Abstract
We first verified that a single chain Fv fragment against prion protein (anti-PrP scFv) was secreted by HEK293T cells and prevented prion replication in infected cells. We then stably expressed anti-PrP scFv in brain-engraftable murine microglial cells and intracerebrally injected these cells into mice before or after infection with prions. Interestingly, the injection before or at an early time point after infection attenuated the infection marginally but significantly prolonged survival times of the mice. These suggest that the ex vivo gene transfer of anti-PrP scFvs using brain-engraftable cells could be a possible immunotherapeutic approach against prion diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prion diseases including Creutzfeldt–Jakob disease in humans are a group of fatal neurodegenerative disorders caused by the so-called prion (Prusiner 1998). Prions consist of the partially proteinase K (PK)-resistant, amyloidogenic isoform of PrP (designated PrPSc), and propagate by catalyzing conformational conversion of the host-encoded PK-sensitive, normal isoform of PrP (PrPC) into PrPSc (Prusiner 1998). The constitutive conversion of PrPC to PrPSc results in marked accumulation of PrPSc in the brain, suggesting that the accumulated PrPSc may be toxic to neurons, thereby causing degenerative neuronal cell death in prion diseases. Indeed, mice devoid of PrPC (Prnp 0/0) are resistant to prions, neither producing PrPSc or prions in the brain nor developing the disease (Bueler et al. 1993; Manson et al. 1994; Prusiner et al. 1993; Sakaguchi et al. 1995). However, the molecular pathogenesis underlying the neurodegeneration remains largely unknown.
It has been shown that certain anti-PrP antibodies (Abs) exhibit an anti-prion activity, reducing PrPSc levels in prion-infected mouse neuroblastoma N2a cells and eventually curing those cells when added to the culture media (Enari et al. 2001; Peretz et al. 2001). These results give rise to the possibility of immunotherapy against prion diseases. Indeed, Song et al. directly infused 31C6 anti-PrP monoclonal Ab (mAb) via an intraventricular route into the brains of mice that had been infected by Chandler or Obihiro scrapie prions, showing that the mAb could attenuate their resulting experimental prion diseases (Song et al. 2008). However, the effectiveness was very marginal, with the survival times extended only by ~10 days. This very limited anti-prion effect of the intraventricularly infused 31C6 mAbs is probably because, due to its large molecular size, the mAb could not efficiently infiltrate into the brain regions that are relevant to the therapy against prion diseases. Therefore, reduction of the molecular size of anti-PrP Abs may be beneficial to augmentation of their anti-prion effects in vivo. Importantly, it was already shown that anti-PrP single chain Fv (scFv) antibodies were effective against prions in infected cells (Campana et al. 2008; Donofrio et al. 2005).
Microglia are glial cells that infiltrate and accumulate at the pathological lesions affected by prions (Kopacek et al. 2000). One of us showed that an immortalized rat microglial cell line expressing the ex vivo transfected-lacZ gene could be engrafted into the rat brain, surviving for at least the 3 weeks it was monitored (Sawada et al. 1998), and also successfully established the immortalized murine microglial Ra2 cell line (Kanzawa et al. 2000). Another colleague produced 3S9 anti-PrP mAb that exhibited strong anti-prion activity (0.6 nM IC50) in infected N2a cells (Miyamoto et al. 2005). We are therefore interested in investigating the possible effects of Ra2 cell-mediated ex vivo gene transfer of 3S9 anti-PrP scFv (3S9scFv) on prion diseases. In this study, we established a Ra2 microglial cell line expressing 3S9scFv tagged with a myc-epitope and showed that intracerebral injection of these cells before or at early time points after experimental prion infection was marginally but still significantly effective, prolonging survival times in mice.
Materials and Methods
Construction of Lentivirus Expression Vectors
To construct lentivirus expression vectors encoding 3S9scFv, each cDNA for the heavy (H) and light (L) chains of 3S9 mAb were first cloned using the SMART™ RACE cDNA Amplification Kit (Clontech, California, USA) according to the user manual (GenBank accession No. HM627495 and HM627496). In brief, total RNA extracted from 3S9 hybridoma was subjected to first strand cDNA synthesis followed by nested polymerase chain reaction (PCR). The first PCR was performed using the Universal Primer (Clontech) as a sense primer and an H chain-specific antisense primer 1 (Table 1) or a kappa L chain-specific antisense primer 1 (Table 1). The second PCR was subsequently done using the Nested Universal Primer (Clontech) as a sense primer and an H chain-specific antisense primer 2 (Table 1) or a kappa L chain-specific antisense primer 2 (Table 1). Each PCR product was cloned into a pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA), resulting in pTOPO-3S9H and pTOPO-3S9L.
