789
Crowder RJ, Freeman RS (1998) Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci 18(8):2933–2943
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y et al (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91(2):231–241
Dietrich P, Rideout HJ, Wang Q, Stefanis L (2003) Lack of p53 delays apoptosis, but increases ubiquitinated inclusions, in proteasomal inhibitor-treated cultured cortical neurons. Mol Cell Neurosci 24(2):430–441
Doble BW, Woodgett JR (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116(Pt 7):1175–1186
Drogat J, Migeot V, Mommaerts E, Mullier C, Dieu M, van Bakel H et al (2012) Cdk11-cyclinL controls the assembly of the RNA polymerase II mediator complex. Cell Rep 2(5):1068–1076
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7(8):606–619
Hirsch HV, Possidente D, Averill S, Despain TP, Buytkins J, Thomas V et al (2009) Variations at a quantitative trait locus (QTL) affect development of behavior in lead-exposed Drosophila melanogaster. Neurotoxicology 30(2):305–311
Jacobs MD, Black J, Futer O, Swenson L, Hare B, Fleming M et al (2005) Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J Biol Chem 280(14):13728–13734
Jiang B, Sohya K, Sarihi A, Yanagawa Y, Tsumoto T (2010) Laminar-specific maturation of GABAergic transmission and susceptibility to visual deprivation are related to endocannabinoid sensitivity in mouse visual cortex. J Neurosci 30(42):14261–14272
Lamarche F, Signorini-Allibe N, Gonthier B, Barret L (2004) Influence of vitamin E, sodium selenite, and astrocyte-conditioned medium on neuronal survival after chronic exposure to ethanol. Alcohol 33(2):127–138
Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC (2001) Protein kinase CK2 in mammary gland tumorigenesis. Oncogene 20(25):3247–3257
Lee JE, Kang JS, Ki YW, Lee SH, Lee SJ, Lee KS et al (2011) Akt/GSK3beta signaling is involved in fipronil-induced apoptotic cell death of human neuroblastoma SH-SY5Y cells. Toxicol Lett 202(2):133–141
Liu X, Cheng C, Shao B, Wu X, Ji Y, Liu Y et al (2012) CDK11(p58) promotes rat astrocyte inflammatory response via activating p38 and JNK pathways induced by lipopolysaccharide. Neurochem Res 37(3):563–573
Mikolajczyk M, Nelson MA (2004) Regulation of stability of cyclin-dependent kinase CDK11p110 and a caspase-processed form, CDK11p46, by Hsp90. Biochem J 384(Pt 3):461–467
Minghetti L, Ajmone-Cat MA, De Berardinis MA, De Simone R (2005) Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Brain Res Rev 48(2):251–256
Ridet JL, Malhotra SK, Privat A, Gage FH (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20(12):570–577
Seifert G, Schilling K, Steinhauser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7(3):194–206
Streit WJ, Walter SA, Pennell NA (1999) Reactive microgliosis. Prog Neurobiol 57(6):563–581
Tolloczko B, Turkewitsch P, Al-Chalabi M, Martin JG (2004) LY-294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one] affects calcium signaling in airway smooth muscle cells independently of phosphoinositide 3-kinase inhibition. J Pharmacol Exp Ther 311(2):787–793
Vedam-Mai V, van Battum EY, Kamphuis W, Feenstra MG, Denys D, Reynolds BA et al (2012) Deep brain stimulation and the role of astrocytes. Mol Psychiatry 17(2):124–131, 115
Wagner-Golbs A, Luhmann HJ (2012) Activity-dependent survival of developing neocortical neurons depends on PI3K signalling. J Neurochem 120(4):495–501
Wang XF, Cynader MS (2001) Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J Neurosci 21(10):3322–3331
Wang X, Chen S, Ma G, Ye M, Lu G (2005) Involvement of proinflammatory factors, apoptosis, caspase-3 activation and Ca2 + disturbance in microglia activation-mediated dopaminergic cell degeneration. Mech Ageing Dev 126(12):1241–1254
Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease–a double-edged sword. Neuron 35(3):419–432
Yano S, Morioka M, Fukunaga K, Kawano T, Hara T, Kai Y et al (2001) Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab 21(4):351–360
Yun X, Wu Y, Yao L, Zong H, Hong Y, Jiang J et al (2007) CDK11(p58) protein kinase activity is associated with Bcl-2 down-regulation in pro-apoptosis pathway. Mol Cell Biochem 304(1–2):213–218
Zhang F, Yin W, Chen J (2004) Apoptosis in cerebral ischemia: executional and regulatory signaling mechanisms. Neurol Res 26(8):835–845
Zhu ZH, Yang R, Fu X, Wang YQ, Wu GC (2006) Astrocyte-conditioned medium protecting hippocampal neurons in primary cultures against corticosterone-induced damages via PI3-K/Akt signal pathway. Brain Res 1114(1):1–10
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (Nos. 31070723, 81070275, 81172879, and 31270802); Key Project Natural Science Foundation of Jiangsu University and College (No. 11KJA310002); A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, X., Cheng, C., Shao, B. et al. LPS-Stimulating Astrocyte-Conditioned Medium Causes Neuronal Apoptosis Via Increasing CDK11p58 Expression in PC12 Cells Through Downregulating AKT Pathway. Cell Mol Neurobiol 33, 779–787 (2013). https://doi.org/10.1007/s10571-013-9945-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-9945-4