To construct CS-CA-3S9scFv, the DNA fragment for the H chain variable region of 3S9 mAb was amplified by PCR using pTOPO-3S9H as a template with a scFv sense primer containing an EcoRI site (Table 1) and a sh-HV-a3 antisense primer including a SalI site as well as a linker-coding sequence (Table 1). The DNA fragment for the L chain variable region of 3S9 mAb was also amplified by PCR using pTOPO-3S9L as a template with a sh-LV-S4 primer containing a SalI site (Table 1) and a scFv-myc-XhoI antisense primer including a myc tag-coding sequence, two copies of a stop codon and an XhoI site (Table 1). The EcoRI–SalI DNA fragment of the H chain variable region and the SalI–XhoI DNA fragment of the L chain variable region with a myc tag were ligated in tandem into the EcoRI–XhoI digested CS-CA-MCA vector (provided from RIKEN BioResource Center, Tsukuba, Japan), resulting in CS-CA-3S9scFv.
For construction of CS-CA-3S9scFv/IRES-hrGFP, the XhoI–Bsp1407 I DNA fragment including an internal ribosome entry site (IRES) sequence followed by the 5′ region of humanized recombinant green fluorescent protein (hrGFP) sequence was isolated from pIRES-hrGFP-2a (Clontech), and the 3′ region of hrGFP was amplified by PCR using pIRES-hrGFP-2a as a template with an hrGFP-Bsp1407 I sense primer (Table 1) and an hrGFP-XhoI antisense primer (Table 1). The isolated XhoI–Bsp1407 I DNA fragment and the amplified Bsp1407 I–Xho I fragment of the 3′ region of hrGFP were ligated in tandem into the XhoI-digested CS-CA-3S9scFv vector, resulting in CS-CA-3S9scFv/IRES-hrGFP. CS-CA-GFP was from RIKEN BioResource Center.
Preparation of Lentiviral Vectors
Lentiviral vectors were prepared as described elsewhere (Miyoshi et al. 1999). In brief, human embryonic kidney HEK293T cells were transfected with CS-CA-3S9scFv/IRES-hrGFP or CS-CA-GFP, together with pCAG-HIVgp (RIKEN BioResource Center) and pCMV-VSV-G-RSV-Rev (RIKEN BioResource Center) using Lipofectamine 2000 reagent (Invitrogen). The culture media containing lentivirus vectors were collected 48 h after transfection and filtered through a 0.45-μm filter. An aliquot of the media was stored at −30°C until use. The others were ultracentrifuged using an SCP85H ultracentrifuge (Hitachi, Tokyo, Japan) and the resulting pellet was suspended in Hank’s Balanced Salt Solution (Invitrogen) and stored at −80°C until use.
Enzyme-Linked Immunosorbent Assay (ELISA)
Mouse recombinant PrP (mo-recPrP) with a 6 × His tag was purified elsewhere (Yamanaka et al. 2006). Each well of a 96-well plate was coated with mo-recPrP by overnight incubation in 50 mM carbonate buffer at 4°C and then blocked with Blocking One reagent (Nacalai tesque, Kyoto, Japan) for 1 h at room temperature (RT). The wells were incubated for 1 h at RT with the culture medium (100 μl/well) from HEK293T cells transfected with either CS-CA-3S9scFv or CS-CA-MCS, and washed with 0.05% Tween 20-containing phosphate-buffered saline (PBS). Immune complexes were detected using mouse anti-c-myc mAb (Biomol, Farmingdale, NY), horseradish peroxidase (HRP)-conjugated sheep anti-mouse Ab (GE Healthcare, Buckinghamshire, England), and 1-Step™ Ultra TMB-ELISA (Pierce, Rockford, IL). Colorimetric values were measured at 450 nm using MULTISKAN JX (Thermo Electron Corporation, Waltham, MA).
Fluorescence-Activated Cell Sorter (FACS) Analysis
PrPC-deficient hippocampal neurons, designated HpL3-4 (Kuwahara et al. 1999), and HpL3-4 cells overexpressing exogenous PrPC, designated HpL3-4TR (Kuwahara et al. 1999) (kindly provided by Prof. Onodera, The University of Tokyo, Japan), were harvested in PBS containing 20 mM EDTA. The cells were then incubated for 1 h on ice with the culture medium from HEK293T cells transfected with either CS-CA-3S9scFv or CS-CA-MCS, and washed with BSS buffer (140 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 1 mM CaCl2, pH 7.0). Immune complexes were detected using mouse anti-c-myc Ab (Biomol) and Alexa Fluor® 546 goat anti-mouse IgG (Invitrogen) and analyzed using a flow cytometer (EPICS® XL-MCL, Beckman Coulter, Brea, CA). Microglial cells were harvested in PBS using rubber scrapers and analyzed for expression of hrGFP using a flow cytometer (BD FACSCanto™ II Flow Cytometer, BD Biosciences, San Jose, CA).
In vitro Anti-Prion Activity Assay
Mouse PrPC-overexpressing N2a cells, designated N2aC24, were established by transfection with pEF1/Myc-His A (Invitrogen) encoding mouse PrPC, designated pEF1-moPrP, and by subsequent cloning of G418 (Nacalai tesque)-resistant colonies using cloning cylinders. The pEF1-moPrP was constructed by insertion of the PCR-amplified mouse PrP ORF (GenBank accession No. M13685). N2aC24 cells were then exposed to 22L and Chandler scrapie prions by incubation with a lysate of 22L or Chandler prion-infected N2a58 cells (kindly provided by Prof. Nishida, Nagasaki University, Japan) (Atarashi et al. 2006), and 22L and Chandler prion-infected N2aC24 clones, designated N2aC24L1-3 and N2aC24Chm, respectively, were obtained by limiting dilution. To assess the in vitro anti-prion activity of 3S9scFv, the infected cells were incubated with the 3S9scFv-containing culture medium for 72 h, lysed in a buffer (150 mM NaCl, 0.5% Triton X-100, 0.5% sodium deoxycholate, 50 mM Tris–HCl, pH 7.5), and subjected to Western blotting for PrPSc.
Cloning of 3S9scFv/GFP-Ra2 and GFP-Ra2
The microglial cell line Ra2 was cultured in Eagle’s MEM (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum, 0.1% of glucose, 2 μg/l of recombinant mouse GM-CSF (R&D Systems, Minneapolis, MN) and 5 mg/l of bovine insulin (Sigma, St. Louis, MO). Ra2 was infected by the concentrated lentivirus encoding Sh3.9scFv/IRES-hrGFP or GFP alone with 1 multiplicity of infection, and 3S9scFv/GFP-Ra2 and GFP-Ra2 cells were cloned by limiting dilution.
Reverse Transcription-PCR (RT-PCR) Analysis
RT-PCR was performed with SuperScript™ III One-Step RT-PCR System with Platinum® Taq High Fidelity (Invitrogen). In brief, total RNA extracted from cells was treated with DNase I for 15 min at RT and heated at 65°C for 10 min to destroy the DNase I activity. The treated RNA was then subjected to 1st strand cDNA synthesis using SuperScript™ III reverse transcriptase with random hexamer. After treatment with RNase H at 37°C for 20 min, the first strand cDNAs for 3S9scFv and mouse glyceraldehyde-3-phosphate dehydrogenase (moGAPDH) were amplified by PCR using primer pairs of 3S9scFv-myc sense and antisense primers (Table 1) or moGAPDH sense and antisense primers, respectively (Table 1).
Western Blot Analysis
Proteins were denatured in Laemli’s sample buffer, separated on a 12% SDS-polyacrylamide gel, and onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA). Thereafter, the membrane was blocked with 5% fat-free dry milk in TBST buffer (10 mM Tris–HCl, pH 7.8, 100 mM NaCl, 0.1% Tween 20) for 1 h at RT and incubated with mouse anti-c-myc mAb (Biomol), rabbit anti-c-myc polyclonal Ab (Medical & Biological Laboratories Co., Ltd., Nagoya, Japan), or goat anti-PrP M-20 Ab (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight in TBST buffer containing 1% fat-free dry milk. Immune complexes were detected using 1 h-incubation with HRP-conjugated anti-mouse IgG (GE Healthcare), anti-rabbit IgG (GE Healthcare), or anti-goat IgG (Millipore), and then visualized by LAS-4000 (Fujifilm, Tokyo, Japan) using ECL Plus Western Blotting System (GE Healthcare) or Immobilon™ Western Chemiluminesent HRP substrate (Millipore).
Prion Inoculation
Brains were removed from ddY mice showing terminal symptoms due to infection with Chandler or 22L scrapie prions and a 10% (w/v) brain homogenate was prepared in MEM (Invitrogen). Five-week-old C57BL/6 mice (CLEA Japan, Tokyo, Japan) were intracerebrally inoculated with a 20 μl-aliquot of the homogenate, containing 1.1 × 104 ID50 RML prions or 0.94 × 103 ID50 22L prions. Mice were cared for in accordance with the Guidelines for Animal Experimentation of The University of Tokushima.
Microglia Inoculation
1 × 106 microglial cells suspended in 20 μl PBS were intracerebrally injected into each mouse at the indicated times before or after inoculation with prions. Mice were cared for in accordance with the Guidelines for Animal Experimentation of The University of Tokushima.
Determination of the Terminal Stage of the Disease
Mice were observed daily and diagnosed as terminal when they developed more than five of the following features: emaciation, decreased locomotion, ruffled body hair, ataxic gait, kyphosis, priapism, upright tail, and paralysis of the hind legs.
Results
Characterization of Recombinant 3S9scFv Produced by HEK293T Cells
We transiently transfected CS-CA-3S9scFv and control CS-CA-MCS expression vectors (Fig. 1a) into HEK293T cells and then carried out western blotting of the cell lysates prepared 2 days after transfection using anti-myc antibody. No signals could be detected in the control CS-CA-MCS-transfected cells (Fig. 1b). In contrast, two strong discrete signals were observed in the CS-CA-3S9scFv-transfected cells (Fig. 1b). They are glycosylated and unglycosylated recombinant 3S9scFvs because the glycosylation signal is found in the variable region of the L chain of 3S9 mAb and the upper band was shifted to the lower band after PNGase F treatment (data not shown). The same signals were detected in the media of CS-CA-3S9scFv-transfected cells (hereafter referred to as the 3S9scFv media), but not in the media of control CS-CA-MCS-transfected cells (hereafter referred to as the control media) (Fig. 1b). These results indicate that recombinant 3S9scFv is produced by HEK293T cells and secreted into the culture media.
3S9scFv is produced by HEK293T cells. a Schematic representations of CS-CA-MCS and CS-CA-3S9scFv lentivirus expression vectors. CMV represents human cytomegalovirus immediate early promoter; R, the R region of the 5′ or 3′ long term repeat (LTR) from human immunodeficiency virus type 1 (HIV-1); U5, the U5 region of the 5′ or 3′ LTR from HIV-1; del U3, deletion of enhancer and promoter sequences in the U3 region of LTR from HIV-1; 5′ SD, 5′ splicing donor site; 3′ SD, 3′ splicing acceptor site; RRE, Rev responsive element; PRE, Woodchuck hepatitis virus posttranscriptional regulatory element; CAG, CMV immediate early enhancer and chicken β-actin gene promoter; BGH pA, bovine growth hormone polyadenylation signal; MCS, multiple cloning site; SS, signal sequence; HV, the variable region of 3S9 heavy chain; LV, the variable region of 3S9 light chain. b Western blotting of 3S9scFv produced by HEK293T cells. Cell lysates and culture media from HEK293T cells transfected with CS-CA-MCS or CS-CA-3S9scFv were subjected to Western blotting with mouse anti-c-myc mAb. Two discrete bands correspond to glycosylated and unglycosylated 3S9scFvs
We then investigated the recombinant 3S9scFv for its binding ability to PrP by an ELISA assay using mo-recPrP. No increase in colorimetric value at 450 nm was detected with the control media (Fig. 2a). In contrast, the colorimetric value of mo-recPrP in 3S9scFv media increased in a dose-dependent manner (Fig. 2a). These results clearly indicate that recombinant 3S9scFv binds to mo-recPrP. We also investigated whether the recombinant 3S9scFv could bind to native PrP or PrPC expressed on the cell surface using HpL3-4 and HpL3-4TR cells by FACS analysis. HpL3-4 cells are devoid of PrPC while HpL3-4TR cells express abundant PrPC on their surface. Both media induced no signal shift of HpL3-4 cells (Fig. 2b). In contrast, the signal peak of HpL3-4TR cells was shifted to the right by the 3S9scFv media in a dose-dependent manner whereas no signal shift caused by the control media could be detected (Fig. 2b). These results indicate that 3S9scFv produced by HEK293T cells recognizes PrPC expressed on the cell surface.
Binding of 3S9scFv produced by HEK293T cells to PrP. a ELISA analysis of 3S9scFv showing its binding activity to mo-recPrP. 3S9scFv-containing culture media showed higher colorimetric values at OD450 in a dose-dependent manner of mo-recPrP. In contrast, no binding was detected with the culture media from control cells. b FACS analysis of 3S9scFv showing its binding activity to PrPC expressed on the cell surface. PrPC-negative HpL3-4 cells showed no signal shift with both the control and the 3S9scFv-containing culture media (upper panels). On the other hand, PrPC-expressing HpL3-4TR cells exhibited positive signals with 3S9scFv-containing culture media, but not with the control media (lower panels)
Finally, we investigated the recombinant 3S9scFv for its anti-prion activity. 22L prion-infected N2aC24L1-3 and Chandler prion-infected N2aC24Chm cells were incubated with either 3S9scFv media or the control media for 3 days and subjected to western blotting for detection of PrPSc. No decrease in PrPSc levels could be detected in both infected cells when incubated with the control media (Fig. 3). In contrast, the 3S9scFv media markedly reduced PrPSc levels in both N2aC24L1-3 and N2aC24Chm cells in a dose-dependent manner (Fig. 3). No cell death was observed in these cells treated with the 3S9scFv media (data not shown). These results clearly indicate that recombinant 3S9scFv produced by HEK293T cells is effective against both prions.
In vitro anti-prion activity of 3S9scFv produced by HEK293T cells. The control and 3S9scFv-containing culture media were added at the indicated ratio into the culture medium of 22L prion-infected N2aC24L1-3 cells (a) or Chandler prion-infected N2aC24Chm cells (b). PrPSc levels in these infected cells were markedly reduced in a dose-dependent manner of 3S9scFv-containing culture media added
Establishment of a Ra2 Microglial Cell Clone Stably Expressing 3S9scFv
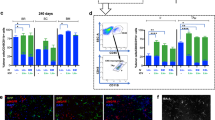
We constructed CS-CA-3S9scFv/IRES-hrGFP expression vector by introducing IRES-hrGFP sequence downstream of the 3S9scFv sequence in CS-CA-3S9scFv expression vector (Fig. 4a) and produced lentivirus vector encoding 3S9scFv and hrGFP. We then infected Ra2 microglial cells with the lentivirus vector and successfully cloned a 3S9scFv/GFP-Ra2 cell line (Fig. 4b) by limiting dilution. Subsequent FACS analysis revealed that about 90% of the cells were positive for hrGFP expression (Fig. 4b). We also cloned control GFP-Ra2 cells, which express GFP alone. The expression of 3S9scFv was confirmed using RT-PCR and western blotting. No 3S9scFv mRNA expression was detected in control GFP-Ra2 cells (Fig. 4c). In contrast, 3S9scFv mRNA was expressed in 3S9scFv/GFP-Ra2 cells (Fig. 4c). Consistently, western blotting showed that 3S9scFv was expressed in 3S9scFv/GFP-Ra2 cells, but not in GFP-Ra2 cells (Fig. 4d).
3S9scFv expressed by Ra2 cells. a A schematic representation of part of the CS-CA-3S9scFv/IRES-hrGFP expression vector. Primer pairs used for RT-PCR are indicated by arrows. b Phase contrast picture (left panel), fluorescent microscopic picture (middle panel), and FACS analysis (right panel) of 3S9scFv/GFP-Ra2 cells. c RT-PCR for 3S9scFv. 3S9scFv mRNA expression was detected in 3S9scFv/GFP-Ra2 cells but not in GFP-Ra2 cells. GAPDH mRNA was similarly expressed in both cells. d Western blotting for 3S9scFv. Lysates from GFP-Ra2 and 3S9scFv/GFP-Ra2 cells were probed with rabbit polyclonal anti-c-myc antibodies. Glycosylated and unglycosylated 3S9scFvs were expressed in 3S9scFv/GFP-Ra2 cells but not in GFP-Ra2 cells
Effects of 3S9scFv/GFP-Ra2 Cells on Scrapie Prions in Mice
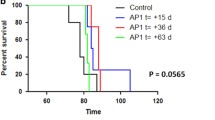
To investigate the possible effects of the brain-engraftable cells-mediated ex vivo gene transfer of 3S9scFv on prions in mice, we directly injected 3S9scFv/GFP-Ra2 microglial cells into the right cerebrum at 1 week and into the left cerebrum at 3 weeks before inoculation of mouse-adapted Chandler prions into the right cerebrum. As controls, we similarly injected GFP-Ra2 microglial cells. Survival times of the mice injected with 3S9scFv/GFP-Ra2 cells were marginally but still significantly elongated, compared to those of the control mice (P = 0.014, Logrank test, Fig. 5a). No differences in PrPSc levels were detected between the brains of mice treated with GFP-Ra2 and 3S9scFv/GFP-Ra2 microglial cells (Fig. 5b). We also injected 3S9scFv/GFP-Ra2 microglial cells into the right cerebrum of mice 7 or 13 weeks after infection with Chandler or 22L scrapie prions. No significant elongation of the survival times could be observed in the mice infected with Chandler prions (Fig. 6). However, 3S9scFv/GFP-Ra2 cells were partially but still significantly effective against 22L prions when injected 7 weeks after infection (P = 0.035, Logrank test, Fig. 6), but not 13 weeks after infection (Fig. 6). Western blotting showed no decrease of PrPSc in the brains of terminally ill mice injected with 3S9scFv/GFP-Ra2 cells (data not shown). We also failed to detect the injected 3S9scFv/GFP-Ra2 cells in the brains of terminally ill mice, even at the injection sites by immunohistochemistry using anti-hrGFP and anti-c-myc Abs (data not shown).
a Survival curves of mice prophylactically injected with 3S9scFv/GFP-Ra2 cells. Mice (n = 10) intracerebrally injected with 3S9scFv/GFP-Ra2 cells before infection with Chandler prions survived significantly longer than those (n = 10) injected with control GFP-Ra2 cells (P = 0.014, Logrank test). b Western blotting of the brains of two mice from each mouse group. No decrease in PrPSc levels was observed in the brains of 3S9scFv/GFP-Ra2 cells-injected mice, compared to the GFP-Ra2 cells-injected control mice
Survival curves of mice intracerebrally injected with 3S9scFv/GFP-Ra2 cells 7 and 12 weeks after infection with prions. No anti-prion effects of 3S9scFv/GFP-Ra2 cells were detected in mice infected with Chandler prions. In contrast, injection of 3S9scFv/GFP-Ra2 cells 7 weeks after infection with 22L prions significantly extended survival times in mice, compared to injection of GFP-Ra2 cells (P = 0.035, Logrank test). No extension in survival times could be detected in 22L prion-infected mice when 3S9scFv/GFP-Ra2 cells were injected 13 weeks after infection commenced. Each group comprises 9–10 mice
Discussion
Since it was shown that anti-PrP mAbs or Fab fragments prevented PrPSc formation and prion propagation in prion-infected N2a cells, eventually rescuing the cells from prion infection (Enari et al. 2001; Peretz et al. 2001), the prospect of immunotherapy against prion diseases has attracted considerable attention. On the other hand, neurotoxic adverse effects were reported for some anti-PrP mAbs and F(ab′)2 fragments when they were injected into the brains of normal and prion-infected mice (Lefebvre-Roque et al. 2007; Solforosi et al. 2004), giving rise to great caution for development of the immunotherapy against prion diseases. However, no adverse effects such as neurotoxicity and autoimmune reactions were observed in mice intraventricularly administered with 31C6 and 3S9 mAbs (Sakaguchi et al. 2009; Song et al. 2008). This therefore indicates that anti-PrP mAb-mediated neurotoxicity appears to be associated with specific epitopes on PrP. Thus, an appropriate selection of non-toxic anti-PrP mAbs, such as 31C6 and 3S9 mAbs, may be required for use in immunotherapy of prion diseases.
No effective regimens for the immunotherapy of prion diseases have been developed. Direct intraventricular infusion of anti-PrP mAbs had very limited or no effect on survival times in mice that had been experimentally infected with mouse-adapted prions (Sakaguchi et al. 2009; Song et al. 2008). Wuertzer et al. (2008) reported the possibility of an alternative regimen using recombinant adeno-associated vector 2 (rAAV2)-mediated gene transfer of anti-PrP scFvs to the brain. They showed that prophylactic injection of the rAAV2 vector encoding anti-PrP scFvs into the thalamus and striatum 1 month before intraperitoneal infection with Chandler prions extended survival times by 50 days in mice. However, no data were available for the therapeutic injection of anti-PrP scFv-encoding rAAV2 vectors. In this study, we presented another possibility for the ex vivo gene transfer of anti-PrP scFvs using brain-engraftable cells as an immunotherapeutic approach against prion diseases.
We showed that recombinant 3S9scFv produced by HEK293T cells could bind to both recombinant PrP and PrPC, native PrP, and prevent PrPSc formation in both 22L and Chandler prion-infected N2a cells, N2aC24L1-3, and N2aC24Chm cells. Similar results were reported by other investigators, showing that 6H4 anti-PrP mAb-derived scFv, which was produced by stably transfected human rhabdomyosarcoma RD-4 cells, reduced PrPSc levels in prion-infected N2a/Bos2 cells (Donofrio et al. 2005). These results clearly indicate that recombinant anti-PrP scFvs secreted from cultured cells are active against prions. We then established a 3S9scFv/GFP-Ra2 cell line, a brain-engraftable Ra2 microgial cell line stably expressing 3S9scFv, and intracerebrally injected these cells into mice before or after infection with Chandler or 22L prions. No autoimmune responses such as lymphocyte infiltration were observed in the brain (data not shown), suggesting that, similarly to parent 3S9 anti-PrP mAb, 3S9scFv might not induce autoimmune reactions. At present, we do not know whether or not 3S9scFv/GFP-Ra2 cells themselves could be infected with prions in the brain. However, transient transfection of the CS-CA-3S9scFv expression vector prevented PrPSc formation in N2aC24L1-3 cells (data not shown), suggesting the unlikelihood of 3S9scFv/GFP-Ra2 cells becoming infected. Nonetheless, the injection of 3S9scFv/GFP-Ra2 cells either before or at an early time point after the infection, significantly prolonged survival times of the mice. Altogether, these results suggest that the ex vivo gene transfer of 3S9scFv using brain-engraftable Ra2 microglial cells might be effective against prions.
The anti-prion effects were different against 22L and Chandler prions. Different anti-prion effects were also reported with 31C6 anti-PrP mAb. The mAb was more effective against Chandler prions than Obihiro prions when intraventricularly infused into mice (Song et al. 2008). This is possibly because different strains of prions affect different brain regions and therefore the brain regions relevant to therapy might be somewhat different from one strain to another. It is also possible that, since 22L prions were inoculated into mice with titers more than tenfold less than the Chandler prions, the different effects of 3S9scFv/GFP-Ra2 cells against 22L and Chandler prions in the mice might result from difference in the inoculation titers. Moreover, the anti-prion effects were very marginal. This might be due to the short lifetime of Ra2 cells in vivo. The injected 3S9scFv/GFP-Ra2 cells were not found in the brains of terminally ill mice by immunohistochemistry using anti-hrGFP and anti-c-myc Abs (data not shown). Alternatively, low expression of 3S9scFv in 3S9scFv/GFP-Ra2 cells might be attributable to the marginal anti-prion effects. Or, both might be mutually associated with the marginal anti-prion effects.
The ex vivo gene transfer of anti-PrP Abs into the brain may be advantageous as an immunotherapeutic approach against prion diseases over the direct intraventricular infusion or the virus vector-mediated gene transfer methods. The intraventricularly infused Abs and the scFvs delivered by virus vectors could spread to only restricted regions that are close to sites where the Abs or virus vectors are injected, resulting in a limited effect on PrPSc formation and prion propagation in the brain. Greater therapeutic effect may be expected by the ex vivo gene transfer of anti-PrP Abs using brain-engraftable cells, because these cells have a potential to migrate to broad regions after engraftment into the brain. Indeed, it was recently reported that bone marrow-derived mesenchymal stem cells could spread widely as differentiated cells including astrocytes, oligodenrocytes and neurons, throughout the brains of mice that had been infected with prions, when engrafted into the hippocampus or even intravenously injected (Song et al. 2009). Taken together with our present results, these indicate that the brain-engraftable cells-mediated ex vivo gene transfer of anti-PrP Abs might be a possible immunotherapeutic approach against prion diseases. However, further studies are necessary for this kind of immunotherapeutic approach against prion diseases to be practically effective.
References
Atarashi R, Sim VL, Nishida N, Caughey B, Katamine S (2006) Prion strain-dependent differences in conversion of mutant prion proteins in cell culture. J Virol 80:7854–7862
Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C (1993) Mice devoid of PrP are resistant to scrapie. Cell 73:1339–1347
Campana V, Zentilin L, Mirabile I, Kranjc A, Casanova P, Giacca M, Prusiner S, Legname G, Zurzolo C (2008) Development of antibody fragments for immunotherapy of prion diseases. Biochem J 418:507–515
Donofrio G, Heppner FL, Polymenidou M, Musahl C, Aguzzi A (2005) Paracrine inhibition of prion propagation by anti-PrP single-chain Fv miniantibodies. J Virol 79:8330–8338
Enari M, Flechsig E, Weissmann C (2001) Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci USA 98:9295–9299
Kanzawa T, Sawada M, Kato K, Yamamoto K, Mori H, Tanaka R (2000) Differentiated regulation of allo-antigen presentation by different types of murine microglial cell lines. J Neurosci Res 62:383–388
Kopacek J, Sakaguchi S, Shigematsu K, Nishida N, Atarashi R, Nakaoke R, Moriuchi R, Niwa M, Katamine S (2000) Upregulation of the genes encoding lysosomal hydrolases, a perforin-like protein, and peroxidases in the brains of mice affected with an experimental prion disease. J Virol 74:411–417
Kuwahara C, Takeuchi AM, Nishimura T, Haraguchi K, Kubosaki A, Matsumoto Y, Saeki K, Matsumoto Y, Yokoyama T, Itohara S, Onodera T (1999) Prions prevent neuronal cell-line death. Nature 400:225–226
Lefebvre-Roque M, Kremmer E, Gilch S, Zou WQ, Feraudet C, Gilles CM, Sales N, Grassi J, Gambetti P, Baron T, Schatzl H, Lasmezas CI (2007) Toxic effects of intracerebral PrP antibody administration during the course of BSE infection in mice. Prion 1:198–206
Manson JC, Clarke AR, McBride PA, McConnell I, Hope J (1994) PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration 3:331–340
Miyamoto K, Nakamura N, Aosasa M, Nishida N, Yokoyama T, Horiuchi H, Furusawa S, Matsuda H (2005) Inhibition of prion propagation in scrapie-infected mouse neuroblastoma cell lines using mouse monoclonal antibodies against prion protein. Biochem Biophys Res Commun 335:197–204
Miyoshi H, Smith KA, Mosier DE, Verma IM, Torbett BE (1999) Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science 283:682–686
Peretz D, Williamson RA, Kaneko K, Vergara J, Leclerc E, Schmitt-Ulms G, Mehlhorn IR, Legname G, Wormald MR, Rudd PM, Dwek RA, Burton DR, Prusiner SB (2001) Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412:739–743
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:13363–13383
Prusiner SB, Groth D, Serban A, Koehler R, Foster D, Torchia M, Burton D, Yang SL, DeArmond SJ (1993) Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc Natl Acad Sci USA 90:10608–10612
Sakaguchi S, Katamine S, Shigematsu K, Nakatani A, Moriuchi R, Nishida N, Kurokawa K, Nakaoke R, Sato H, Jishage K, Kuno J, Noda T, Miyamoto T (1995) Accumulation of proteinase K-resistant prion protein (PrP) is restricted by the expression level of normal PrP in mice inoculated with a mouse-adapted strain of the Creutzfeldt-Jakob disease agent. J Virol 69:7586–7592
Sakaguchi S, Ishibashi D, Matsuda H (2009) Antibody-based immunotherapeutic attempts in experimental animal models of prion diseases. Expert Opin Ther Pat 19:907–917
Sawada M, Imai F, Suzuki H, Hayakawa M, Kanno T, Nagatsu T (1998) Brain-specific gene expression by immortalized microglial cell-mediated gene transfer in the mammalian brain. FEBS Lett 433:37–40
Solforosi L, Criado JR, McGavern DB, Wirz S, Sanchez-Alavez M, Sugama S, DeGiorgio LA, Volpe BT, Wiseman E, Abalos G, Masliah E, Gilden D, Oldstone MB, Conti B, Williamson RA (2004) Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 303:1514–1516
Song CH, Furuoka H, Kim CL, Ogino M, Suzuki A, Hasebe R, Horiuchi M (2008) Effect of intraventricular infusion of anti-prion protein monoclonal antibodies on disease progression in prion-infected mice. J Gen Virol 89:1533–1544
Song CH, Honmou O, Ohsawa N, Nakamura K, Hamada H, Furuoka H, Hasebe R, Horiuchi M (2009) Effect of transplantation of bone marrow-derived mesenchymal stem cells on mice infected with prions. J Virol 83:5918–5927
Wuertzer CA, Sullivan MA, Qiu X, Federoff HJ (2008) CNS delivery of vectored prion-specific single-chain antibodies delays disease onset. Mol Ther 16:481–486
Yamanaka H, Ishibashi D, Yamaguchi N, Yoshikawa D, Nakamura R, Okimura N, Arakawa T, Tsuji T, Katamine S, Sakaguchi S (2006) Enhanced mucosal immunogenicity of prion protein following fusion with B subunit of Escherichia coli heat-labile enterotoxin. Vaccine 24:2815–2823
Acknowledgments
This work was supported by a grant from the Ministry of Health and Welfare of Japan in part and a Cooperative Research Grant of the Institute for Enzyme Research, the University of Tokushima.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, K., Yamaguchi, Y., Mori, T. et al. Effects of a Brain-Engraftable Microglial Cell Line Expressing Anti-Prion scFv Antibodies on Survival Times of Mice Infected with Scrapie Prions. Cell Mol Neurobiol 31, 999–1008 (2011). https://doi.org/10.1007/s10571-011-9696-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9696-z